Abstract
The plasma membrane (PM) contains redox enzymes that provide electrons for energy metabolism and recycling of antioxidants such as coenzyme Q and α-tocopherol. Brain aging and neurodegenerative disorders involve impaired energy metabolism and oxidative damage, but the involvement of the PM redox system (PMRS) in these processes is unknown. Caloric restriction (CR), a manipulation that protects the brain against aging and disease, increased activities of PMRS enzymes (NADH-ascorbate free radical reductase, NADH-quinone oxidoreductase 1, NADH-ferrocyanide reductase, NADH-coenzyme Q10 reductase, and NADH-cytochrome c reductase) and antioxidant levels (α-tocopherol and coenzyme Q10) in brain PM during aging. Age-related increases in PM lipid peroxidation, protein carbonyls, and nitrotyrosine were attenuated by CR, levels of PMRS enzyme activities were higher, and markers of oxidative stress were lower in cultured neuronal cells treated with CR serum compared with those treated with ad libitum serum. These findings suggest important roles for the PMRS in protecting brain cells against age-related increases in oxidative and metabolic stress.
Keywords: Alzheimer's disease, reactive oxygen species, coenzyme Q10, oxidoreductase
Aging is a complex process involving progressive and deleterious changes in cell functions that are believed to result, in part, from the production of reactive oxygen species resulting in cell senescence, dysfunction, and/or death (1–5). The oxidative and metabolic alterations that occur during aging increase the vulnerability of cells and organ systems to various diseases including cancers, cardiovascular disease, and neurodegenerative disorders (6–8). The aging process (9) and disease pathogenesis (10, 11) can be retarded by dietary calorie (energy) restriction (CR). CR has been shown to lower the rate of production of free radicals by mitochondria and to protect cells against oxidative stress (12–17), a mechanism consistent with the free radical theory of aging. CR can attenuate age-related deficits in brain function and can protect neurons against dysfunction and death in animal models of Alzheimer's disease, Parkinson's disease, Huntington's disease, and stroke (11, 18–22). The mechanisms by which CR protects brain cells during aging are unknown but may involve induction of the expression of neurotrophic factors (20, 23), protein chaperones (22), and mitochondrial uncoupling proteins (24).
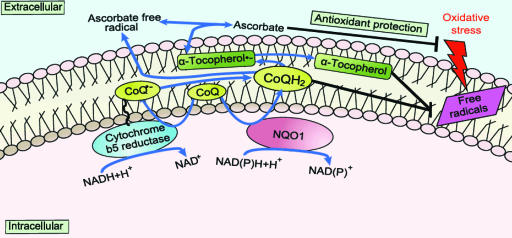
The plasma membrane (PM) regulates numerous aspects of cell physiology and signaling and also protects cells against oxidative stress. Analogous to the mitochondrial inner membrane, the PM contains enzymes involved in electron transport and energy metabolism (Fig. 1) (25). Cells respond to oxidative stress by transferring electrons from NAD(P)H and ascorbate to extracellular free radicals and/or oxidants (26, 27). Coenzyme Q10 (CoQ), a key molecule in the PM redox system (PMRS), can be reduced at the PM by either NAD(P)H-quinone oxidoreductase 1 (NQO1) (28) or NADH-cytochrome b5 reductase (b5R) (29). NQO1 is a NAD(P)H-dependent reductase that is translocated to the inner surface of the PM under stress conditions (30). Dietary CoQ is important in maintaining levels of reduced CoQ and α-tocopherol in the PM, thereby protecting the PM from lipid peroxidation (31). In the present study the effects of aging and CR on the brain PMRS and PM-associated oxidative stress were investigated in rats. In addition, an in vitro model of CR in which brain cells were incubated in medium containing serum from animals fed ad libitum (AL) or CR diets (32) was used to elucidate humoral and cellular responses of neural cells to CR.
Fig. 1.
Key components of the PMRS. Diverse antioxidants protect the cell under stress conditions, including ascorbate at the hydrophilic cell surface and both CoQ and α-tocopherol in the hydrophobic phospholipid bilayer. Both NADH-b5R reductase and NQO1 act at the PM to reduce CoQ. These two enzymes contribute to the PMRS, providing the electrons that are required to maintain its antioxidant properties. NADH-AFR reductase, a trans-oriented activity shows a strong dependency on the CoQ status of the PM, and NQO1 also contribute to the PMRS and are responsive to oxidative stress and aging (29, 58).
