Abstract
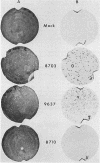
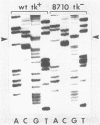
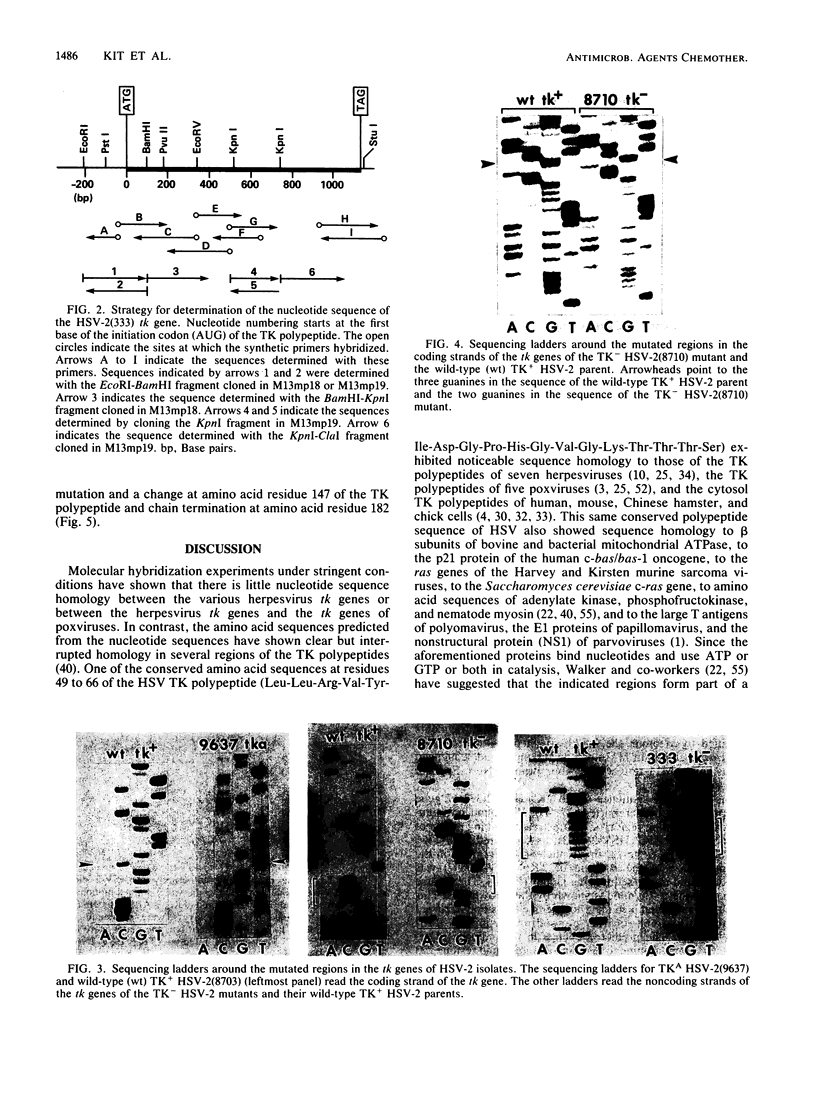
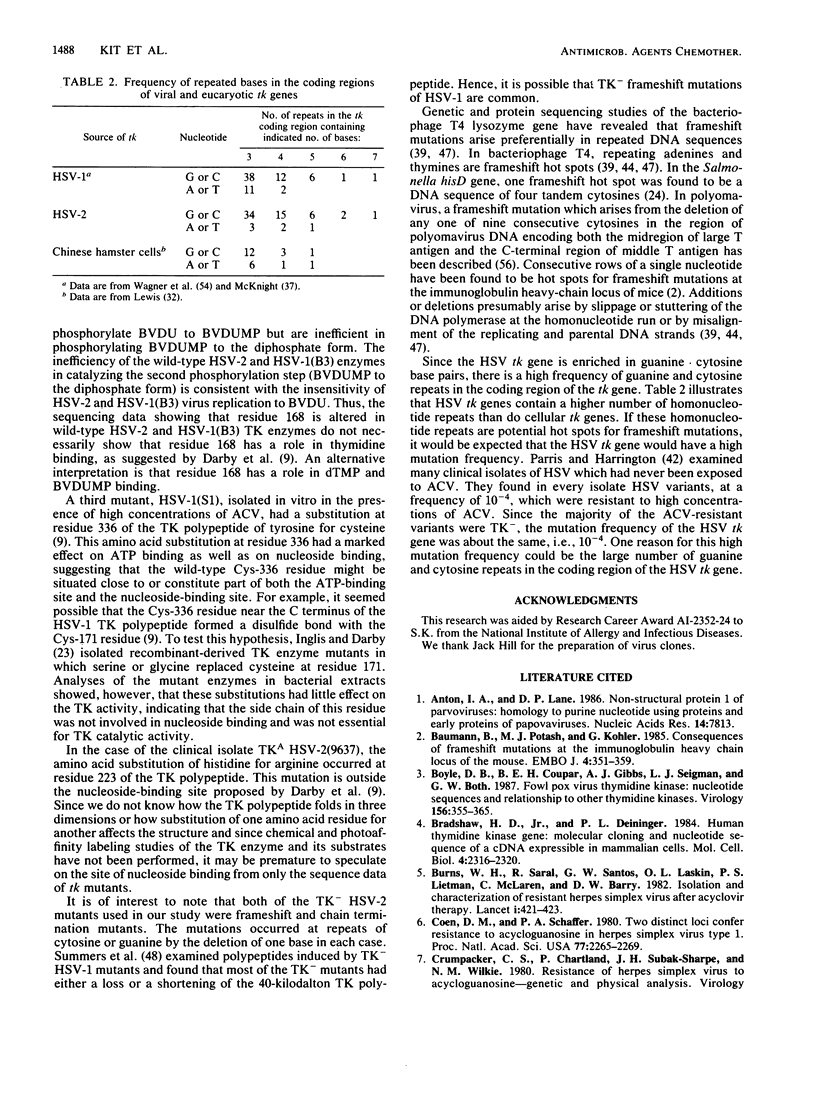
To identify the nucleotide changes that occur in drug-induced thymidine kinase (TK) mutants of herpes simplex virus type 2 (HSV-2), we compared the nucleotide sequences of the tk genes of two mutant HSV-2 clones isolated from a patient who had been treated with acyclovir [9-(2-hydroxyethoxymethyl)guanine; ACV] with the nucleotide sequence of the parental TK+ HSV-2(8703) strain isolated from the same patient. One of the mutants, TK-altered (TKA) HSV-2(9637), was ACV resistant but induced the incorporation of [14C]thymidine into the DNA of infected rabbit skin cells. The nucleotide sequence of the tk gene of mutant TKA HSV-2(9637) had a single change (G to A) at nucleotide 668, which would cause an arginine-to-histidine substitution at amino acid residue 223 of the TK polypeptide. The second ACV-resistant mutant, TK- HSV-2(8710), did not induce detectable incorporation of [14C]thymidine into the DNA of infected rabbit skin cells. This mutant exhibited a deletion of a single base at nucleotide 217 of its nucleotide sequence. This deletion would cause a frameshift mutation at amino acid residue 73 and chain termination at amino acid residue 86 of the TK polypeptide. The nucleotide sequence of TK+ HSV-2(8703) was the same as that of the laboratory strain, TK+ HSV-2(333). The nucleotide sequence of a bromodeoxyuridine-resistant TK- HSV-2(333) mutant of TK+ HSV-2(333) also exhibited a single-base deletion, but at nucleotide 439. This deletion would cause a frameshift mutation at amino acid residue 147 and chain termination at amino acid residue 182. The frameshift mutations of TK- HSV(8710) and TK- HSV-2(333), respectively, occurred in sequences in which C was repeated three times and G was repeated seven times. The results raise the possibility that TK- frameshift mutations of HSV-2 may be common.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anton I. A., Lane D. P. Non-structural protein 1 of parvoviruses: homology to purine nucleotide using proteins and early proteins of papovaviruses. Nucleic Acids Res. 1986 Oct 10;14(19):7813–7813. doi: 10.1093/nar/14.19.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E., Gibbs A. J., Seigman L. J., Both G. W. Fowlpox virus thymidine kinase: nucleotide sequence and relationships to other thymidine kinases. Virology. 1987 Feb;156(2):355–365. doi: 10.1016/0042-6822(87)90415-6. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Deininger P. L. Human thymidine kinase gene: molecular cloning and nucleotide sequence of a cDNA expressible in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2316–2320. doi: 10.1128/mcb.4.11.