Abstract
Chaperones (stress proteins) are essential proteins to help the formation and maintenance of the proper conformation of other proteins and to promote cell survival after a large variety of environmental stresses. Therefore, normal chaperone function is a key factor for endogenous stress adaptation of several tissues. However, altered chaperone function has been associated with the development of several diseases; therefore, modulators of chaperone activities became a new and emerging field of drug development. Inhibition of the 90 kDa heat shock protein (Hsp)90 recently emerged as a very promising tool to combat various forms of cancer. On the other hand, the induction of the 70 kDa Hsp70 has been proved to be an efficient help in the recovery from a large number of diseases, such as, for example, ischemic heart disease, diabetes and neurodegeneration. Development of membrane-interacting drugs to modify specific membrane domains, thereby modulating heat shock response, may be of considerable therapeutic benefit as well. In this review, we give an overview of the therapeutic approaches and list some of the key questions of drug development in this novel and promising therapeutic approach.
Keywords: Chaperone coinducers; chaperones; Hsp90; Hsp70, heat shock proteins; geldanamycin; stress proteins; membrane fluidity
Molecular chaperones
Molecular chaperones (1) protect other proteins against aggregation, (2) solubilize initial, loose protein aggregates, (3) assist in folding of nascent proteins or in refolding of damaged proteins, (4) target severely damaged proteins to degradation and (5) in case of excessive damage, sequester damaged proteins to larger aggregates. Chaperones are ubiquitous, highly conserved proteins, which utilize a cycle of ATP-driven conformational changes to refold their targets, and which probably played a major role in the molecular evolution of modern enzymes (Hartl, 1996; Csermely, 1997, 1999; Thirumalai & Lorimer, 2001). Due to the significant overlap in their functions, the major classes of molecular chaperones are best classified by their molecular weights, thus, for example, the abbreviation Hsp90 refers to the 90 kDa heat shock protein (Hsp).
Cellular stress leads to the expression of Hsp's. Stress can be any sudden change in the cellular environment, to which the cell is not prepared to respond, such as heat shock. However, almost all types of cellular stress induce Hsp's. Owing to the generality of this phenomenon, Hsp's are often called stress proteins. The rationale behind this phenomenon is that after stress there is an increased need for the chaperone function of Hsp's, which triggers their induction. This need is caused by the increased amount of damaged proteins, by the inhibition of their elimination via the proteasome as well as by the damage of the chaperones themselves. Hsp induction might help to renature chaperones and, therefore, Hsp induction might lead to a ‘cascading amplification' of available chaperone activity.
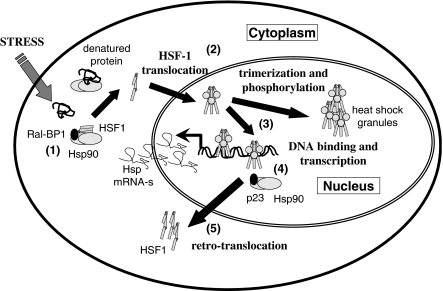
Hsp synthesis is induced by the activation of the heat shock factor (HSF)-1. In resting cells several chaperones, most importantly Hsp90, were shown to bind to HSF-1 and keep it in an inactive form. During stress, these repressing chaperones become occupied by misfolded proteins, which results in the dissociation of the cytoplasmic chaperone/HSF-1 complex. Dissociation of HSF-1 from Hsp90 uncovers the nuclear localization signal of this transcription factor and allows its translocation to the cell nucleus. During this process, the trimerization and phosphorylation of HSF-1 occurs (Morimoto, 2002). Though the exact sequence of these events has not been clearly established, recent studies uncovered the polo-like kinase 1 as an important actor in the phosphorylation and consequent nuclear translocation of HSF-1 at the Ser-419 residue (Kim et al., 2005). However, other studies (Guettouche et al., 2005) found Ser-326 but not Ser-419 as an important site of activation-related phosphorylation of HSF-1. Part of the nuclear HSF-1 is assembled to heat shock granules, which may modify the chromatin structure (Jolly et al., 2004). Binding of HSF-1 to the heat shock elements of the heat shock-inducible genes unlocks the RNA polymerase, which becomes arrested (‘pauses') in most of these genes after transcribing the initial segment of the mRNA in the absence of HSF-1. HSF-1 is released from the DNA by a nuclear complex of Hsp90, which is probably followed by its retrotranslocation to the cytoplasm. The details of the nuclear translocation of Hsp90 during stress, their complex formation in the nucleus and consequent recruitment to the DNA-bound HSF-1 are not entirely known. All elements of the HSF-1 activation and downregulation cascade, such as additional proteins of the cytoplasmic or nuclear HSF-1/Hsp90 complexes, for example, the Ral-binding protein 1 and tubulin in the cytoplasmic HSF-1/Hsp90 complex (Hu & Mivechi, 2003) or p23 in the nuclear HSF-1/Hsp90 complex (Guo et al., 2001), are of great interest as potential drug targets (Figure 1).
Figure 1.
Elements of HSF-1 activation: potential drug targets. The figure contains the major elements of the activation of HSF-1, the major transcription factor leading to the induction of Hsp synthesis. The potential drug targets are the following: (1) cytoplasmic complex of HSF-1 and Hsp90; (2) HSF-1 translocation to the nucleus; (3) intranuclear distribution of HSF-1; (4) nuclear complex of HSF-1 and Hsp90; (5) retrotranslocation of HSF-1 to the cytoplasm. RalBP-1, Ral-binding protein-1; p23, cochaperone of Hsp90.
Hsp's never work alone. They are always forming a complex with each other and recruit various smaller proteins, called cochaperones, which regulate their ATP-ase cycle, therefore the speed of Hsp-assisted refolding. A central chaperone complex of the cytoplasm is assembled around Hsp90 and is called foldosome (Pratt & Toft, 2003).
Therapeutic potential of Hsp90 inhibition
Inhibition of Hsp90 as an efficient tool for anticancer therapies
Hsp90 is one of the most abundant proteins of eukaryotic cells, comprising 1–2% of total proteins under nonstress conditions. It is evolutionarily conserved among species, and is essential for cell survival. Hsp90 exerts its chaperone activity together with a number of cochaperones, playing an important role in the folding of at least 200 specific proteins of various signaling pathways, and in the refolding of denatured proteins after stress (Csermely et al., 1998; Buchner, 1999; Pratt & Toft, 2003).
