Abstract
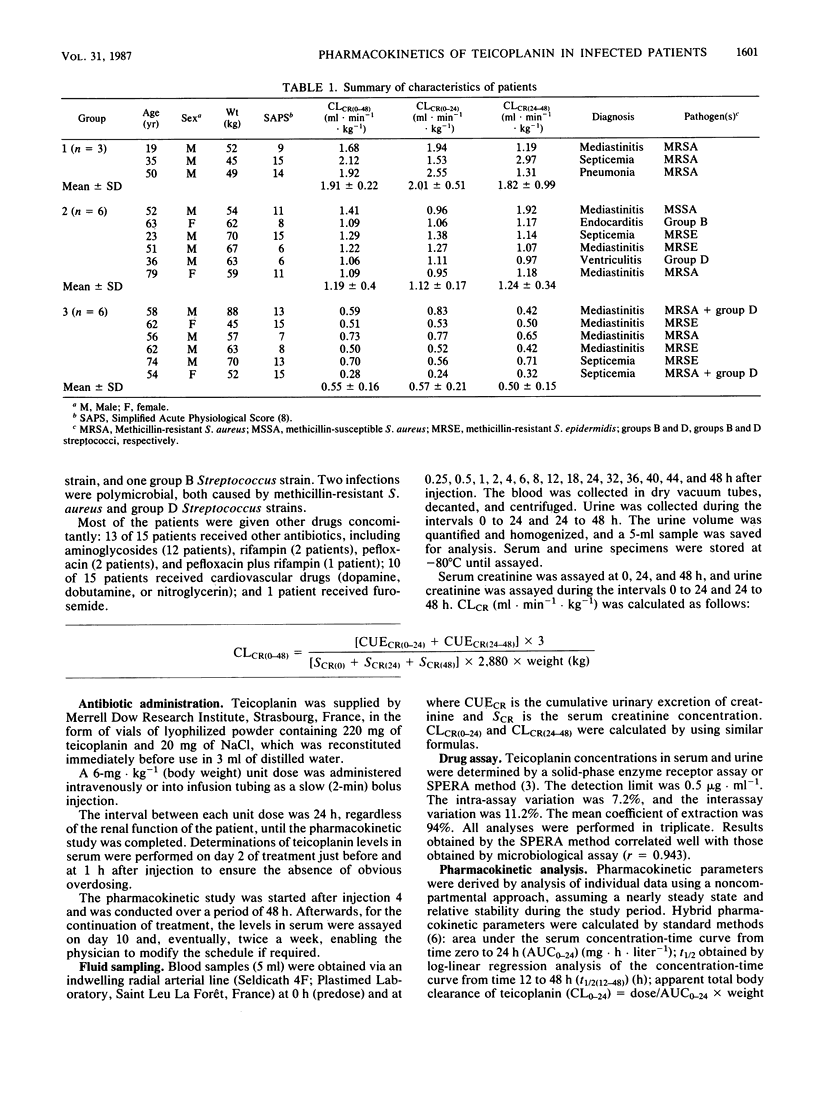
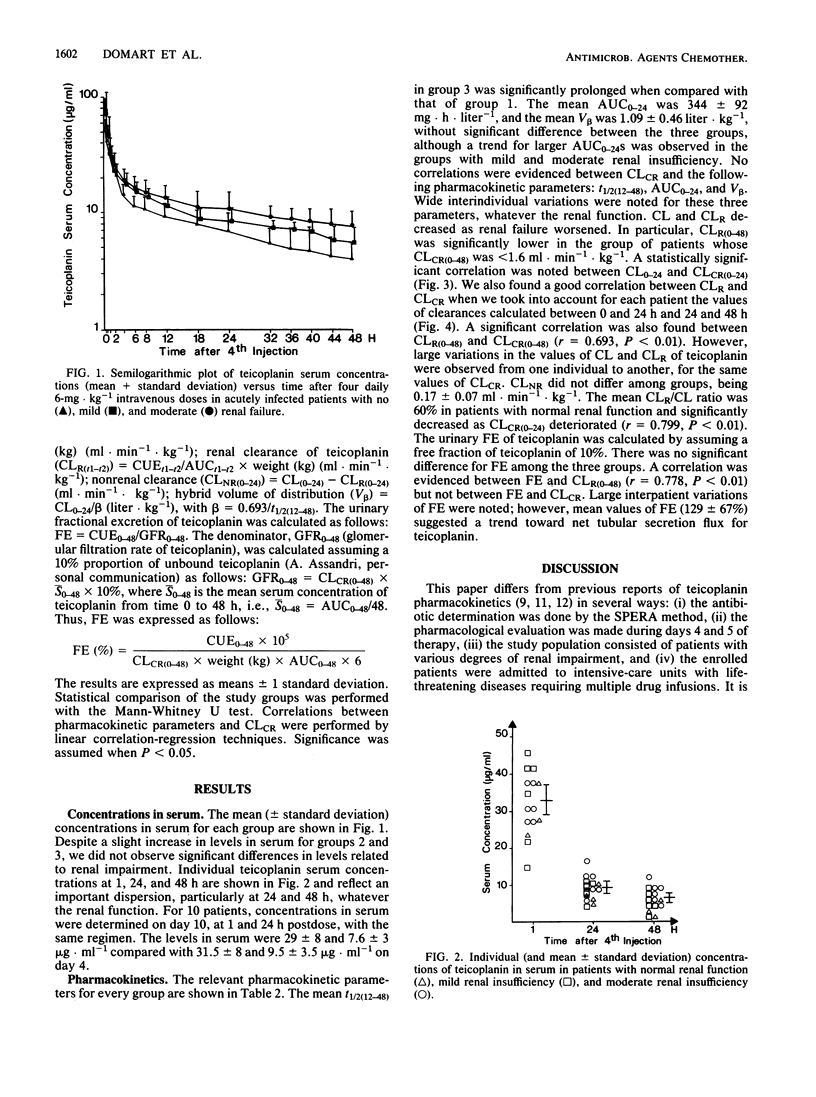
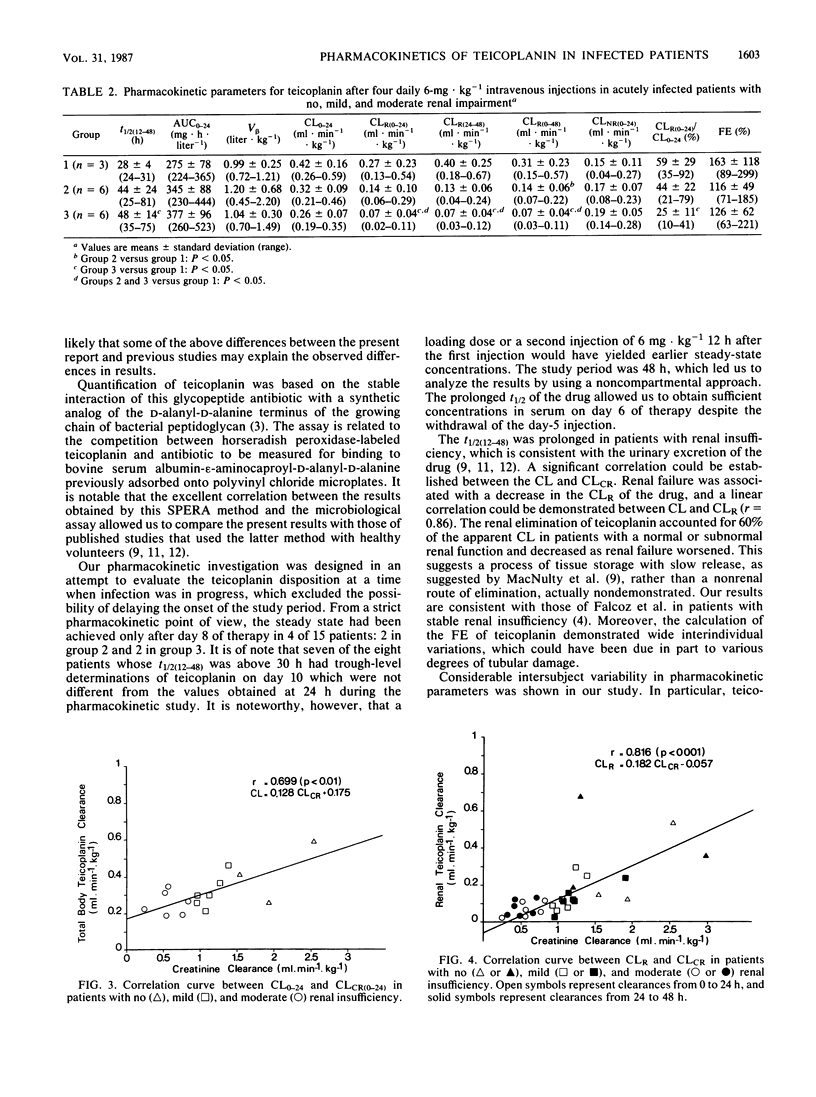
The pharmacokinetics of teicoplanin were studied in 15 adult patients in the acute phase of severe infections caused by gram-positive cocci. All the subjects were given a daily intravenous bolus dose of 6 mg of teicoplanin kg-1 (body weight). The pharmacokinetic study was performed over a 48-h period after injection 4. The subjects were categorized according to their mean creatinine clearances (ml.min-1.kg-1) during the study period: group 1 (n = 3), greater than 1.6; group 2 (n = 6), 0.8 to 1.6; and group 3 (n = 6), 0.15 to 0.8. Mean concentrations of teicoplanin in serum at 1, 24, and 48 h were 33 +/- 8, 9 +/- 3, and 6 +/- 2.5 micrograms.ml-1, respectively. The mean half-lives of the concentration-time curve from 12 to 48 h were 28 +/- 4, 44 +/- 24, and 48 +/- 14 h in groups 1, 2, and 3, respectively (group 3 versus group 1: P less than 0.05). The mean area under the serum concentration-time curve from time zero to 24 h was 344 +/- 92 mg.h.liter-1, and the mean hybrid volume of distribution was 1.09 +/- 0.46 liter.kg-1. These values were similar for the three groups, with a trend for larger areas under the curve in group 3. Creatinine clearance correlated directly with the total body clearance of teicoplanin (r = 0.70) and with the