Abstract
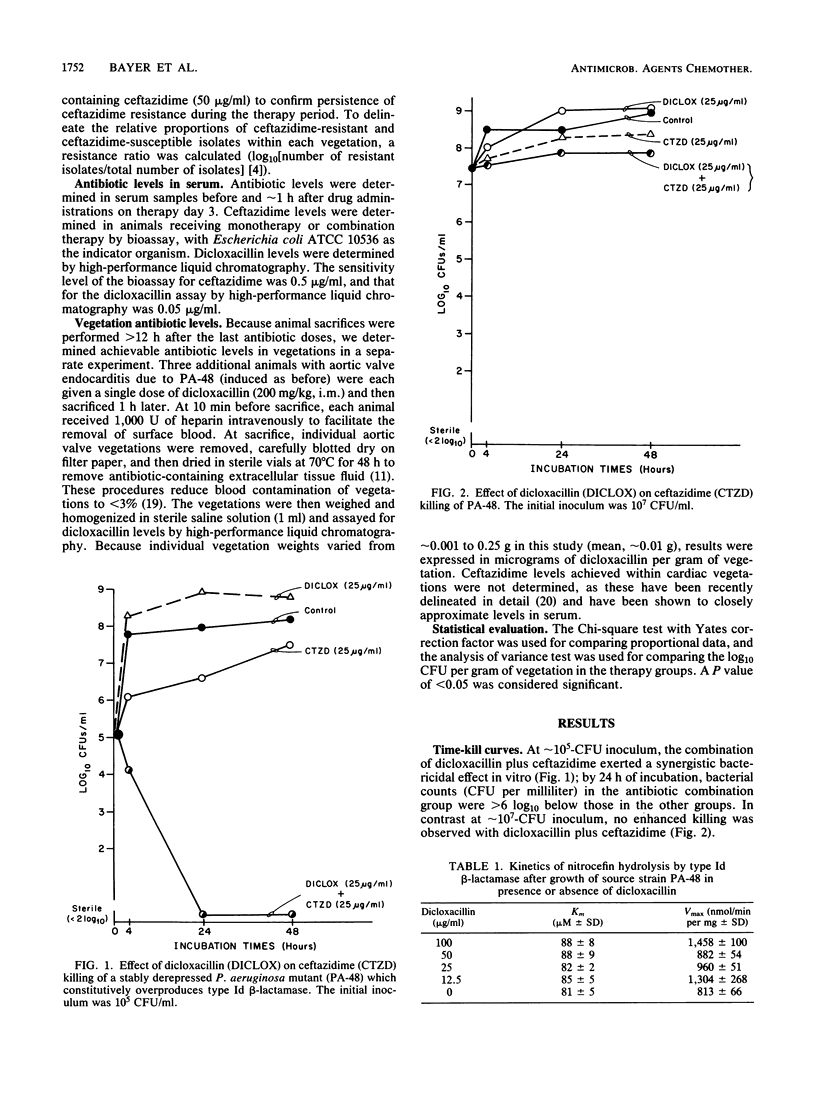
We investigated the in vitro and in vivo effects of a combination of a beta-lactam (ceftazidime) and a beta-lactamase inhibitor (dicloxacillin) to synergistically kill a ceftazidime-resistant variant, Pseudomonas aeruginosa PA-48, which overproduces type Id cephalosporinase constitutively. In vitro, dicloxacillin plus ceftazidime exerted bactericidal synergy at approximately 10(5) CFU/ml of inoculum (but not at approximately 10(7)-CFU inoculum), whereas other beta-lactamase inhibitors (sulbactam, clavulanic acid) showed no enhanced killing of PA-48 when combined with ceftazidime at clinically achievable levels for each agent. Dicloxacillin was a potent competitive inhibitor of the extracted Id cephalosporinase from strain PA-48 in short-term comixture studies (less than 10 min [Ki = 2 nM]); in contrast, longer-term comixture studies (90 min) indicated that dicloxacillin functions as a competitive substrate for the enzyme. Growth of PA-48 cells in the presence of dicloxacillin (12.5 to 100 micrograms/ml) had no significant effect on the production rates or functional activity of the Id enzyme. In experimental aortic valve endocarditis due to the ceftazidime-resistant variant (PA-48), rabbits received either no therapy, ceftazidime (25 mg/kg intramuscularly, every 4 h), or ceftazidime plus dicloxacillin (200 mg/kg intramuscularly, every 4 h). The combination regimen reduced mean bacterial densities of PA-48 within cardiac vegetations significantly below those in the other groups at both days 3 and 6 of treatment (P less than 0.005). However, mean vegetation bacterial densities remained greater than 6 log10 CFU/g in the combined treatment group. This modest in vivo synergistic effect (as compared to striking in vitro synergy at approximately 10(5)-CFU inoculum) most likely reflects the high densities of PA-48 achieved in vivo within cardiac vegetations (greater than 8 log10 CFU/g).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer A. S., Norman D. C., Kim K. S. Characterization of impermeability variants of Pseudomonas aeruginosa isolated during unsuccessful therapy of experimental endocarditis. Antimicrob Agents Chemother. 1987 Jan;31(1):70–75. doi: 10.1128/aac.31.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Norman D., Kim K. S. Efficacy of amikacin and ceftazidime in experimental aortic valve endocarditis due to Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1985 Dec;28(6):781–785. doi: 10.1128/aac.28.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Peters J., Parr T. R., Jr, Chan L., Hancock R. E. Role of beta-lactamase in in vivo development of ceftazidime resistance in experimental Pseudomonas aeruginosa endocarditis. Antimicrob Agents Chemother. 1987 Feb;31(2):253–258. doi: 10.1128/aac.31.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J., Drake T. A., Rusnak M. G., Sande M. A. Endocarditis due to methicillin-resistant Staphylococcus aureus in rabbits: expression of resistance to beta-lactam antibiotics in vivo and in vitro. J Infect Dis. 1984 Jun;149(6):894–903. doi: 10.1093/infdis/149.6.894. [DOI] [PubMed] [Google Scholar]
- Choi C., Bayer A. S., Fujita N. K., Lam K., Guze L. B., Yoshikawa T. T. Therapy of experimental Pseudomonas endocarditis with high-dose amikacin and ticarcillin. Chemotherapy. 1983;29(4):303–312. doi: 10.1159/000238213. [DOI] [PubMed] [Google Scholar]