Hsp90 is an ATP-dependent chaperone. The N-terminal domain of Hsp90 contains a rather unique ATP-binding site, the Bergerat-fold, characteristic of only some bacterial gyrases, topoisomerases and histidine-kinases besides Hsp90 (Prodromou et al., 1997; Stebbins et al., 1997). The unique ATP-binding site allowed the development of specific Hsp90 inhibitors. Recently, it was shown that Hsp90 contains a second nucleotide-binding site at its C-terminal domain, which may open new possibilities for the inhibition of this chaperone (Marcu et al., 2000; Garnier et al., 2002; Soti et al., 2002).
Hsp90 interacts with and stabilizes a growing list of various kinases including several key members of malignant transformation, such as the ErbB2, Src, Abl or Met tyrosine kinases, or the Raf, Akt and cyclin-dependent serine kinases. Besides these, Hsp90 is necessary for the maturation of several transcription factors, like the nuclear hormone receptors and the hypoxia-inducible factor-1. Additionally, Hsp90 binding has been shown to contribute to the accumulation of mutant forms of the tumor suppressor transcription factor p53. Hsp90 is associated with nitric oxide synthases, the antiapoptotic protein, Apaf-1, etc. (Csermely et al., 1998; Buchner, 1999; Pratt & Toft, 2003; Zhao et al., 2005).
The above examples show that a large number of proteins need the help of molecular chaperones to maintain their activation-competent conformation. ‘Conventional' inhibitors interact with their target, directly inhibiting its function. However, chaperone-based inhibitors do not interact with the effector proteins, but inhibit the ability of the associated chaperone(s) to maintain their activation-competent conformation. As a result, the client proteins became degraded by the proteasome (Schulte et al., 1997). In contrast to most direct inhibitors, which are often fairly specific for a given protein, chaperone-based inhibitors diminish the level of many protein targets in parallel (Sreedhar et al., 2004b; Neckers & Neckers, 2005). Thus, chaperone inhibitors behave as typical multi-target drugs, which were suggested to be more efficient than highly selective single-target drugs in many applications (Csermely et al., 2005).
The most important Hsp90 inhibitors are geldanamycin (Whitesell et al., 1994), its less toxic analog, 17-allylamino-17-demethoxy-geldanamycin (17AAG) (Schulte & Neckers, 1998) as well as radicicol, and its more stable oxime derivatives (Soga et al., 1998; Agatsuma et al., 2002; Ikuina et al., 2003), which have a higher affinity for Hsp90 than geldanamycin (Roe et al., 1999). Recently, new geldanamycin analogs (Hargreaves et al., 2003) as well as a third class of tumor-specific Hsp90 inhibitors, the purine-scaffold inhibitors, were developed (Chiosis et al., 2002; Vilenchik et al., 2004), and there are ongoing efforts to synthesize even more Hsp90-interacting drug candidates (Banerji et al., 2003; Maloney et al., 2003; Workman, 2004; Dymock et al., 2005; Kreusch et al., 2005) (Table 1). Hsp90 inhibition was achieved in an unusual way, using the antitumor agent histone deacetylase inhibitors. These compounds, such as Trichostatin-A, induce the acetylation and consequent inhibition of Hsp90 (Yu et al., 2002).
Table 1.
Hsp modulator compounds (a few examples)
| Compound | Modulation | Targets | Potential indications | References |
|---|---|---|---|---|
| Carbenoxonole | Inducer | Hsp70 | Ulcer | Nagayama et al. (2001) |
| 2-Cyclopenten-1-one | Inducer | HSF-1 | Viral infections | Rossi et al. (1996) |
| Dexamethasone | Inducer | HSF-1 | Ischemic heart disease, inflammation | Sun et al. (2000) |
| Geranyl-geranyl acetone | Inducer | Hsp70 | Cerebral and cardiac ischemia | Ooie et al. (2001), Yasuda et al. (2005) |
| Paeoniflorin | Inducer | Hsp27, Hsp40, Hsp70 | Not identified yet | Yan et al. (2004) |
| Proteasome inhibitors | Inducer | Hsp70 | Neuro-degeneration | Kim et al. (2004) |
| Stannous chloride | Inducer | Hsp70 | Tissue transplantation | House et al. (2001) |
| Glycyrrhizin | Co-inducer | HSF-1 | Not identified yet | Yan et al. (2004) |
| Bimoclomol, arimoclomol, BRX-220 | Co-inducer | HSF-1, membranes | ALS, diabetic neurophaty, pancreatitis, ischemic heart disease | Vigh et al. (1997), Jednakovits et al. (2000), Kurthy et al. (2002), Rakonczay Jr. et al. (2002), Kieran et al. (2004) |
| Geldanamycin, 17AAG | Inhibitor | Hsp90 | Cancer | Whitesell et al. (1994), Neckers & Neckers (2005) |
| Radicicol, cyclo-proparadicicol | Inhibitor | Hsp90 | Cancer | Soga et al. (1998), Yang et al. (2004) |
| PU3, PU24F-Cl (purin-based molecules) | Inhibitor | Hsp90 | Cancer | Chiosis et al. (2001) |
| Dihydroxy-phenylpyrazole | Inhibitor | Hsp90 | Cancer | Kreusch et al. (2005) |
| Trichostatin-A (histone deacetylase inhibitors) | Inhibitor | Hsp90 | Cancer | Yu et al. (2002) |
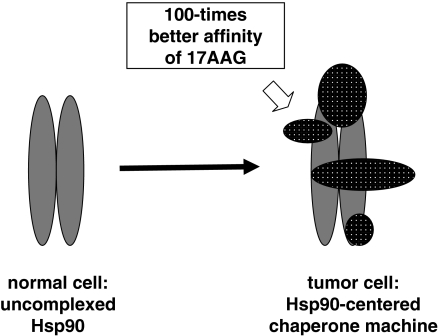
The tumor specificity of Hsp90 inhibitors is helped by an unusual mechanism (Figure 2; Kamal et al., 2003). Hsp90 is largely in a latent, uncomplexed state in normal cells. However, Hsp90 becomes activated, forming a large complex with various cochaperones in tumor cells. 17AAG binds to the tumor-specific, complex form of Hsp90 with a 100-fold higher affinity than to the latent form (Kamal et al., 2003), raising the possibility that active Hsp90 behaves as a tumor-selective catalyst to convert geldanamycin derivatives to their active conformation (Lee et al., 2004). The increased affinity of Hsp90 inhibitors towards tumor-specific Hsp90 is also true for purine-based inhibitors (Vilenchik et al., 2004).