Results
CR Increases PM Antioxidant Levels.
After the two-phase partition process, the PM fractions were immunoreactive against a PM-specific Na+/K+-ATPase α-subunit antibody, whereas cytochrome c oxidase (mitochondrial protein) and ribophorin (endoplasmic reticulum protein) were not detected [supporting information (SI) Fig. 6A]. The Na+/K+-ATPase was prominent in the fractions isolated from SH-SY5Y cells, whereas ribophorin and cytochrome c oxidase were not detected (SI Fig. 6B).
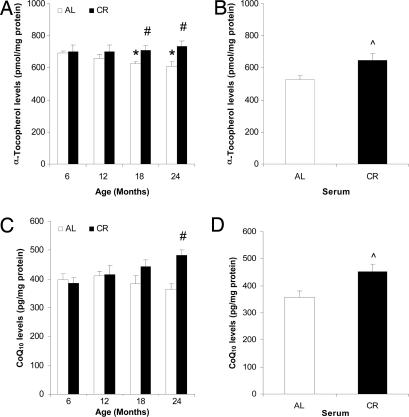
Levels of PM α-tocopherol declined with age but were maintained by CR (Fig. 2A). Levels of CoQ also tended to decrease with age, although the change was not statistically significant (Fig. 2C). However, the levels of CoQ were significantly increased by CR. When cultured neuronal cells were treated with serum from CR rats, levels of α-tocopherol (Fig. 2B) and CoQ (Fig. 2D) in the PMs were significantly increased compared with neuronal cells treated with serum from AL rats.
Fig. 2.
CR increases levels of CoQ and α-tocopherol in neuronal PMs. CoQ (A and B) and α-tocopherol (C and D) in the PM from brains of rats fed AL or CR diets (A and C) and cultured neuronal cells incubated with serum from AL or CR rats (B and D) were analyzed by HPLC. Open bars, AL; filled bars, CR. Values are the means ± SEM; n = 4 (rat brains) or 6 (cultured cells). ∗, P < 0.01 compared with the value for 6-month-old rats; #, P < 0.01 compared with the AL value for the same age group; ⋀, P < 0.01 compared with the value for cells cultured with AL serum.
CR Elevates Activities of Multiple PM Redox Enzymes.
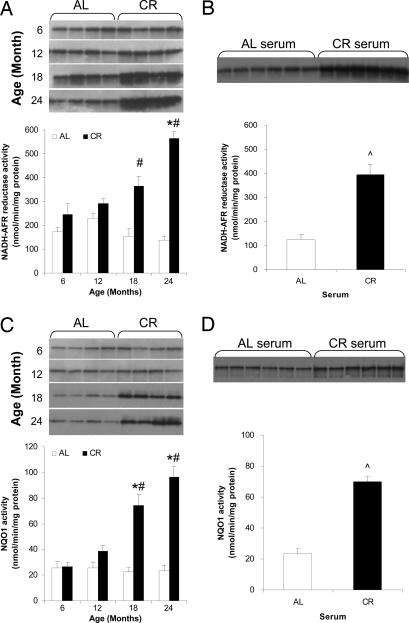
In AL-fed rats NADH-ascorbate free radical (NADH-AFR) reductase activity, which is mainly due to b5R, decreased with age resulting in a significant reduction in 24-month-old rats (Fig. 3A). In contrast, CR resulted in an increase in NADH-AFR reductase activity that was significant at 18 months and reached a level approximately three times higher than in AL brains at 24 months (Fig. 3A). Dicoumarol-sensitive PM-associated NQO1 activity increased with age in rats maintained on CR, whereas there was no age-related change in PM NQO1 activity in brains from rats fed AL (Fig. 3C). The difference in NQO1 activity between the AL and CR groups became significant at 18 months. Immunoblot analysis showed that levels of NADH-AFR reductase and NQO1 proteins were increased during aging in PMs from the brains of CR rats compared with AL rats (Fig. 3 A and C).
Fig. 3.