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns W. H., Saral R., Santos G. W., Laskin O. L., Lietman P. S., McLaren C., Barry D. W. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982 Feb 20;1(8269):421–423. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Dekker C., Ellis M. N., McLaren C., Hunter G., Rogers J., Barry D. W. Virus resistance in clinical practice. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):137–152. doi: 10.1093/jac/12.suppl_b.137. [DOI] [PubMed] [Google Scholar]
- Ellis M. N., Keller P. M., Fyfe J. A., Martin J. L., Rooney J. F., Straus S. E., Lehrman S. N., Barry D. W. Clinical isolate of herpes simplex virus type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob Agents Chemother. 1987 Jul;31(7):1117–1125. doi: 10.1128/aac.31.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G., Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980 Jul;49(1):115–124. doi: 10.1099/0022-1317-49-1-115. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2'-deoxyuridine monophosphate by thymidylate kinases from herpes simplex viruses types 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982 Mar;21(2):432–437. [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Fyfe J. A., McKee S. A., Keller P. M. Altered thymidine-thymidylate kinases from strains of herpes simplex virus with modified drug sensitivities to acyclovir and (E)-5-(2-bromovinyl)-2'-deoxyuridine. Mol Pharmacol. 1983 Sep;24(2):316–323. [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Homology between human bladder carcinoma oncogene product and mitochondrial ATP-synthase. Nature. 1983 Jan 20;301(5897):262–264. doi: 10.1038/301262a0. [DOI] [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Hyman A. L., Chapnick B. M., Kadowitz P. J., Lands W. E., Crawford C. G., Fried J., Barton J. Unusual pulmonary vasodilator activity of 13,14-dehydroprostacyclin methyl ester: comparison with endoperoxides and other prostanoids. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5711–5715. doi: 10.1073/pnas.74.12.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis M. M., Darby G. Analysis of the role of the cysteine 171 residue in the activity of herpes simplex virus type 1 thymidine kinase by oligonucleotide-directed mutagenesis. J Gen Virol. 1987 Jan;68(Pt 1):39–46. doi: 10.1099/0022-1317-68-1-39. [DOI] [PubMed] [Google Scholar]
- Isono K., Yourno J. Chemical carcinogens as frameshift mutagens: Salmonella DNA sequence sensitive to mutagenesis by polycyclic carcinogens. Proc Natl Acad Sci U S A. 1974 May;71(5):1612–1617. doi: 10.1073/pnas.71.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- Kit S., Kit M., Qavi H., Trkula D., Otsuka H. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim Biophys Acta. 1983 Nov 17;741(2):158–170. doi: 10.1016/0167-4781(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Qavi H. Thymidine kinase (TK) induction after infection of TK-deficient rabbit cell mutants with bovine herpesvirus type 1 (BHV-1): isolation of TK- BHV-1 mutants. Virology. 1983 Oct 30;130(2):381–389. doi: 10.1016/0042-6822(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kit S. Thymidine kinase. Microbiol Sci. 1985 Dec;2(12):369–375. [PubMed] [Google Scholar]
- Kit S., Trkula D., Qavi H., Dreesman G., Kennedy R. C., Adler-Storthz K., Kaufman R., Adam E. Sequential genital infections by herpes simplex viruses types 1 and 2: restriction nuclease analyses of viruses from recurrent infections. Sex Transm Dis. 1983 Apr-Jun;10(2):67–71. doi: 10.1097/00007435-198304000-00004. [DOI] [PubMed] [Google Scholar]
- Kwoh T. J., Engler J. A. The nucleotide sequence of the chicken thymidine kinase gene and the relationship of its predicted polypeptide to that of the vaccinia virus thymidine kinase. Nucleic Acids Res. 1984 May 11;12(9):3959–3971. doi: 10.1093/nar/12.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Cheng Y. C., Darby G. Characterization of abnormal thymidine kinases induced by drug-resistant strains of herpes simplex virus type 1. J Gen Virol. 1983 Mar;64(Pt 3):523–532. doi: 10.1099/0022-1317-64-3-523. [DOI] [PubMed] [Google Scholar]
- Lewis J. A. Structure and expression of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1986 Jun;6(6):1998–2010. doi: 10.1128/mcb.6.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Lieberman H. B., Yeh D. B., Xu T., Zhao S. Y., Ruddle F. H. Molecular cloning and structural analysis of murine thymidine kinase genomic and cDNA sequences. Mol Cell Biol. 1985 Nov;5(11):3149–3156. doi: 10.1128/mcb.5.11.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Zeuthen J., McBride A. A., Trøst Sørensen E., Powell K. L., Walsh-Arrand J. E., Arrand J. R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986 Aug;5(8):1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Watanabe K. A., Fox J. J. 2'-fluoro-5-iodo-aracytosine, a potent and selective anti-herpesvirus agent. Antimicrob Agents Chemother. 1980 May;17(5):803–806. doi: 10.1128/aac.17.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Ellis M. N., Keller P. M., Biron K. K., Lehrman S. N., Barry D. W., Furman P. A. Plaque autoradiography assay for the detection and quantitation of thymidine kinase-deficient and thymidine kinase-altered mutants of herpes simplex virus in clinical isolates. Antimicrob Agents Chemother. 1985 Aug;28(2):181–187. doi: 10.1128/aac.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Okada Y., Streisinger G., Owen J. E., Newton J., Tsugita A., Inouye M. Molecular basis of a mutational hot spot in the lysozyme gene of bacteriophage T4. Nature. 1972 Apr 14;236(5346):338–341. doi: 10.1038/236338a0. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Hazen M., Kit M., Qavi H., Kit S. Cloning of the marmoset herpesvirus thymidine kinase gene and analyses of the boundaries of the coding region. Virology. 1981 Aug;113(1):196–213. doi: 10.1016/0042-6822(81)90148-3. [DOI] [PubMed] [Google Scholar]