Figure 2.
The tumor specificity of 17AAG, a geldanamycin analog inhibitor of Hsp90. 17AAG has a 100-times higher affinity towards the tumor-specific Hsp90 complexed by a large number of cochaperones than to the Hsp90 dimer, which is the predominant form of this chaperone in normal cells (Kamal et al., 2003; Lee et al., 2004).
Inhibition of Hsp90 induces apoptosis of various tumor cells (Sreedhar & Csermely, 2004). Hsp90 inhibition also leads to a defect in a number of proliferative signals, including the Akt-dependent survival pathway (Munster et al., 2001, 2002; Basso et al., 2002). Hsp90 inhibition may also sensitize tumor cells against various attacks by helping their lysis under hypoxia, complement attack or mild detergent treatment (Sreedhar et al., 2003, 2004a).
Importantly, there are additional drugs which interact with Hsp90, such as the widely used chemotherapeutic agents, cisplatin (Itoh et al., 1999), taxol (Byrd et al., 1999), as well as the antibactericid novobiocin (Marcu et al., 2000). Cisplatin was recently shown to bind to a novel nucleotide-binding site of Hsp90 at its C-terminus (Soti et al., 2002), while novobiocin binds to several domains of Hsp90 (Marcu et al., 2000; Soti et al., 2002). The C-terminal nucleotide-binding pocket has a unique nucleotide-binding specificity (Soti et al., 2003), as well as a differential effect on Hsp90 client proteins (Soti et al., 2002), which gives hopes that selective inhibitors against this segment of Hsp90 can be developed, showing novel properties in various anticancer protocols. This can be an important approach all the more, since recently a new Hsp90 isoform, Hsp90N, has been reported, which lacks the N-terminal domain; thus, the tumor cells which accumulate this chaperone are resistant against any conventional Hsp90 inhibitors (Grammatikakis et al., 2002).
Hsp90 inhibitors enter clinical trials
Though the very first Hsp90 inhibitor geldanamycin showed clear antitumor effects, it encountered difficulties in clinical trials due to its high hepatotoxicity in some of the human tumor models (Supko et al., 1995). Thus, a search for new classes of Hsp90 inhibitors with lower toxicity began, and was successful in developing the analog 17AAG. 17AAG possesses all the Hsp90-related characteristics of geldanamycin (Schulte & Neckers, 1998) with lower toxicity (Brunton et al., 1998; Chiosis et al., 2003; Workman, 2003) and completed five Phase I clinical trials and enters to phase II trials soon (Banerji et al., 2005; Neckers & Neckers, 2005). Both geldanamycin and 17AAG can be metabolized by NADH quinone oxidoreductase 1 (DT-diaphorase), which is known to potentiate antitumor activity by stabilizing the tumor suppressor p53. NADH quinone oxidoreductase 1 may be a major factor in conferring on 17AAG as well as its parent compound the advantage that they specifically accumulate in tumor cells (Chiosis et al., 2003; Workman, 2003), which also contributes to the explanation why Hsp90 inhibitors are not so generally toxic to patients as one would expect from the pleiotropic roles of Hsp90 inhibited by them besides their higher affinity to the tumor-specific, complexed form of Hsp90 mentioned before (Kamal et al., 2003; Vilenchik et al., 2004).
Combination therapies, applying low doses of Hsp90 inhibitors together with conventional chemotherapeutic agents, seem to be an effective way to target various cancers. For example, in the case of Bcr–Abl-expressing leukemias, a low dose of geldanamycin is sufficient to sensitize these cells to apoptosis in the presence of ineffective concentrations of doxorubicin (Blagosklonny et al., 2001). 17AAG in combination with taxol showed enhanced cytotoxic effects on taxol-resistant ErbB2-overexpressing breast cancer cells (Munster et al., 2001; Sausville, 2001). Another approach is to combine 17AAG with angiogenesis inhibitors, which was proven to be highly successful in breast tumors (de Candia et al., 2003). As an alternative strategy the synthesis of geldanamycin hybrids, such as the adduct with steroids (Kuduk et al., 1999, 2000) as well as with inhibitors of the PI-3-kinase related survival pathway (Chiosis et al., 2001), conferred further selectivity and efficiency for these drugs besides their tumor-specific accumulation. Besides the success of 17AAG, other Hsp90 inhibitors also entered phase I trials, which shows the high therapeutic potential of these drugs (Neckers & Neckers, 2005).
Hsp90 inhibitors as Hsp inducers?
Hsp synthesis is induced by the activation of HSF-1. Hsp90 dissociates during HSF-1 activation due to a competitive binding of misfolded proteins to this chaperone (Morimoto, 2002). Hsp90 inhibitors may also cause the transcriptional activation of HSF-1 by disrupting Hsp/HSF-1 complexes. Indeed, geldanamycin and 17AAG were shown to activate HSF-1 (Kim et al., 1999b; Bagatell et al., 2000) and the expression of Hsp40, Hsp70 and Hsp90 (Sittler et al., 2001). Thus, the inhibition of Hsp90, paradoxically, leads to an increase in their overall amount as well, which should be taken into account as a potential disadvantage, when clinical applications of these drugs are designed. Indeed, both geldanamycin and 17AAG were shown to antagonize the action of cisplatin in human colon adenocarcinoma cells (Vasilevskaya et al., 2003).
Inhibition or activation of Hsp90 is cardioprotective?
Griffin et al. (2004) has recently demonstrated that radicicol, which activates Hsp expression by binding to Hsp90, induces Hsp expression in neonatal rat cardiomyocytes, and this increase in Hsp's confers cardioprotection to these cardiomyocytes. However, they found that radicicol induction of the Hsp's and cardioprotection are dependent on the inhibition of Hsp90 in cardiomyocytes, and concluded that inhibitors of the function of Hsp90's in the cell may represent potential cardioprotective agents. In contrast, another recent study shows that in vivo gene transfer of Hsp90 in the myocardium leads to a protection of the ischemic myocardium in pigs via a direct stimulation of eNOS by Hsp90 (Kupatt et al., 2004). It is well known that NO is cardioprotective; however, increased NO synthesis with concomitant superoxide synthesis leads to the formation of the cytotoxic peroxynitrite in certain cardiovascular disorders (Ferdinandy & Schulz, 2003). Therefore, further studies are necessary to evaluate the possible role of Hsp90 inhibitors in the therapy of ischemic heart disease.