CR increases the activities of NADH-AFR reductase and NQO1 in brain PMs during aging. The PM fractions isolated from rat brains (A and C) and SH-SY5Y cells cultured with AL or CR serum (B and D) were used to measure activity and expression levels of NADH-AFR reductase (A and B) and NQO1 (C and D). Open bars, AL; filled bars, CR. Values are the means ± SEM; n = 4 (rat brains) or 6 (SH-SY5Y cells). ∗, P < 0.01 compared with the value for 6-month-old rats; #, P < 0.01 compared with the AL value for the same age group; ⋀, P < 0.01 compared with the value for cells cultured with AL serum.
To determine whether the in vitro model of CR would reproduce the above results, cells were incubated with serum from either AL or CR rats. PM isolated from CR serum-treated cells displayed a 3-fold elevation of NADH-AFR reductase activity and a 2.5-fold increase in NQO1 activity compared with PM fractions isolated from AL serum-treated cells (Fig. 3 B and D). Levels of NADH-AFR reductase and NQO1 proteins were greater in membranes from cultured cells treated with CR serum compared with those treated with AL serum (Fig. 3 B and D).
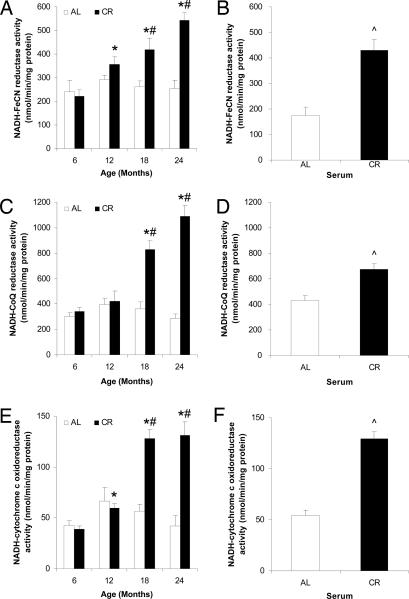
The activities of the PMRS enzymes NADH-ferricyanide (FeCN) reductase, NADH-CoQ reductase, and NADH-cytochrome c oxidoreductase were also significantly elevated in PMs from the brains of CR rats at 18 and 24 months compared with PMs from AL fed rats (Fig. 4 A, C, and E) and were also significantly increased in the PMs of CR serum-treated neuronal cells compared with AL serum-treated cells (Fig. 4 B, D, and F). Therefore, CR elicits a coordinated increase in the PMRS of neural cells.
Fig. 4.
CR increases the activities of NADH-FeCN reductase, NADH-CoQ reductase, and NADH-cytochrome c oxidoreductase during aging. The PM fractions isolated from rat brains (A, C, and E) and SH-SY5Y cells cultured with AL or CR serum (B, D, and F) were used to measure activity of NADH-FeCN reductase (A and B), NADH-CoQ reductase (C and D), and NADH-cytochrome c oxidoreductase (E and F). Open bars, AL; filled bars, CR. Values are the means ± SEM; n = 4 (rat brains) or 6 (SH-SY5Y cells). ∗, P < 0.01 compared with the value for 6-month-old rats; #, P < 0.01 compared with the AL value for the same age group; ⋀, P < 0.01 compared with the value for cells cultured with AL serum.
CR Suppresses Age-Related Increases in Markers of PM Oxidative Stress.