renal clearance of teicoplanin (r = 0.82). However, in critically ill patients, the wide interindividual variations in pharmacokinetic parameters are more relevant than those related to the variations in renal function when creatinine clearance is above 0.30 ml.min-1.kg-1. We concluded that, in such conditions, monitoring of concentrations of teicoplanin in serum is mandatory.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibler M. R., Frame P. T., Hagler D. N., Bode R. B., Staneck J. L., Thamlikitkul V., Harris J. E., Haregewoin A., Bullock W. E., Jr Clinical evaluation of efficacy, pharmacokinetics, and safety of teicoplanin for serious gram-positive infections. Antimicrob Agents Chemother. 1987 Feb;31(2):207–212. doi: 10.1128/aac.31.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Sande M. A. Teicoplanin versus nafcillin and vancomycin in the treatment of experimental endocarditis caused by methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1984 Jul;26(1):61–64. doi: 10.1128/aac.26.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti A., Rurali C., Borghi A., Cassani G. Solid-phase enzyme-receptor assay (SPERA): a competitive-binding assay for glycopeptide antibiotics of the vancomycin class. Clin Chem. 1985 Oct;31(10):1606–1610. [PubMed] [Google Scholar]
- Falcoz C., Ferry N., Pozet N., Cuisinaud G., Zech P. Y., Sassard J. Pharmacokinetics of teicoplanin in renal failure. Antimicrob Agents Chemother. 1987 Aug;31(8):1255–1262. doi: 10.1128/aac.31.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto D. W., Boscia J. A., Kobasa W. D., Kaye D. Teicoplanin compared with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus epidermidis. J Infect Dis. 1986 Jul;154(1):69–75. doi: 10.1093/infdis/154.1.69. [DOI] [PubMed] [Google Scholar]
- Glupczynski Y., Lagast H., Van der Auwera P., Thys J. P., Crokaert F., Yourassowsky E., Meunier-Carpentier F., Klastersky J., Kains J. P., Serruys-Schoutens E. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 1986 Jan;29(1):52–57. doi: 10.1128/aac.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall J. R., Loirat P., Alperovitch A. Simplified acute physiological score for intensive care patients. Lancet. 1983 Sep 24;2(8352):741–741. doi: 10.1016/s0140-6736(83)92278-x. [DOI] [PubMed] [Google Scholar]
- McNulty C. A., Garden G. M., Wise R., Andrews J. M. The pharmacokinetics and tissue penetration of teicoplanin. J Antimicrob Chemother. 1985 Dec;16(6):743–749. doi: 10.1093/jac/16.6.743. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Täuber M. G., Hackbarth C. J., Sande M. A. Therapeutic efficacy of teicoplanin in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1985 Jan;27(1):135–136. doi: 10.1128/aac.27.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]