Therapeutic potential of upregulation of Hsp's
While the inhibitors of Hsp90 are of potential therapeutic interest primarily in cancer therapy, upregulation of other Hsp's, especially that of Hsp70, has been shown to be of great therapeutic potential in a variety of diseases.
Hsp-based immunotherapies of cancers and infections
The ability of Hsp's to interact with a wide range of proteins and peptides, a property that is shared by major histocompatibility complex molecules, has made the Hsp's to be used in new immunotherapies of cancers and infections. Increased Hsp60, Hsp70 and Hsp72 may lead to tumor cell sensitization for immune attacks by two mechanisms: tumor cells may express Hsp's on their surface, which leads to their enhanced recognition by the natural killer cells of the native immune system (Multhoff et al., 1997; Multhoff, 2002), as well as a specific antitumor immunity may be induced by Hsp-related antitumor vaccination (Chu et al., 2000; Baker-LePain et al., 2003; Shigapova et al., 2005). Hsp induction may help these processes and may be overcome by the limitations of aging- (Wick & Grubeck-Loebenstein, 1997; Pawelec et al., 2002) and chaperone overload-induced (Csermely, 2001) immunosuppression. As these topics have been extensively reviewed recently (Ranford & Henderson, 2002; Srivastava, 2002; Manjili et al., 2004; Oki & Younes, 2004), no further details are given in our present review.
Tissue protection by induction and ‘coinduction' of Hsp70 and other Hsp's
From the now classical observations of Currie et al. (1988), we know that heat-shock response has a significant role in cardioprotection. Similarly, Hsp72 induction helps the survival of neurons after stroke (Yenari et al., 1998), as well as improves the efficiency of tissue transplantation (Perdrizet et al., 1993). Hsp70 induction eases the deleterious consequences of chronic diseases, such as diabetes, as revealed by the application of the Hsp coinducer compounds bimoclomol and BRX-220 (Nanasi & Jednakovits, 2001; Kurthy et al., 2002). Conditions like Alzheimer's, Parkinson's, Huntington's or prion disease, where the accumulation of misfolded proteins is the major cause of neurodegeneration (Warrick et al., 1999; Carmichael et al., 2000; Sittler et al., 2001), as well as conditions such as trauma, where neuroregeneration becomes necessary (Kalmar et al., 2002, 2003), also have beneficial effects from overexpression of Hsp40 and Hsp70.
There are various approaches to induce Hsp's using pharmacological interventions without any traditional forms of stress, such as heat shock. Proteasome inhibitors upregulate Hsp synthesis by increasing the amount of misfolded proteins, which compete for Hsp's with HSF-1 (Kim et al., 1999a, 2004). Some of the protein kinase inhibitors were also shown to induce Hsp27 induction (Kawamura et al., 1999). Stannous chloride has been shown as a nontoxic, efficient inducer of Hsp70, improving the success rate of tissue transplantations (House et al., 2001). Geranyl-geranyl acetone, a nontoxic Hsp70 inducer, has been shown to protect neurons against cerebral ischemia (Yasuda et al., 2005). Similarly, the antiulcer drug carbenoxolone has also been shown to be an inducer of Hsp70 (Nagayama et al., 2001) (Table 1).
Most of the above methods introduce a certain level of stress to the cells and thus provoke Hsp synthesis. However, in most of the diseases it seems to be more efficient if the administered drug does not induce Hsp's, and just helps the natural Hsp induction provoked by the natural stimuli on the cell. This help in Hsp induction was termed as chaperone ‘coinduction' (Vigh et al., 1997). Chaperone coinducers act like ‘smart drugs' by a selective interaction with only those cells which are in danger, and may provide an important novel therapy in a number of acute and chronic diseases.
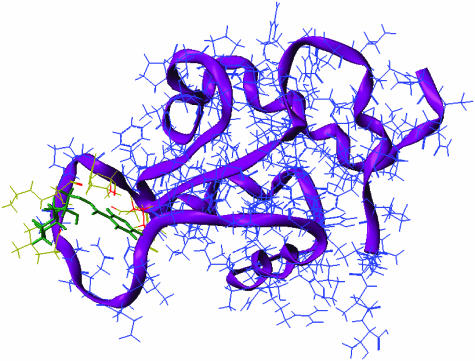
The best-known chaperone coinducer is aspirin, which enhances Hsp70 synthesis (Jurivich et al., 1992). Recently, certain herbal extracts were also shown to possess Hsp coinducer activity (Yan et al., 2004). Another family of drug candidates exemplified by Bimoclomol helps the induction of Hsp70 synthesis by both perturbing various membrane structures and helping the release of putative lipid-signaling molecules, as well as by the prolongation of the binding of HSF-1 to the heat shock elements on the DNA (Vigh et al., 1997; Hargitai et al., 2003; Torok et al., 2003; Kieran et al., 2004). Bimoclomol binds to HSF-1 with a low affinity (Figure 3), which may contribute to its effect to prolong HSF-1 binding to DNA. Chaperone coinducers also stabilize membranes, which may be of special importance to prevent apoptotic events related to the decomposition of cardiolipin and the consecutive instabilization of the mitochondrial membrane (Torok et al., 2003). Recently, a successful application of the chaperone coinducer arimoclomol has been shown to delay the onset of amyotrophic lateral sclerosis (Kieran et al., 2004). Another chaperone coinducer, BRX-220, has been shown to protect against CCK-induced acute pancreatitis (Fujiwara et al., 2004) (Table 1).
Figure 3.
Bimoclomol binds to the HSF-1 with a low affinity (Hargitai et al., 2003). Fitting of /R/-bimoclomol to the 3D model of the DNA-binding domain of human HSF-1. The SiteID, FlexX and the FlexiDock modules of the Sybyl package were used sequentially to identify the loop domain as the active center (yellow–red amino acids) of the DNA-binding domain of human HSF-1 and dock bimoclomol (green) into this site. The calculated binding energy for the /R/-bimoclomol was −26 kcal mol−1. The backbone of the polypeptide is shown (purple).
Diminished heat stress response in hyperlipidemia and aging: potential use of Hsp inducers as anti-ischemic agents?