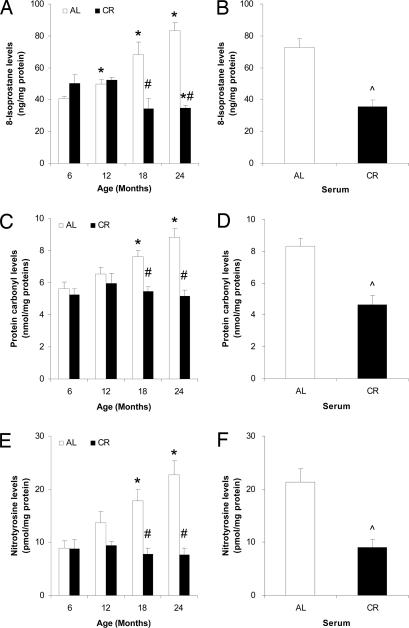
The levels of 8-isoprostane in PM, a marker for lipid peroxidation (33), were increased significantly at 18 and 24 months in AL fed rats whereas they decreased in the CR rats (Fig. 5A). Protein carbonyl levels, a biomarker of protein oxidation (34), were increased during aging in PM from AL rat brains, but not in PM from CR rat brains (Fig. 5C). Peroxynitrite (ONOO−) and other reactive nitrogen species are believed to play a role in brain aging and the pathogenesis of neurodegenerative disorders (35, 36). Levels of nitrotyrosine, a marker of reactive nitrogen species activity, were not increased with age in the PM from the brains of CR rats, whereas they were significantly elevated with age in AL rat brain PM (Fig. 5D). In the in vitro model of CR, levels of 8-isoprostane, protein carbonyls, and nitrotyrosine were significantly lower in cultures treated with CR serum compared with those treated with AL serum (Fig. 5 B, D, and F).
Fig. 5.
CR decreases levels of PM lipid peroxidation and protein carbonylation and nitration. PM fractions isolated from rat brains (A, C, and E) and SH-SY5Y cells cultured with AL or CR serum (B, D, and F) were used to measure each of the isoprostanes (A and B), protein carbonyls (C and D), and nitrotyrosine (E and F). Open bars, AL; filled bars, CR. Values are the means ± SEM; n = 4 (rat brains) or 6 (SH-SY5Y cells). ∗, P < 0.01 compared with the value for 6-month-old rats; #, P < 0.01 compared with the AL value for the same age group; ⋀, P < 0.01 compared with the value for cells cultured with AL serum.
Discussion
The PM plays fundamental roles in regulating cellular ion homeostasis, nutrient transport, cell adhesion, and signal transduction. The proteins and lipids involved in these functions of the PM are susceptible to oxidative modifications that may contribute to the dysfunction and degeneration of neurons that occur in aging and neurodegenerative disorders (37). For example, lipid peroxidation and oxidative modifications of PM ion-motive ATPases, glucose transporters, and G protein-coupled receptor signaling are implicated in the pathogenesis of Alzheimer's disease (38–40). The PMRS, which includes CoQ and multiple redox enzymes (Fig. 1), is increasingly recognized as a major mechanism for reducing PM-associated oxidative stress and, in compensating for mitochondrial dysfunction, as an alternative source of ATP production by increasing NAD levels and glycolysis (28, 29). The biochemical characteristics of this system, mainly the dependence on intracellular NADH and CoQ as an electron transfer intermediary, sets it apart from the NOX 1–5 family of enzymes (41). We found that multiple enzymes of the PMRS are up-regulated in the brain in response to CR and that these changes in the PMRS were associated with decreased markers of oxidative stress and increased levels of CoQ and α-tocopherol. Our findings suggest that that enhancement of the PMRS is a mechanism by which CR may counteract mitochondrial dysfunction and oxidative stress in the brain during aging. In light of previous results demonstrating links among cellular energy metabolism, sirtuin activity, and aging (42, 43), our data suggest that the NAD+/NADH ratio and incorporation of CoQ into the PMs can be increased by CR, which would be expected to increase SIRT1 activity and attenuate the accumulation of damaged proteins during aging. Because the PM prepared from the brain represent a mixture of neuronal and glial PM, the relative effects of CR on the PMRS of these different cell types remain to be determined. However, we found that exposure of cultured neuronal cells to a “CR environment” results in changes in PMRS enzymes similar to those seen in animals maintained on CR in vivo, suggesting an effect of CR on the neuronal PMRS.
