Abstract
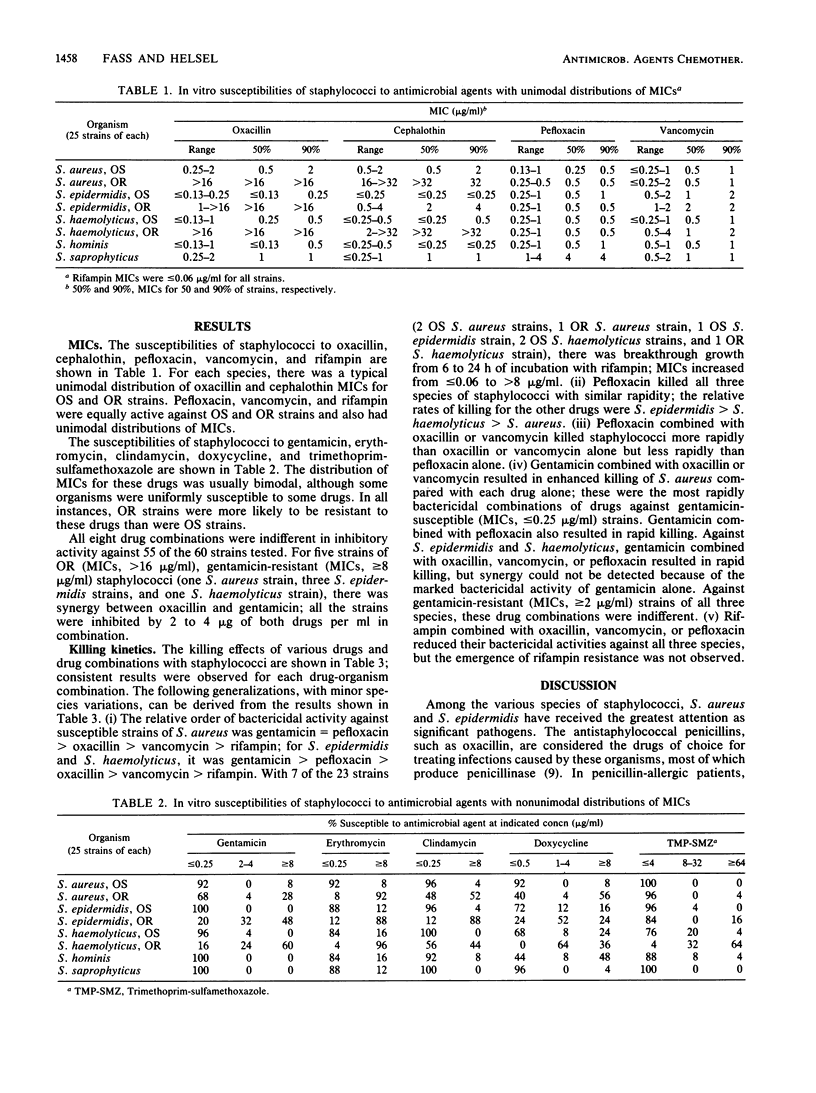
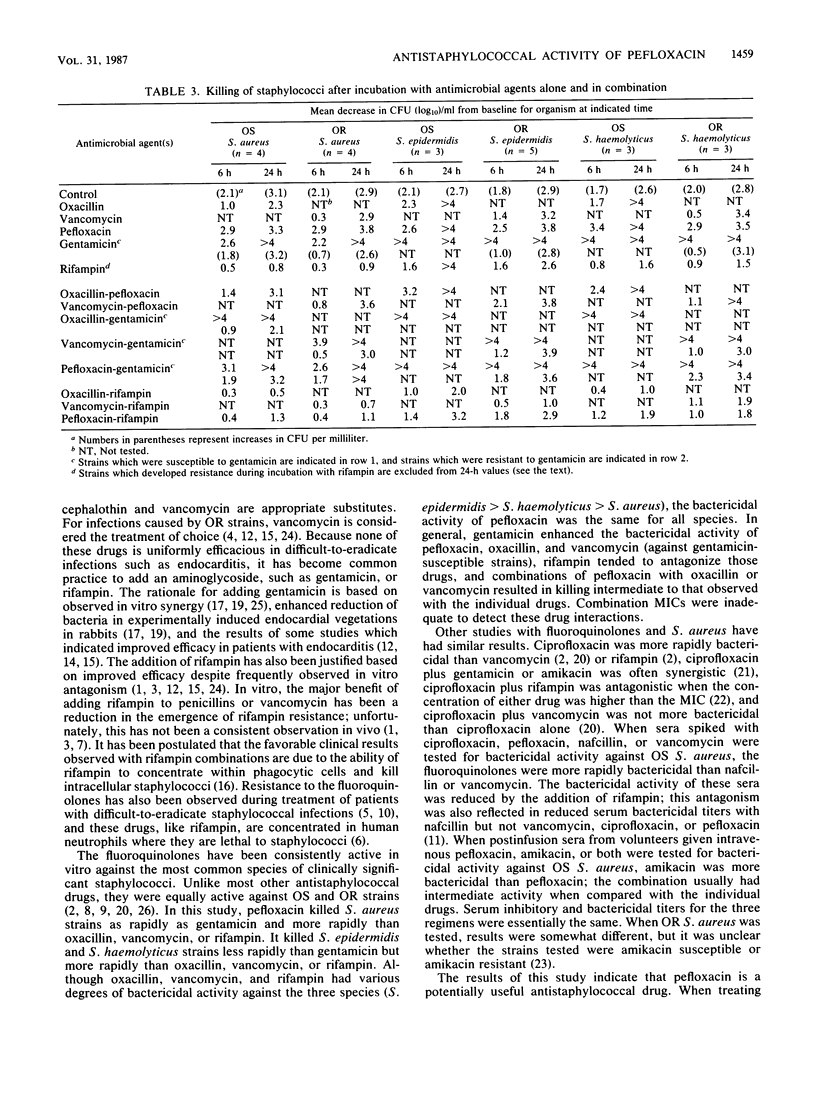
MICs of pefloxacin and nine antistaphylococcal drugs were determined for 200 isolates of Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, and Staphylococcus saprophyticus. All the strains were susceptible to pefloxacin, vancomycin, and rifampin. Oxacillin-resistant strains were uniformly resistant to cephalothin and were more likely to be resistant to gentamicin, erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole than were oxacillin-susceptible strains. Time-kill studies with 23 strains of S. aureus, S. epidermidis, and S. haemolyticus indicated that the relative order of bactericidal activities was gentamicin greater than or equal to pefloxacin greater than oxacillin greater than vancomycin greater than rifampin. Pefloxacin combined with oxacillin or vancomycin killed staphylococci more rapidly than oxacillin or vancomycin alone but less rapidly than pefloxacin alone. Gentamicin combined with oxacillin, vancomycin, or pefloxacin resulted in the most rapid killing of gentamicin-susceptible strains. Rifampin combined with oxacillin, vancomycin, or pefloxacin reduced the bactericidal activities of those drugs, but rifampin resistance was not observed as it was with rifampin alone. Pefloxacin is a potentially useful antistaphylococcal agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Janney A., Sanders C. V. Comparison of the activities of coumermycin, ciprofloxacin, teicoplanin, and other non-beta-lactam antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus from various geographical locations. Antimicrob Agents Chemother. 1985 Nov;28(5):634–638. doi: 10.1128/aac.28.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven D. E., Kollisch N. R., Hsieh C. R., Connolly M. G., Jr, McCabe W. R. Vancomycin treatment of bacteremia caused by oxacillin-resistant Staphylococcus aureus: comparison with beta-lactam antibiotic treatment of bacteremia caused by oxacillin-sensitive Staphylococcus aureus. J Infect Dis. 1983 Jan;147(1):137–143. doi: 10.1093/infdis/147.1.137. [DOI] [PubMed] [Google Scholar]