It is well known that aging leads to a diminished heat shock response in several tissues, thereby resulting in the deterioration of stress adaptation (Demirel et al., 2003) (for a review, see Soti & Csermely, 2003). We have recently reported that experimental hyperlipidemia also leads to diminished heat-stress response, as measured by Hsp70 expression in the ischemic heart of rats (Csont et al., 2002). It is of great interest that both aging and hyperlipidemia have been shown to decrease the cardioprotective effects of the ischemic preconditioning, that is, the endogenous adaptive response of the heart to ischemic stress (for reviews, see Ferdinandy et al., 1998; Baxter & Ferdinandy, 2001; Ferdinandy, 2003). The role of Hsp's in the mechanism of preconditioning has been extensively reviewed elsewhere (Latchman, 2001; Snoeckx et al., 2001). It has been shown that the cardioprotective effect of preconditioning is linked to the functions of Hsp70, Hsp27, and alphaB-crystalline. Heat stress or Hsp70 gene transfection into rat hearts has been shown to protect the ischemic myocardium (Arnaud et al., 2001; Jayakumar et al., 2001). Therefore, it is plausible to speculate that the loss of the protective effect of preconditioning in these disease states is related – at least in part – to diminished heat stress response. We have recently shown using DNA-microarray assay that hyperlipidemia alters the expression of several chaperones in the rat myocardium, including upregulation of Hsp86, metallothionein and glutathione-S-transferase, as well as downregulation of proteasome component C9, ubiquitin-like protein FUBI, Hsp105, calreticulin and chaperonin subunit 5 epsilon (Puskas et al., 2004).
The mechanisms by which hyperlipidemia and aging lead to diminished heat stress response are not known. It is suggested, however, that the altered membrane lipid composition and physical state (fluidity) of membranes are decisive factors in the processes of perception and transduction of stress into a signal that triggers the transcriptional activation of stress protein genes. Nevertheless, Hsp inducers and coinducers may have great therapeutic potential in aging and hyperlipidemia to regain the endogenous adaptation of the heart to ischemic stress.
Cellular membranes act as alternative thermosensors to regulate Hsp expression
Elucidation of primary sensors that perceive various stress stimuli and transducers that carry signals culminating in the expression of a particular Hsp is of great interest for the development of novel Hsp inducers and coinducers. According to the classical model, the common primary signal for induction of Hsp's is an increase in the amount of denatured proteins within the cell during stress and their competition with HSF (Morimoto, 1998) (see above for more refs). Hsp's are present in abnormal levels in a variety of human diseases and aging, but there is no evidence for concomitant modification of the kinetics of accumulation of denatured proteins that could justify the changes observed in the expression of Hsp's (Vigh et al., 1998; Vigh & Maresca, 2002). For example, curcumin, a well-known dietary pigment with potent antiinflammatory, antioxidant and anticancer effects, causes heat shock response in transformed cell lines such as leukemia, breast, colon, etc.; however, it has no effect on nontransformed cell lines (Khar et al., 2001). Since no differences in the pattern of protein denaturation have been reported between normal and transformed cell lines, these data imply that the protein denaturation is not (or not only) the mechanism by which Hsp's are upregulated. There is also evidence for differential expression patterns of different Hsp's in the same cell type in response to the same stimulus (Heads et al., 1995). For example, herbimycin-A provokes Hsp70 synthesis and protects against a subsequent lethal heat stress; however, it does not activate the transcription of other Hsp genes such as hsp90, hsp60, hsp27 and grp78 in cultured neonatal cardiomyocytes (Morris et al., 1996). Monophosphoryl lipid A, a nontoxic derivative of endotoxin with potent anti-ischemic effect, induced a remarkable accumulation of Hsp70, but did not change the expression of Hsp27, Hsp32 and Hsp90 in adult rat cardiac myocytes (Nayeem et al., 1997). Selective stimulation of small Hsp's, Hsp27, and alfaB-crystalline but not Hsp70 was shown by anisomycin following heat stress in C6 glioma cells (Kato et al., 1999), by sphingosine1-phospate in osteoblasts (Kozawa et al., 1999), by hydroxyurea in B16 murine melanoma cells (Eskenazi et al., 1998), by short-chain fatty acids in the rat intestinal epithelial cells (Ren et al., 2001) or by thrombin in aortic smooth muscle A10 cells (Hirade et al., 2002), respectively. These observations indicate that Hsp expression in response to various stressors is regulated by differential control mechanisms rather than by uniform mechanisms.
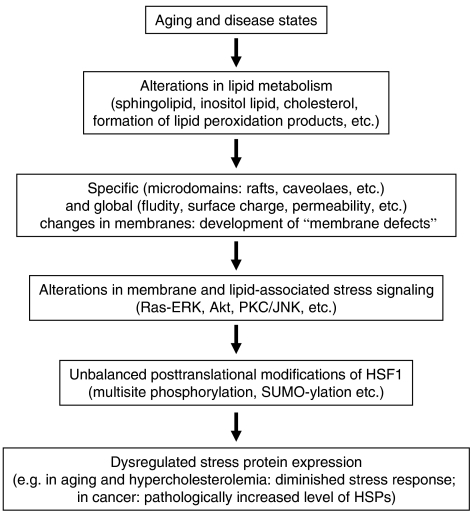
On the basis of experimental evidence, it is proposed that, besides protein denaturation, specific membrane domains may act as alternative sensors or ‘cellular thermometers'. Stress-induced membrane perturbations are converted into signal(s) leading to activation of heat-shock genes (Carratu et al., 1996; Horvath et al., 1998; Vigh et al., 1998; Vigh & Maresca, 2002; Shigapova et al., 2005). Changes in membrane composition and/or fluidity alter the ‘set point' for Hsp expression, with expression initiated at lower temperatures in cells with more fluid membranes (Carratu et al., 1996; Horvath et al., 1998; Chatterjee et al., 2000; Shigapova et al., 2005) (Figure 4).
Figure 4.
Cascade of events from membrane defects to dysregulated stress protein response.