- Crosby M. A., Gump D. W. Activity of cefoperazone and two beta-lactamase inhibitors, sulbactam and clavulanic acid, against Bacteroides spp. correlated with beta-lactamase production. Antimicrob Agents Chemother. 1982 Sep;22(3):398–405. doi: 10.1128/aac.22.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File T. M., Jr, Tan J. S., Salstrom S. J., Johnson L. A., Douglas G. F. Timentin versus piperacillin or moxalactam in the therapy of acute bacterial infections. Antimicrob Agents Chemother. 1984 Sep;26(3):310–313. doi: 10.1128/aac.26.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Stankewich J. P., Marshall D. C., O'Brien M. M., Hayes S. L., Weidler D. J., McMahon F. G. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother. 1983 May;23(5):692–699. doi: 10.1128/aac.23.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. Comparative inhibition beta-lactamases by novel beta-lactam compounds. Antimicrob Agents Chemother. 1979 Feb;15(2):171–176. doi: 10.1128/aac.15.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo F. M., Schentag J. J. Rate of methicillin penetration into normal heart valve and experimental endocarditis lesions. Antimicrob Agents Chemother. 1982 Mar;21(3):456–459. doi: 10.1128/aac.21.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwith M. J., Stein G. E., Gurwith D. Prospective comparison of amoxicillin-clavulanic acid and cefaclor in treatment of uncomplicated urinary tract infections. Antimicrob Agents Chemother. 1983 Nov;24(5):716–719. doi: 10.1128/aac.24.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R. A model system to demonstrate that beta-lactamase-associated antibiotic trapping could be a potential means of resistance. J Infect Dis. 1983 Aug;148(2):316–321. doi: 10.1093/infdis/148.2.316. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Lucho V. E., Saravolatz L. D., Medeiros A. A., Pohlod D. Failure of therapy in pseudomonas endocarditis: selection of resistant mutants. J Infect Dis. 1986 Jul;154(1):64–68. doi: 10.1093/infdis/154.1.64. [DOI] [PubMed] [Google Scholar]
- King A., Shannon K., Eykyn S., Phillips I. Reduced sensitivity to beta-lactam antibiotics arising during ceftazidime treatment of Pseudomonas aeruginosa infections. J Antimicrob Chemother. 1983 Oct;12(4):363–370. doi: 10.1093/jac/12.4.363. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Do beta-lactamases 'trap' cephalosporins? J Antimicrob Chemother. 1985 May;15(5):511–514. doi: 10.1093/jac/15.5.511. [DOI] [PubMed] [Google Scholar]
- McColm A. A., Ryan D. M. Comparative pharmacokinetics of ceftazidime in fibrin clots and cardiac vegetations in rabbits with Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1985 Jun;27(6):925–927. doi: 10.1128/aac.27.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preheim L. C., Penn R. G., Sanders C. C., Goering R. V., Giger D. K. Emergence of resistance to beta-lactam and aminoglycoside antibiotics during moxalactam therapy of Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 1982 Dec;22(6):1037–1041. doi: 10.1128/aac.22.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M. P., Brown W. J., Lerner A. M. Treatment of patients with pseudomonas endocarditis with high dose aminoglycoside and carbenicillin therapy. Medicine (Baltimore) 1978 Jan;57(1):57–67. doi: 10.1097/00005792-197801000-00004. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Current problems in the treatment of infective endocarditis due to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):314–321. doi: 10.1093/clinids/5.2.314. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Microbial resistance to newer generation beta-lactam antibiotics: clinical and laboratory implications. J Infect Dis. 1985 Mar;151(3):399–406. doi: 10.1093/infdis/151.3.399. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Casey P. A., Cooper D. L. Single-dose pharmacokinetics of intravenous clavulanic acid with amoxicillin in pediatric patients. Antimicrob Agents Chemother. 1983 Feb;23(2):252–255. doi: 10.1128/aac.23.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