Previous studies have shown that CR has global anti-aging effects on cells and tissues throughout the body that involve lower levels of oxidative stress and increased expression of stress resistance proteins such as HSP-70 (22). Numerous changes in blood chemistry have also been documented in animals and humans maintained on CR including decreased levels of insulin, glucose, triglycerides, and markers of oxidative stress (44). We previously reported that when cultured cells are incubated in serum from CR animals they exhibit increased resistance to oxidative stress, which was associated with up-regulation of the expression of HSP-70 compared with cells incubated in serum from AL animals (32). The latter study provided evidence that reduced levels of insulin and IGF-1 in CR serum contribute to the anti-aging stress-resistant phenotype, consistent with the insulin signaling hypothesis of aging (45). In the present study we found that incubation of neuronal cells in serum from CR animals resulted in the up-regulation of multiple PMRS enzymes and reduced levels of markers of oxidative stress. These findings suggest that the PMRS is regulated by a pathway or pathways that play a fundamental role in aging. The insulin signaling pathway is one potential regulator of the PMRS. Another possibility is that the up-regulation of the PRMS is secondary to a primary effect of CR on energy metabolism. Indeed, it was recently reported that CR results in an increase in the expression of the mitochondrial uncoupling protein UCP4, resulting in decreased mitochondrial ATP and reactive oxygen species production (24), an effect of CR that would be expected to up-regulate the PMRS (46).
The PMRS is stimulated when mitochondrial function is impaired (47–50). Considerable evidence suggests that during normal aging mitochondrial function declines in neurons in the brain, as indicated by decreased levels of activity of mitochondrial metabolic enzymes (51, 52). We found that PMRS enzyme activities were maintained in brain cells during aging suggesting that, in contrast to mitochondrial redox systems, the PM is not compromised. However, there was no adaptive compensatory up-regulation of the PMRS during aging in rats fed AL. Nevertheless, the PMRS was capable of responding to an environmental condition (CR) that preserves brain function during aging. Previous studies have shown that neurons in the brains of rats and mice maintained on CR regimens are relatively resistant to oxidative and metabolic stress compared with control animals fed AL in models of Alzheimer's disease, Parkinson's disease, Huntington's disease, and stroke (18, 19, 22, 53). The mechanism by which CR protects neurons against oxidative injury is unknown. Our findings suggest that CR may protect neurons, in part, by enhancing PMRS enzyme activities resulting in decreased levels of reactive oxygen species-mediated damage to membrane lipids and proteins.
Materials and Methods
Animals and Dietary Manipulation.
Male Fischer-344 (F344) rats were fed AL or maintained on a 40% CR regimen beginning at 1 month of age as described (13). The AL diet consisted of NIH-31 standard diet, and the CR diet was a vitamin- and mineral-fortified version of the same diet at a level 40% less (by weight) than the average AL consumption.
Cell Culture.
Human SH-SY5Y neuroblastoma cells (54) were purchased from American Type Culture Collection (Manassas, VA) and maintained in 175-cm2 tissue culture flasks (Nalge Nunc International, Rochester, NY) containing DMEM (Invitrogen, Carlsbad, CA) supplemented 10% with serum from AL- or CR-fed rats, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were maintained at 37°C under a humidified 5% CO2 and 95% O2 air atmosphere. AL and CR sera were prepared as described previously (32).
Isolation and Characterization of PM Fractions.
PMs from brain tissue or cultured cells were isolated by using a two-phase partition as described (13). Immunoblotting and enzyme activity assays using markers for PMs, mitochondria, and ER were performed to establish the purity of the isolated fractions as described (13). For immunoblotting, anti-Na+/K+-ATPase α-subunit monoclonal antibody (1:1,000 dilution; Affinity BioReagents, Golden, CO), anti-cytochrome c oxidase subunit I monoclonal antibody (1:1,000 dilution; Molecular Probes, Eugene, OR), and anti-ribophorin I antibody (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) were used.
Measurement of PM CoQ and α-Tocopherol Levels.
CoQ and α-tocopherol levels were assessed as described (32). Briefly, lipid fractions were extracted from the isolated PMs by using hexane or NaOH and then analyzed by HPLC with a reverse-phase C18 column (25 cm × 5 mm, 5-mm particle size; Supelco, Bellefonte, PA) and quantified by comparing integration of peak area with internal standards.
PMRS Enzyme Activities.
NADH-AFR reductase and NQO1 activities were determined in vitro by using different electron donors as described (13). Immunoblot analysis was carried out by using antibodies against b5R (1:1,000 dilution; obtained from J. M. Villalba, University of Cordoba, Cordoba, Spain) (29) and NQO1 (1:1,000 dilution; gift from D. Ross, University of Colorado, Denver, CO) (55).