Cellular membranes as potential targets for Hsp inducers
Although the Hsp coinducing effects of bimoclomol were shown to be mediated via HSF-1 as described above, bimoclomol does not affect protein denaturation in the cells (Vigh et al., 1997; Hargitai et al., 2003). It has been shown that bimoclomol and its analogs specifically interact with and significantly increase the fluidity of negatively charged membrane lipids. Accordingly, the Hsp coinducing activity of bimoclomol is highly susceptible to the fatty acid composition and fluidity of membrane of target cells. In addition, bimoclomol is an efficient inhibitor of bilayer-to-nonbilayer lipid-phase transitions. Consequently, while sensitizing the cellular membranes at mild heat shock conditions, the drug protects against irreversible membrane damage at higher temperatures (Torok et al., 2003). Thus, even subtle alterations of the lipid phase of membranes caused by aging or pathophysiological conditions may influence membrane-initiated signaling processes either through fluidity changes or by specific interactions of membrane lipids with receptor proteins localized in the membrane. Taken together, it is highly conceivable that plasma membrane, which is the barrier to the external environment and well suited for sensing stress, acts also as an important regulatory interface. In line of this concept, recently it was speculated that the reduced HSF-1 and Hsp levels in diabetes are the result of reduced membrane fluidity (Hooper & Hooper, 2005). In fact, as a result of glycation, oxidative stress and insulin deficiency, diabetes is associated with less fluid membranes in human mononuclear leucocytes and platelets (Tong et al., 1995; Srivastava, 2002). Since heat treatment itself causes membrane hyperfluidization, it is not surprising that the daily hot water immersions in patents with type II diabetes improve glycemic control and reduce neuropathic symptoms (Hooper, 1999).
Perturbation of the organization and phase state of acidic glycerophospholipids, the major determinants of the activity of membrane proteins (van Klompenburg et al., 1997), by nontoxic drugs like bimoclomol may influence the structure/activity of membrane-bound proteins without direct drug–protein interaction. Protein function influenced by membrane fluidity and/or microheterogeneity has been suggested for phospholipase A2, which is stimulated by heat shock, resulting in arachidonic acid release (Honger et al., 1996; Samples et al., 1999). Arachidonic acid stimulates HSF–DNA binding, increases phosphorylation of HSF-1 and upregulates transcription of the Hsp70 gene in HeLa cells (Jurivich et al., 1994). Elevated activity of membrane-bound phospholipases and the resultant release of free fatty acids and diacylglycerols could also enhance the membrane association and activation of protein kinase C (PKC) isoforms responsible for the phosphorylation of HSFs. In agreement with these data, administration of a PKC activator phorbol ester compound in combination with heat shock markedly enhanced Hsp70 expression in K562 cells (Holmberg et al., 1997). Several other pharmacological modulators of kinase/phosphatase activities can alter the different regulatory steps of the heat shock response. Highlighting the complexity of this point, overexpression of inducible Hsp70 downregulated the basal activities of protein kinase A, various free and membrane-associated PKC isoforms and the MAP kinase pathways including c-Jun N-terminal kinase and p38 stress-activated protein kinase (p38 SAPK) (Kiang et al., 2002). Through the activation of the glycosylation of membrane sterols, cholesterol glucoside is rapidly accumulating in cells from mold to humans by exposure to environmental stress, and cholesterol glucoside production is followed by the activation of certain PKCs and induction of Hsp's (Kunimoto et al., 2002). Cholesterol glucoside accelerated the binding of HSF-1 to HSE and upregulated Hsp70 synthesis in human fibroblast (Kunimoto et al., 2002). Orally administered cholesterol glucoside apparently showed antiulcer activity in rats via HSF activation and Hsp70 induction (Kunimoto et al., 2003). A bimoclomol-related compound, BRX-235, was shown to induce phosphorylation of p38 SAPK, implying that the molecule acted upstream of p38 SAPK (Denes et al., 2002; Kabakov et al., 2005). HSF-1 appears to have several additional layers of regulation in the cell, including Ras/ERK1/GSK3/14-3-3 pathway (Hamaguchi et al., 2003; Wang et al., 2003), Akt-induced inhibition of GSK-3β (Bijur & Jope, 2000), small G-protein signalling such as Ras (Engelberg et al., 1994; Murakoshi et al., 2004) and oxidative stress-induced membrane translocation of Rac1 (Xu et al., 2000; Han et al., 2001), all potential targets for Hsp modulator development. Noteworthy that simvastatin, the known hydroxymethyl-glutaryl-CoA reductase inhibitor antihyperlipidemic drug, blocked the oxidative stress-induced membrane translocation of Rac1 (Negre-Aminou et al., 2002).
It is highly conceivable that the above findings are linked to those hypothetic signal transduction pathways which transmit the heat stress signal from membranes to DNA to induce expression of Hsp's. However, a lipid-selective association of a subpopulation of Hsp's with membranes, leading to increased molecular order, may in turn lead to downregulation of the heat shock gene expression (Torok et al., 1997, 2001; Tsvetkova et al., 2002). Such a ‘crosstalk' between the primary stress sensor in the membranes and Hsp's suggests a feedback mechanism in the regulation of heat-shock genes, explaining the known temporality of induction of Hsp's. These findings show that knowledge on pathways of stress signaling will provide several molecular targets for further development of Hsp modulators.
Conclusions and future perspectives
Chaperones play a major role in the mechanism of endogenous stress adaptation of several tissues. However, altered chaperone function has been associated with the development of several pathologies; therefore, chaperone modulators became a new and emerging field of drug development. Inhibitors of Hsp90 recently emerged as a very promising tool to combat various forms of cancer. On the other hand, activation of chaperone induction proved to be an efficient tool for the recovery from a large number of diseases, such as, for example, ischemic heart disease, diabetes and neurodegeneration. Development of several Hsp modulators has already reached clinical phases. Due to the promising results, specific chaperone modulators could be one of the future blockbuster drugs on the market for several different therapeutic indications.
Acknowledgments
The help of Andras Fiser and Timea Rácz in providing Figure 3 is greatly acknowledged. We acknowledge the support from grants from the EU 6th Framework program (FP6-506850, FP6-016003), the Hungarian Science Foundation (OTKA T37357, T46417, F47281, TS44836), the Hungarian Ministry of Social Welfare (ETT-32/03, ETT 616/03), the Hungarian National Research Initiative (NKFP-1A/056/2004 and KKK-0015/3.0) and from the National Office for Research and Technology (NKTH-RET2004). P.F. holds an István Széchenyi Professorship of the Hungarian Academy of Sciences.
