Abstract
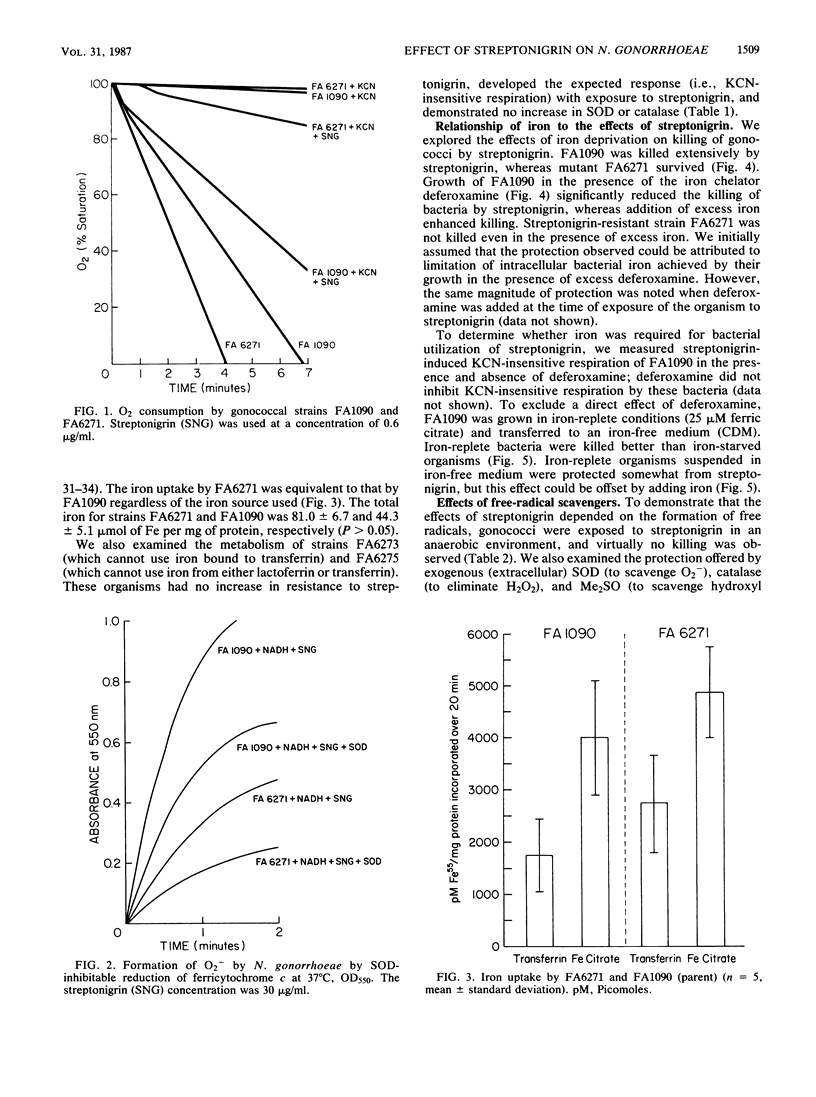
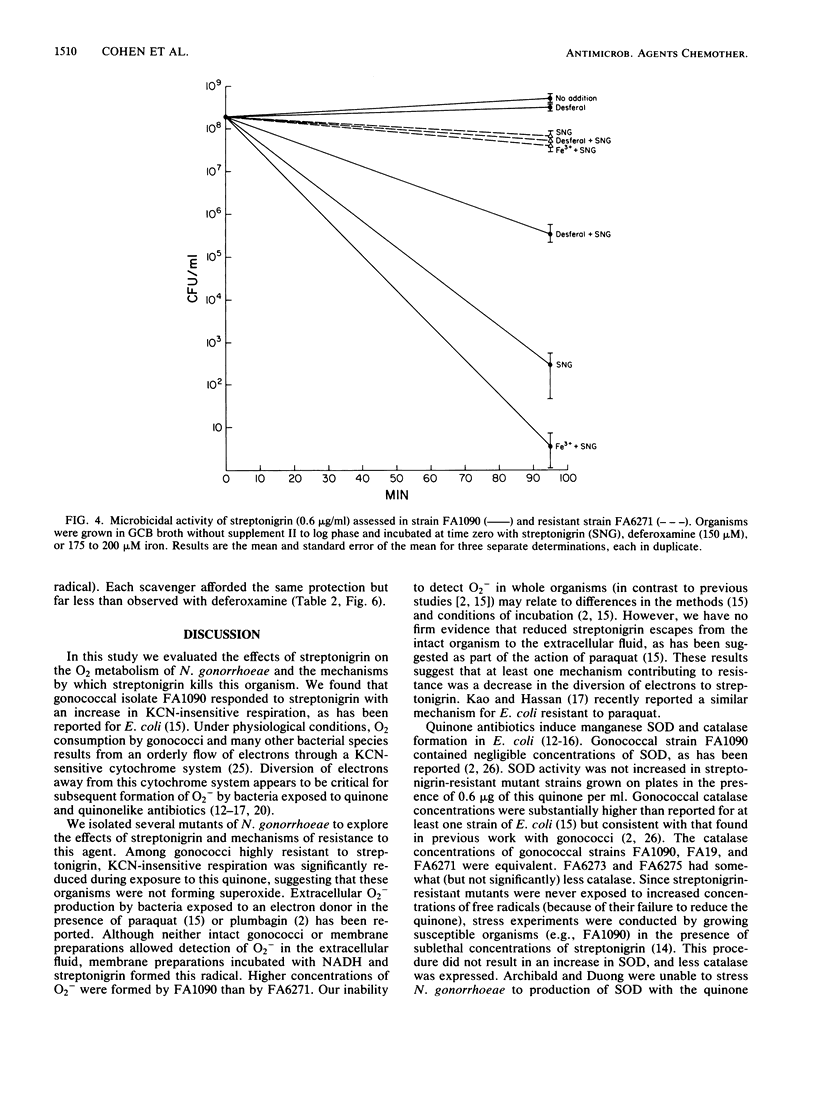
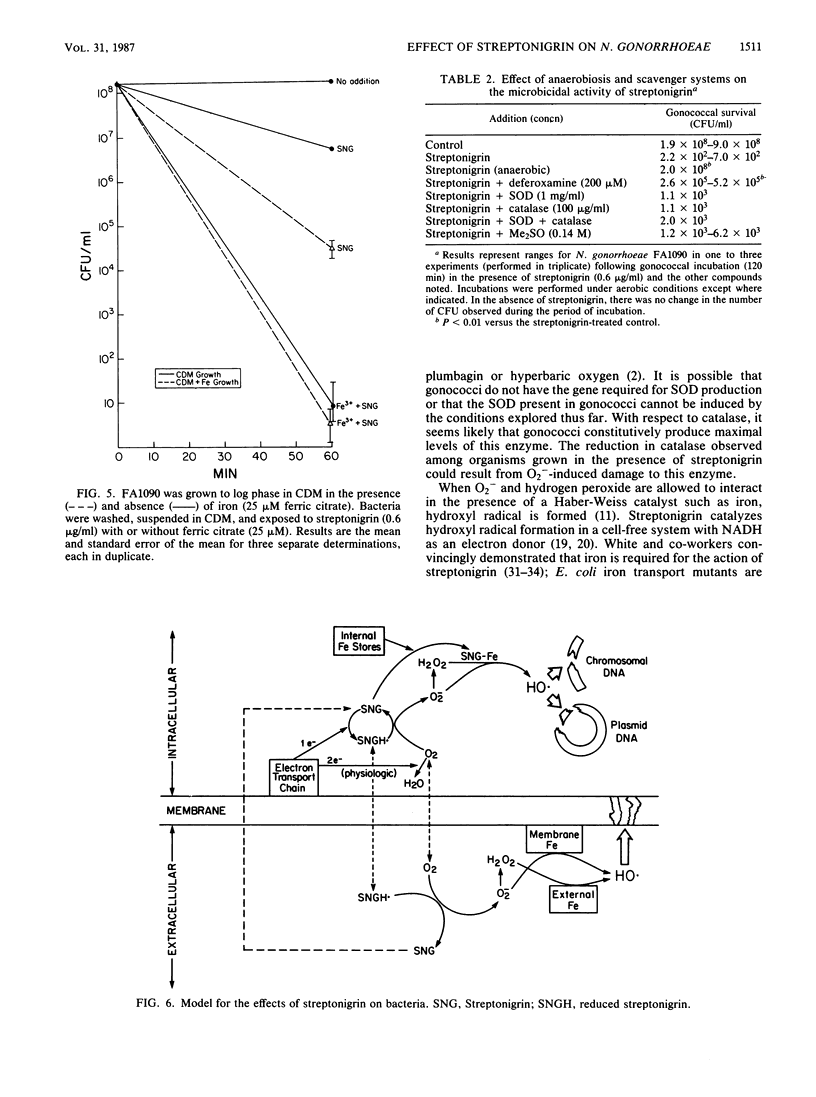
The quinone antibiotic streptonigrin is believed to kill bacteria by promoting formation of oxygen radicals. This antibiotic has also been used to select resistant bacterial mutants, some of which vary in iron utilization. We examined the effects of streptonigrin on Neisseria gonorrhoeae and several types of gonococcal mutants. Streptonigrin (0.025 microgram/ml) efficiently killed gonococcal strain FA1090, and this effect depended on iron. Streptonigrin-resistant mutant FA6271 had normal iron uptake but was moderately deficient in total iron. Resistance most likely resulted from failure of FA6271 to divert electrons to streptonigrin, as demonstrated by a reduction in KCN-insensitive respiration (a hallmark of the action of quinones) and superoxide formation. Other mutants selected for inability to use human iron-binding proteins (strains FA6273 and FA6275) had no increase in streptonigrin MIC and no decrease in KCN-insensitive respiration. Mutants did not demonstrate an increase in superoxide dismutase or catalase. Streptonigrin killing of gonococci depended on a reaction(s) in which extracellular iron was important, presumably because iron was required for catalysis of hydroxyl radical. The results suggest that a membrane component may be a target for the actions of streptonigrin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrich J. M., Gilbaugh J. H., 3rd, Callahan K. B., Hurst J. K. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J Clin Invest. 1986 Jul;78(1):177–184. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986 Feb;51(2):631–641. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gross R., Köster W., Zimmermann L. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol Gen Genet. 1983;192(1-2):131–139. doi: 10.1007/BF00327658. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Cone R., Hasan S. K., Lown J. W., Morgan A. R. The mechanism of the degradation of DNA by streptonigrin. Can J Biochem. 1976 Mar;54(3):219–223. doi: 10.1139/o76-034. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 1976 Feb;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Streptonigrin-induced deoxyribose degradation: inhibition by superoxide dismutase, hydroxyl radical scavengers and iron chelators. Biochem Pharmacol. 1984 Oct 1;33(19):3059–3062. doi: 10.1016/0006-2952(84)90609-9. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. M., Hassan H. M. Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10478–10481. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lown J. W., Sim S. K., Chen H. H. Hydroxyl radical production by free and DNA-bound aminoquinone antibiotics and its role in DNA degradation. Electron spin resonance detection of hydroxyl radicals by spin trapping. Can J Biochem. 1978 Nov;56(11):1042–1047. doi: 10.1139/o78-164. [DOI] [PubMed] [Google Scholar]
- Lown J. W. The mechanism of action of quinone antibiotics. Mol Cell Biochem. 1983;55(1):17–40. doi: 10.1007/BF00229240. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Luginbuhl G. H., Sandstrom E., Morse S. A. Identification of an iron-regulated 37,000-dalton protein in the cell envelope of Neisseria gonorrhoeae. Infect Immun. 1984 Aug;45(2):410–416. doi: 10.1128/iai.45.2.410-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Cacciapuoti A. F., Lysko P. G. Physiology of Neisseria gonorrhoeae. Adv Microb Physiol. 1979;20:251–320. doi: 10.1016/s0065-2911(08)60209-x. [DOI] [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur J Biochem. 1983 Dec 1;137(1-2):119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R. Streptonigrin-transition metal complexes: binding to DNA and biological activity. Biochem Biophys Res Commun. 1977 Jul 11;77(1):387–391. doi: 10.1016/s0006-291x(77)80209-x. [DOI] [PubMed] [Google Scholar]
- White J. R., Yeowell H. N. Iron enhances the bactericidal action of streptonigrin. Biochem Biophys Res Commun. 1982 May 31;106(2):407–411. doi: 10.1016/0006-291x(82)91125-1. [DOI] [PubMed] [Google Scholar]
- Yeowell H. N., White J. R. Changes in streptonigrin lethality during adaptation of Escherichia coli to picolinic acid. Correlation with intracellular picolinate and iron uptake. Biochim Biophys Acta. 1984 Mar 1;797(3):302–311. doi: 10.1016/0304-4165(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Yeowell H. N., White J. R. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982 Dec;22(6):961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]