Activities of NADH-FeCN reductase, NADH-CoQ reductase, and NADH-cytochrome c oxidoreductase were examined in a buffer containing 50 mM Tris·HCl (pH 7.6), 0.2 mM NADH, and electron donors (0.1 mM potassium FeCN, 0.2 mM CoQ, or 20 μM cytochrome c). The reaction was started after the addition of NADH and followed by measuring changes in absorbance at 420 nm (for NADH-FeCN and NADH-CoQ reductase) or 550 nm (NADH-cytochrome c oxidoreductase). The extinction coefficients used for the specific activity calculation were 1 (NADH-FeCN reductase), 0.7 (NADH-CoQ reductase), and 29.5 (NADH-cytochrome c oxidoreductase) mM−1·cm−1.
Markers of Oxidative Stress.
Lipid peroxidation levels were assessed by using the 8-Isoprostane assay kit (OxisResearch, Portland, OR). Briefly, PM fractions (100 μl) were added to a 96-well plate and incubated with 100 μl of horseradish peroxidase-conjugated antibody at room temperature for 1 h, 200 μl of substrate was added to the plate, and it was incubated for 30 min. Absorbance was read at 450 nm after stopping the reaction by 50 μl of 3 M sulfuric acid. Protein carbonyl content was determined as described (56), except that the final PM protein pellets were dissolved in 1 ml of 6 M guanidinium hydrochloride. Carbonyl content was calculated as nmol/mg of protein (57). Measurement of protein-bound nitrotyrosine content of isolated PM was performed by using the Nitrotyrosine Assay Kit (OxisResearch).
Statistical Analysis.
Statistical differences were determined by one-way ANOVA. Pairwise comparisons were performed by using a post hoc Bonferroni t test.
Supplementary Material
Acknowledgments
We thank Jose Manuel Villalba and David Ross for providing antibodies against b5R and NQO1, respectively. This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations
- AFR
ascorbate free radical
- AL
ad libitum
- b5R
cytochrome b5 reductase
- CoQ
coenzyme Q10
- CR
calorie restriction
- FeCN
ferricyanide
- NQO1
NAD(P)H-quinone oxidoreductase 1
- PM
plasma membrane
- PMRS
PM redox system.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608008103/DC1.
References
- 1.Halliwell B. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T, Holbrook NJ. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 5.Balaban RS, Nemoto S, Finkel T. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedlander RM. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 8.Mattson MP, Magnus T. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weindruch R, Sohal RS. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cefalu WT, Wagner JD, Wang ZQ, Bell-Farrow AD, Collins J, Haskell D, Bechtold R, Morgan T. J Gerontol A Biol Sci Med Sci. 1997;52:B10–B19. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- 11.Martin B, Mattson MP, Maudsley S. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal S, Sharma S, Agrawal V, Roy N. Free Radical Res. 2005;39:55–62. doi: 10.1080/10715760400022343. [DOI] [PubMed] [Google Scholar]
- 13.de Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Guo ZM, Yang H, Hamilton ML, VanRemmen H, Richardson A. Mech Ageing Dev. 2001;122:1771–1786. doi: 10.1016/s0047-6374(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 15.Merry BJ. Aging Cell. 2004;3:7–12. doi: 10.1046/j.1474-9728.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 16.Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. FASEB J. 2000;14:1825–1836. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Mutcherson R, II, Helfand SL. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 18.Duan W, Mattson MP. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, et al. Proc Natl Acad Sci USA. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Yu ZF, Mattson MP. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 23.Lee J, Duan W, Mattson MP. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP. Neuromol Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 25.Ly JD, Lawen A. Redox Rep. 2003;8:3–21. doi: 10.1179/135100003125001198. [DOI] [PubMed] [Google Scholar]
- 26.del Castillo-Olivares A, Nunez de Castro I, Medina MA. Crit Rev Biochem Mol Biol. 2000;35:197–220. doi: 10.1080/10409230091169203. [DOI] [PubMed] [Google Scholar]