- Otsuka H., Kit S. Nucleotide sequence of the marmoset herpesvirus thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide. Virology. 1984 Jun;135(2):316–330. doi: 10.1016/0042-6822(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Harrington J. E. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob Agents Chemother. 1982 Jul;22(1):71–77. doi: 10.1128/aac.22.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti P. F., Cassai E., Meneguzzi G., Chenciner N., Milanesi G. Herpes simplex virus DNA isolation from infected cells with a novel procedure. Virology. 1979 Feb;93(1):260–264. doi: 10.1016/0042-6822(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Pribnow D., Sigurdson D. C., Gold L., Singer B. S., Napoli C., Brosius J., Dull T. J., Noller H. F. rII cistrons of bacteriophage T4. DNA sequence around the intercistronic divide and positions of genetic landmarks. J Mol Biol. 1981 Jul 5;149(3):337–376. doi: 10.1016/0022-2836(81)90477-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. R., Kit S., Myers M. G. Thymidine kinase-deficient herpes simplex virus type 2 genital infection in guinea pigs. J Virol. 1985 Aug;55(2):322–328. doi: 10.1128/jvi.55.2.322-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Swain M. A., Galloway D. A. Nucleotide sequence of the herpes simplex virus type 2 thymidine kinase gene. J Virol. 1983 Jun;46(3):1045–1050. doi: 10.1128/jvi.46.3.1045-1050.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Jones J. C., Ressel S. J., Fralish F. A. Thymidine plaque autoradiography of thymidine kinase-positive and thymidine kinase-negative herpesviruses. J Clin Microbiol. 1983 Jan;17(1):122–127. doi: 10.1128/jcm.17.1.122-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Upton C., McFadden G. Identification and nucleotide sequence of the thymidine kinase gene of Shope fibroma virus. J Virol. 1986 Dec;60(3):920–927. doi: 10.1128/jvi.60.3.920-927.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. C., McLaren C., Meyers J. D. Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J Infect Dis. 1983 Dec;148(6):1077–1082. doi: 10.1093/infdis/148.6.1077. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. B., Hayday A., Courtneidge S., Fried M. A frameshift at a mutational hotspot in the polyoma virus early region generates two new proteins that define T-antigen functional domains. Cell. 1986 Feb 14;44(3):477–487. doi: 10.1016/0092-8674(86)90469-1. [DOI] [PubMed] [Google Scholar]