Abbreviations
- 17AAG
17-allylamino-17-demethoxy-geldanamycin
- HSF
heat shock factor
- Hsp
heat shock protein
- PKC
protein kinase C
References
- AGATSUMA T., OGAWA H., AKASAKA K., ASAI A., YAMASHITA Y., MIZUKAMI T., AKINAGA S., SAITOH Y. Halohydrin and oxime derivatives of radicicol: synthesis and antitumor activities. Bioorg. Med. Chem. 2002;10:3445–3454. doi: 10.1016/s0968-0896(02)00260-2. [DOI] [PubMed] [Google Scholar]
- ARNAUD C., LAUBRIET A., JOYEUX M., GODIN-RIBUOT D., ROCHETTE L., DEMENGE P., RIBUOT C. Role of nitric oxide synthases in the infarct size-reducing effect conferred by heat stress in isolated rat hearts. Br. J. Pharmacol. 2001;132:1845–1851. doi: 10.1038/sj.bjp.0703942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAGATELL R., PAINE-MURRIETA G.D., TAYLOR C.W., PULCINI E.J., AKINAGA S., BENJAMIN I.J., WHITESELL L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin. Cancer Res. 2000;6:3312–3318. [PubMed] [Google Scholar]
- BAKER-LEPAIN J.C., REED R.C., NICCHITTA C.V. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr. Opin. Immunol. 2003;15:89–94. doi: 10.1016/s0952791502000067. [DOI] [PubMed] [Google Scholar]
- BANERJI U., JUDSON I., WORKMAN P. The clinical applications of heat shock protein inhibitors in cancer – present and future. Curr. Cancer Drug Targets. 2003;3:385–390. doi: 10.2174/1568009033481813. [DOI] [PubMed] [Google Scholar]
- BANERJI U., O'DONNELL A., SCURR M., PACEY S., STAPLETON S., ASAD Y., SIMMONS L., MALONEY A., RAYNAUD F., CAMPBELL M., WALTON M., LAKHANI S., KAYE S., WORKMAN P., JUDSON I. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J. Clin. Oncol. 2005;23:4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- BASSO A.D., SOLIT D.B., CHIOSIS G., GIRI B., TSICHLIS P., ROSEN N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F., FERDINANDY P. Delayed preconditioning of myocardium: current perspectives. Basic Res. Cardiol. 2001;96:329–344. doi: 10.1007/s003950170041. [DOI] [PubMed] [Google Scholar]
- BIJUR G.N., JOPE R.S. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J. Neurochem. 2000;75:2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- BLAGOSKLONNY M.V., FOJO T., BHALLA K.N., KIM J.S., TREPEL J.B., FIGG W.D., RIVERA Y., NECKERS L.M. The Hsp90 inhibitor geldanamycin selectively sensitizes Bcr–Abl-expressing leukemia cells to cytotoxic chemotherapy. Leukemia. 2001;15:1537–1543. doi: 10.1038/sj.leu.2402257. [DOI] [PubMed] [Google Scholar]
- BRUNTON V.G., STEELE G., LEWIS A.D., WORKMAN P. Geldanamycin-induced cytotoxicity in human colon-cancer cell lines: evidence against the involvement of c-Src or DT-diaphorase. Cancer Chemother. Pharmacol. 1998;41:417–422. doi: 10.1007/s002800050760. [DOI] [PubMed] [Google Scholar]
- BUCHNER J. Hsp90 & Co. a holding for folding. Trends Biochem. Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- BYRD C.A., BORNMANN W., ERDJUMENT-BROMAGE H., TEMPST P., PAVLETICH N., ROSEN N., NATHAN C.F., DING A. Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5645–5650. doi: 10.1073/pnas.96.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMICHAEL J., CHATELLIER J., WOOLFSON A., MILSTEIN C., FERSHT A.R., RUBINSZTEIN D.C. Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington's disease. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9701–9705. doi: 10.1073/pnas.170280697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRATU L., FRANCESCHELLI S., PARDINI C.L., KOBAYASHI G.S., HORVATH I., VIGH L., MARESCA B. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE M.T., KHALAWAN S.A., CURRAN B.P. Cellular lipid composition influences stress activation of the yeast general stress response element (STRE) Microbiology. 2000;146:877–884. doi: 10.1099/00221287-146-4-877. [DOI] [PubMed] [Google Scholar]
- CHIOSIS G., HUEZO H., ROSEN N., MIMNAUGH E., WHITESELL L., NECKERS L. 17AAG: low target binding affinity and potent cell activity – finding an explanation. Mol. Cancer Ther. 2003;2:123–129. [PubMed] [Google Scholar]
- CHIOSIS G., LUCAS B., SHTIL A., HUEZO H., ROSEN N. Development of a purine-scaffold novel class of Hsp90 binders that inhibit the proliferation of cancer cells and induce the degradation of Her2 tyrosine kinase. Bioorg. Med. Chem. 2002;10:3555–3564. doi: 10.1016/s0968-0896(02)00253-5. [DOI] [PubMed] [Google Scholar]
- CHIOSIS G., ROSEN N., SEPP-LORENZINO L. LY294002-geldanamycin heterodimers as selective inhibitors of the PI3K and PI3K-related family. Bioorg. Med. Chem. Lett. 2001;11:909–913. doi: 10.1016/s0960-894x(01)00099-3. [DOI] [PubMed] [Google Scholar]
- CHU N.R., WU H.B., WU T.C., BOUX L.J., MIZZEN L.A., SIEGEL M.I. Immunotherapy of a human papillomavirus type 16 E7-expressing tumor by administration of fusion protein comprised of Mycobacterium bovis BCG Hsp65 and HPV16 E7. Cell Stress Chaperones. 2000;5:401–405. doi: 10.1379/1466-1268(2000)005<0401:ioahpt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSERMELY P. Proteins, RNAs and chaperones in enzyme evolution: a folding perspective. Trends Biochem. Sci. 1997;22:147–149. doi: 10.1016/s0968-0004(97)01026-8. [DOI] [PubMed] [Google Scholar]
- CSERMELY P. Chaperone-percolator model: a possible molecular mechanism of Anfinsen-cage-type chaperones. BioEssays. 1999;21:959–965. doi: 10.1002/(SICI)1521-1878(199911)21:11<959::AID-BIES8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- CSERMELY P. Chaperone overload is a possible contributor to ‘civilization diseases'. Trends Genet. 2001;17:701–704. doi: 10.1016/s0168-9525(01)02495-7. [DOI] [PubMed] [Google Scholar]
