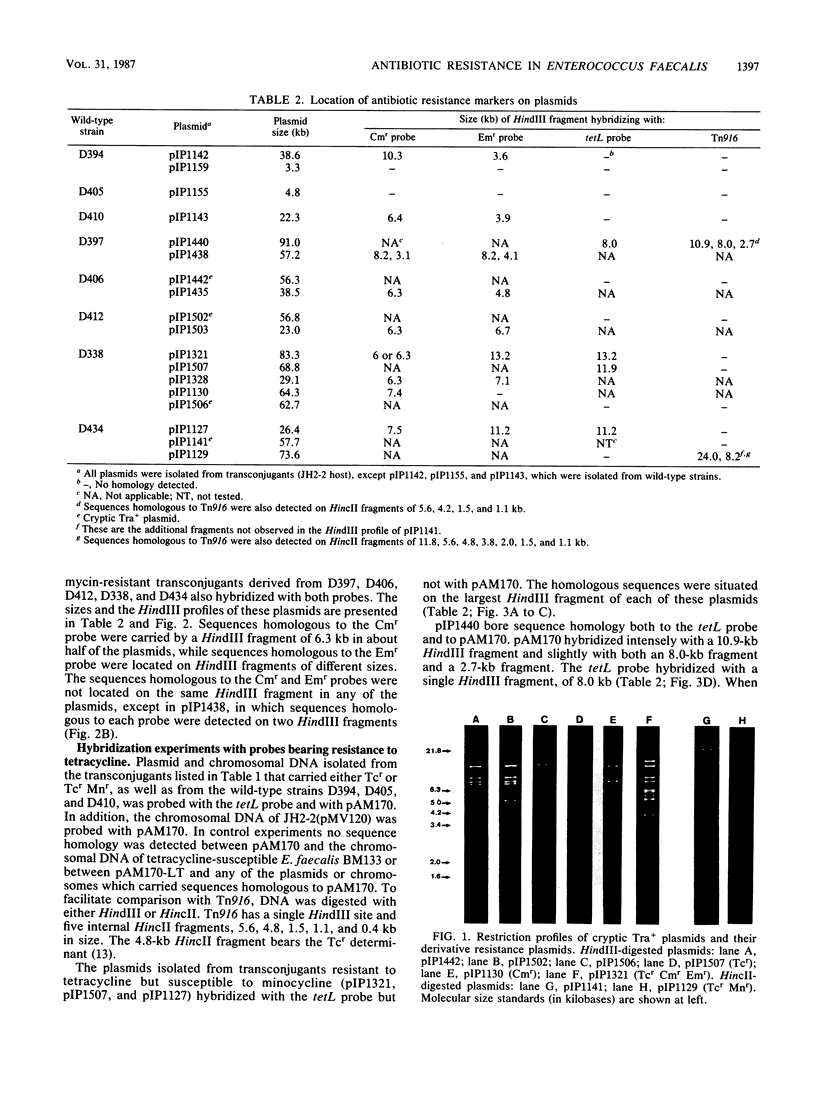
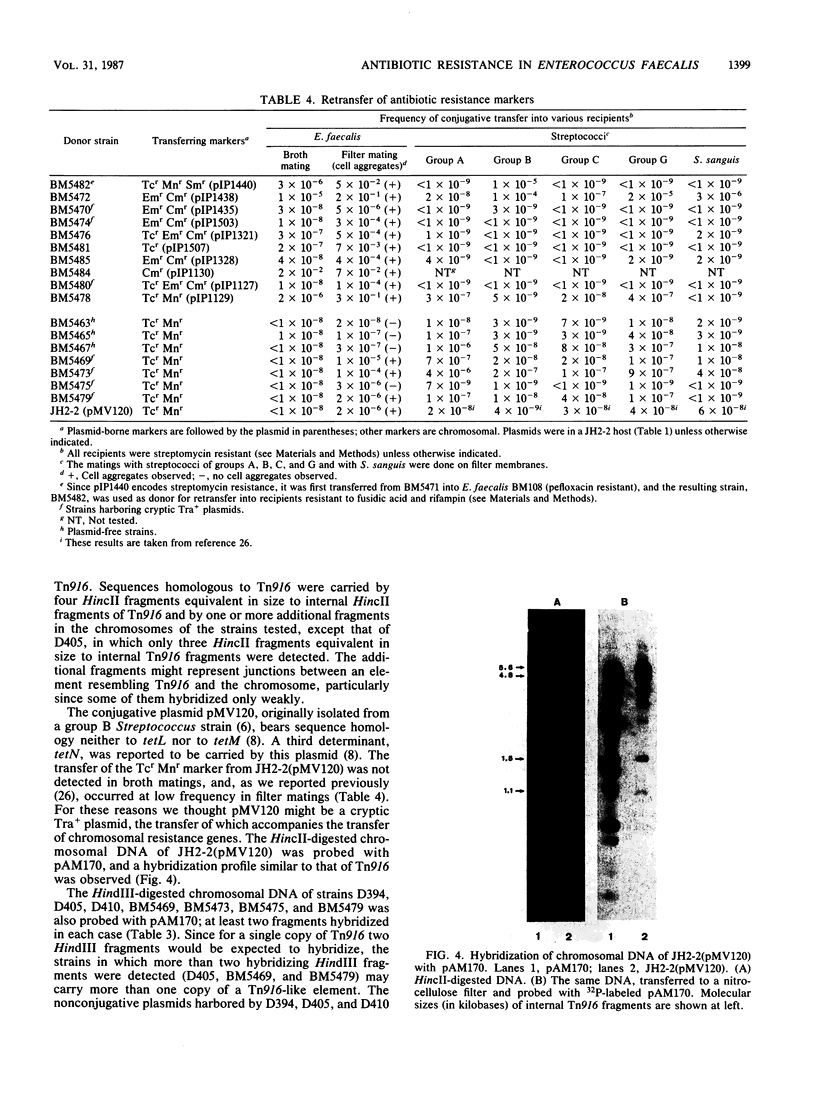
Abstract
Eight wild-type strains of Enterococcus faecalis, resistant to chloramphenicol (Cmr), erythromycin (Emr), tetracycline (Tcr), and minocycline (Mnr), were examined for the genetic basis of their antibiotic resistance, Five of the strains transferred all of their antibiotic resistance markers by conjugation, while the other three strains transferred only Tcr and Mnr. Cmr and Emr determinants were localized by DNA-DNA hybridization experiments, in which the Cmr gene of plasmid pIP501, of group B Streptococcus origin, and the Emr gene of transposon Tn917, of E. faecalis origin, served as probes. A chromosomal location was found for the nonconjugative Cmr and Emr markers of one wild-type strain. In two strains these markers were carried by nonconjugative plasmids, and in the other strains they were carried by plasmids that transferred by conjugation. Plasmids isolated from three transconjugants resistant to tetracycline but susceptible to minocycline bore nucleotide sequences homologous to the tetL gene. Nucleotide sequences homologous to conjugative transposon Tn916, of E. faecalis origin, were detected by hybridization in the tetracycline-minocycline-resistant transconjugants. Three of these transconjugants were plasmid free, while four harbored conjugative cryptic plasmids. Sequences homologous to Tn916 were also found on two conjugative plasmids, one of which appeared to be a conjugative cryptic plasmid that had acquired chromosomal Tcr Mnr markers during transfer.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banai M., LeBlanc D. J. Streptococcus faecalis R plasmid pJH1 contains an erythromycin resistance transposon (Tn3871) similar to transposon Tn917. J Bacteriol. 1984 Jun;158(3):1172–1174. doi: 10.1128/jb.158.3.1172-1174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Gilmore M. S. Location of antibiotic resistance determinants, copy control, and replication functions on the double-selective streptococcal cloning vector pGB301. Mol Gen Genet. 1981;184(1):115–120. doi: 10.1007/BF00271206. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1980 Nov;18(5):753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Streptococcal tetracycline resistance mediated at the level of protein synthesis. J Bacteriol. 1986 Feb;165(2):564–569. doi: 10.1128/jb.165.2.564-569.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Dunny G. M. Identification of regions of the Streptococcus faecalis plasmid pCF-10 that encode antibiotic resistance and pheromone response functions. Plasmid. 1986 May;15(3):230–241. doi: 10.1016/0147-619x(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmar I., Horaud T. Enterococcus faecalis hemolysin-bacteriocin plasmids belong to the same incompatibility group. Appl Environ Microbiol. 1987 Mar;53(3):567–570. doi: 10.1128/aem.53.3.567-570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. F., Clewell D. B. A conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun. 1985 Feb;47(2):415–420. doi: 10.1128/iai.47.2.415-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horaud T., Le Bouguenec C., Pepper K. Molecular genetics of resistance to macrolides, lincosamides and streptogramin B (MLS) in streptococci. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):111–135. doi: 10.1093/jac/16.suppl_a.111. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H., Bieth G., Chabbert Y. A. R plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1976 Nov;10(5):795–801. doi: 10.1128/aac.10.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bouanchaud D. H., Chabbert Y. A. Conjugative R plasmids in Streptococcus agalactiae (group B). Plasmid. 1979 Apr;2(2):197–206. doi: 10.1016/0147-619x(79)90038-6. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Le Bouguenec C., Bieth G. Narrow host range of some streptococcal R plasmids. Plasmid. 1982 Sep;8(2):199–206. doi: 10.1016/0147-619x(82)90057-9. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Delbos F. Les streptocoques du groupe D dans les infections humaines: identification et sensibilité aux antibiotiques. Ann Microbiol (Paris) 1980 Sep-Oct;131B(2):131–144. [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Horaud T., Bieth G., Colimon R., Dauguet C. Translocation of antibiotic resistance markers of a plasmid-free Streptococcus pyogenes (group A) strain into different streptococcal hemolysin plasmids. Mol Gen Genet. 1984;194(3):377–387. doi: 10.1007/BF00425548. [DOI] [PubMed] [Google Scholar]