- Desplaces N., Gutmann L., Carlet J., Guibert J., Acar J. F. The new quinolones and their combinations with other agents for therapy of severe infections. J Antimicrob Chemother. 1986 Mar;17 (Suppl A):25–39. doi: 10.1093/jac/17.suppl_a.25. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Crane J. P. Uptake of ciprofloxacin by human neutrophils. J Antimicrob Chemother. 1985 Jul;16(1):67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Smith S. M., Tillem M., Cherubin C. Rifampin resistance. Development during the therapy of methicillin-resistant Staphylococcus aureus infection. Arch Intern Med. 1985 Jan;145(1):146–148. doi: 10.1001/archinte.145.1.146. [DOI] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L., Barnishan J., Ayers L. W. In vitro susceptibilities of four species of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1986 Oct;30(4):545–552. doi: 10.1128/aac.30.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro activity of RO 23-6240 (AM-833): a new fluoroquinolone. Diagn Microbiol Infect Dis. 1987 Apr;6(4):293–299. doi: 10.1016/0732-8893(87)90178-7. [DOI] [PubMed] [Google Scholar]
- Greenberg R. N., Kennedy D. J., Reilly P. M., Luppen K. L., Weinandt W. J., Bollinger M. R., Aguirre F., Kodesch F., Saeed A. M. Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):151–155. doi: 10.1128/aac.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F., Sande M. A. Serum bactericidal activity of rifampin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):611–613. doi: 10.1128/aac.29.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A. R., Kirby C. D., Eddins D. L., Orenstein W. A., Bernier R. H., Turner P. M., Bart K. J. Elimination of indigenous measles from the United States. Rev Infect Dis. 1983 May-Jun;5(3):538–545. doi: 10.1093/clinids/5.3.538. [DOI] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski O., Sande M. A. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: A prospective study. Ann Intern Med. 1982 Oct;97(4):496–503. doi: 10.7326/0003-4819-97-4-496. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Hammer S. M. Staphylococcus epidermidis infections. Ann Intern Med. 1983 Dec;99(6):834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Vest T. K. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis. 1972 May;125(5):486–490. doi: 10.1093/infdis/125.5.486. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Wexler M. A., Steigbigel N. H. Single and combination antibiotic therapy of Staphylococcus aureus experimental endocarditis: emergence of gentamicin-resistant mutants. Antimicrob Agents Chemother. 1978 Sep;14(3):336–343. doi: 10.1128/aac.14.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Courtney K. B. Nafcillin-gentamicin synergism in experimental staphylococcal endocarditis. J Lab Clin Med. 1976 Jul;88(1):118–124. [PubMed] [Google Scholar]
- Smith S. M., Eng R. H. Activity of ciprofloxacin against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):688–691. doi: 10.1128/aac.27.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Berman E. The effect of ciprofloxacin on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1986 Mar;17(3):287–295. doi: 10.1093/jac/17.3.287. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Joly P. Comparative in-vitro activities of teicoplanin, vancomycin, coumermycin and ciprofloxacin, alone and in combination with rifampicin or LM 427, against Staphylococcus aureus. J Antimicrob Chemother. 1987 Mar;19(3):313–320. doi: 10.1093/jac/19.3.313. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Klastersky J., Lieppe S., Husson M., Lauzon D., Lopez A. P. Bactericidal activity and killing rate of serum from volunteers receiving pefloxacin alone or in combination with amikacin. Antimicrob Agents Chemother. 1986 Feb;29(2):230–234. doi: 10.1128/aac.29.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C., Tisone J. C. Synergism between vancomycin and gentamicin or tobramycin for methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1982 Nov;22(5):903–905. doi: 10.1128/aac.22.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