- 27.May JM, Qu ZC. Biochim Biophys Acta. 1999;1421:19–31. doi: 10.1016/s0005-2736(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 28.Beyer RE, Segura-Aguilar J, Di Bernardo S, Cavazzoni M, Fato R, Fiorentini D, Galli MC, Setti M, Landi L, Lenaz G. Proc Natl Acad Sci USA. 1996;93:2528–2532. doi: 10.1073/pnas.93.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro F, Villalba JM, Crane FL, Mackellar WC, Navas P. Biochem Biophys Res Commun. 1995;212:138–413. doi: 10.1006/bbrc.1995.1947. [DOI] [PubMed] [Google Scholar]
- 30.Navarro F, Navas P, Burgess JR, Bello RI, De Cabo R, Arroyo A, Villalba JM. FASEB J. 1998;12:1665–1673. doi: 10.1096/fasebj.12.15.1665. [DOI] [PubMed] [Google Scholar]
- 31.Villalba JM, Navas P. Antioxid Redox Signal. 2000;2:213–230. doi: 10.1089/ars.2000.2.2-213. [DOI] [PubMed] [Google Scholar]
- 32.de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. Exp Gerontol. 2003;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 33.Morrow JD, Harris TM, Roberts LJ., II Anal Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 34.Stadtman ER, Berlett BS. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B, Jenner P. Lancet. 1998;351:1510. doi: 10.1016/S0140-6736(05)78898-X. [DOI] [PubMed] [Google Scholar]
- 36.Ischiropoulos H. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 37.Mattson MP. Trends Neurosci. 1998;21:53–57. doi: 10.1016/s0166-2236(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 38.Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mark RJ, Hensley K, Butterfield DA, Mattson MP. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly JF, Furukawa K, Barger SW, Rengen MR, Mark RJ, Blanc EM, Roth GS, Mattson MP. Proc Natl Acad Sci USA. 1996;93:6753–6758. doi: 10.1073/pnas.93.13.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Infanger DW, Sharma RV, Davisson RL. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 42.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordone L, Guarente L. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 44.Heilbronn LK, Ravussin E. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 45.Rincon M, Rudin E, Barzilai N. Exp Gerontol. 2005;40:873–877. doi: 10.1016/j.exger.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Hyun DH, Hernandez JO, Mattson MP, de Cabo R. Ageing Res Rev. 2006;5:209–220. doi: 10.1016/j.arr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Barroso MP, Gomez-Diaz C, Villalba JM, Buron MI, Lopez-Lluch G, Navas P. J Bionenerg Biomembr. 1997;29:259–267. doi: 10.1023/a:1022462111175. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Diaz C, Villalba JM, Perez-Vicente R, Crane FL, Navas P. Biochem Biophys Res Commun. 1997;234:79–81. doi: 10.1006/bbrc.1997.6582. [DOI] [PubMed] [Google Scholar]
- 49.Larm JA, Vaillant F, Linnane AW, Lawen A. J Biol Chem. 1994;269:30097–30100. [PubMed] [Google Scholar]
- 50.Scarlett DJ, Herst P, Tan A, Prata C, Berridge M. Biofactors. 2004;20:199–206. doi: 10.1002/biof.5520200404. [DOI] [PubMed] [Google Scholar]
- 51.Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, Cai J, Pierce WM, Klein JB, Butterfield DA. Neurobiol Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Bertoni-Freddari C, Fattoretti P, Caselli U, Paoloni R, Meier-Ruge W. Mech Ageing Dev. 1996;90:53–62. doi: 10.1016/0047-6374(96)01753-8. [DOI] [PubMed] [Google Scholar]
- 53.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 54.Ross RA, Spengler BA, Biedler JL. J Natl Cancer Inst. 1983;71:741–747. [PubMed] [Google Scholar]
- 55.Anwar A, Dehn D, Siegel D, Kepa JK, Tang LJ, Pietenpol JA, Ross D. J Biol Chem. 2003;278:10368–10373. doi: 10.1074/jbc.M211981200. [DOI] [PubMed] [Google Scholar]
- 56.Lyras L, Evans PJ, Shaw PJ, Ince PG, Halliwell B. Free Radical Res. 1996;24:397–406. doi: 10.3109/10715769609088038. [DOI] [PubMed] [Google Scholar]
- 57.Reznick AZ, Packer L. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 58.De Cabo R, Cabello R, Rios M, Lopez-Lluch G, Ingram DK, Lane MA, Navas P. Exp Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.