- Le Bouguenec C., Horodniceanu T. Conjugative R plasmids in Streptococcus faecium (group D). Antimicrob Agents Chemother. 1982 May;21(5):698–705. doi: 10.1128/aac.21.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N. Characterization of two tetracycline resistance determinants in Streptococcus faecalis JH1. J Bacteriol. 1982 May;150(2):835–843. doi: 10.1128/jb.150.2.835-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper K., Le Bouguénec C., de Cespédès G., Horaud T. Dispersal of a plasmid-borne chloramphenicol resistance gene in streptococcal and enterococcal plasmids. Plasmid. 1986 Nov;16(3):195–203. doi: 10.1016/0147-619x(86)90057-0. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. Streptococcus plasmid pAM alpha 1 is a composite of two separable replicons, one of which is closely related to Bacillus plasmid pBC16. J Bacteriol. 1983 Aug;155(2):607–615. doi: 10.1128/jb.155.2.607-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose S. B., Ehrlich S. D. Isolation of plasmid deletion Mutants and study of their instability. Plasmid. 1981 Sep;6(2):193–201. doi: 10.1016/0147-619x(81)90066-4. [DOI] [PubMed] [Google Scholar]
- Rollins L. D., Lee L. N., LeBlanc D. J. Evidence for a disseminated erythromycin resistance determinant mediated by Tn917-like sequences among group D streptococci isolated from pigs, chickens, and humans. Antimicrob Agents Chemother. 1985 Apr;27(4):439–444. doi: 10.1128/aac.27.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brenner D. G., LeGrice S. F., Skinner S. E., Hawkins A. R. Chloramphenicol acetyltransferase gene of staphylococcal plasmid pC221. Nucleotide sequence analysis and expression studies. FEBS Lett. 1985 Jan 1;179(1):101–106. doi: 10.1016/0014-5793(85)80200-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]