- CSERMELY P., AGOSTON V., PONGOR S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol. Sci. 2005;26:178–182. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- CSERMELY P., SCHNAIDER T., SOTI C., PROHASZKA Z., NARDAI G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- CSONT T., BALOGH G., CSONKA C., BOROS I., HORVATH I., VIGH L., FERDINANDY P. Hyperlipidemia induced by high cholesterol diet inhibits heat shock response in rat hearts. Biochem. Biophys. Res. Commun. 2002;290:1535–1538. doi: 10.1006/bbrc.2002.6377. [DOI] [PubMed] [Google Scholar]
- CURRIE R.W., KARMAZYN M., KLOC M., MAILER K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ. Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- DE CANDIA P., SOLIT D.B., GIRI D., BROGI E., SIEGEL P.M., OLSHEN A.B., MULLER W.J., ROSEN N., BENEZRA R. Angiogenesis impairment in Id-deficient mice cooperates with an Hsp90 inhibitor to completely suppress HER2/neu-dependent breast tumors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12337–12342. doi: 10.1073/pnas.2031337100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMIREL H.A., HAMILTON K.L., SHANELY R.A., TUMER N., KOROLY M.J., POWERS S.K. Age and attenuation of exercise-induced myocardial HSP72 accumulation. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1609–H1615. doi: 10.1152/ajpheart.00982.2002. [DOI] [PubMed] [Google Scholar]
- DENES L., JEDNAKOVITS A., HARGITAI J., PENZES Z., BALLA A., TALOSI L., KRAJCSI P., CSERMELY P. Pharmacologically activated migration of aortic endothelial cells is mediated through p38 SAPK. Br. J. Pharmacol. 2002;136:597–603. doi: 10.1038/sj.bjp.0704738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYMOCK B.W., BARRIL X., BROUGH P.A., CANSFIELD J.E., MASSEY A., MCDONALD E., HUBBARD R.E., SURGENOR A., ROUGHLEY S.D., WEBB P., WORKMAN P., WRIGHT L., DRYSDALE M.J. Novel, potent small-molecule inhibitors of the molecular chaperone Hsp90 discovered through structure-based design. J. Med. Chem. 2005;48:4212–4215. doi: 10.1021/jm050355z. [DOI] [PubMed] [Google Scholar]
- ENGELBERG D., ZANDI E., PARKER C.S., KARIN M. The yeast and mammalian Ras pathways control transcription of heat shock genes independently of heat shock transcription factor. Mol. Cell. Biol. 1994;14:4929–4937. doi: 10.1128/mcb.14.7.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESKENAZI A.E., POWERS J., PINKAS J., OESTERREICH S., FUQUA S.A., FRANTZ C.N. Induction of heat shock protein 27 by hydroxyurea and its relationship to experimental metastasis. Clin. Exp. Metastasis. 1998;16:283–290. doi: 10.1023/a:1006553127695. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P. Myocardial ischaemia/reperfusion injury and preconditioning: effects of hypercholesterolaemia/hyperlipidaemia. Br. J. Pharmacol. 2003;138:283–285. doi: 10.1038/sj.bjp.0705097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERDINANDY P., SZILVASSY Z., BAXTER G.F. Adaptation to myocardial stress in disease states: is preconditioning a healthy heart phenomenon. Trends Pharmacol. Sci. 1998;19:223–229. doi: 10.1016/s0165-6147(98)01212-7. [DOI] [PubMed] [Google Scholar]
- FUJIWARA H., YAMAKUNI T., UENO M., ISHIZUKA M., SHINKAWA T., ISOBE T., OHIZUMI Y. IC101 induces apoptosis by Akt dephosphorylation via an inhibition of heat shock protein 90-ATP binding activity accompanied by preventing the interaction with Akt in L1210 cells. J. Pharmacol. Exp. Ther. 2004;310:1288–1295. doi: 10.1124/jpet.104.065979. [DOI] [PubMed] [Google Scholar]
- GARNIER C., LAFITTE D., TSVETKOV P.O., BARBIER P., LECLERC-DEVIN J., MILLOT J.M., BRIAND C., MAKAROV A.A., CATELLI M.G., PEYROT V. Binding of ATP to heat shock protein 90: evidence for an ATP-binding site in the C-terminal domain. J. Biol. Chem. 2002;277:12208–12214. doi: 10.1074/jbc.M111874200. [DOI] [PubMed] [Google Scholar]
- GRAMMATIKAKIS N., VULTUR A., RAMANA C.V., SIGANOU A., SCHWEINFEST C.W., WATSON D.K., RAPTIS L. The role of Hsp90N, a new member of the Hsp90 family, in signal transduction and neoplastic transformation. J. Biol. Chem. 2002;277:8312–8320. doi: 10.1074/jbc.M109200200. [DOI] [PubMed] [Google Scholar]
- GRIFFIN T.M., VALDEZ T.V., MESTRIL R. Radicicol activates heat shock protein expression and cardioprotection in neonatal rat cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2004;287:1081–1088. doi: 10.1152/ajpheart.00921.2003. [DOI] [PubMed] [Google Scholar]
- GUETTOUCHE T., BOELLMANN F., LANE W.S., VOELLMY R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO Y., GUETTOUCHE T., FENNA M., BOELLMANN F., PRATT W.B., TOFT D.O., SMITH D.F., VOELLMY R. Evidence for a mechanism of repression of heat shock factor 1 transcriptional activity by a multichaperone complex. J. Biol. Chem. 2001;276:45791–45799. doi: 10.1074/jbc.M105931200. [DOI] [PubMed] [Google Scholar]
- HAMAGUCHI A., SUZUKI E., MURAYAMA K., FUJIMURA T., HIKITA T., IWABUCHI K., HANDA K., WITHERS D.A., MASTERS S.C., FU H., HAKOMORI S. A sphingosine-dependent protein kinase that specifically phosphorylates 14-3-3 (SDK1) is identified as the kinase domain of PKCdelta: a preliminary note. Biochem. Biophys. Res. Commun. 2003;307:589–594. doi: 10.1016/s0006-291x(03)01070-2. [DOI] [PubMed] [Google Scholar]
- HAN S.I., OH S.Y., WOO S.H., KIM K.H., KIM J.H., KIM H.D., KANG H.S. Implication of a small GTPase Rac1 in the activation of c-Jun N-terminal kinase and heat shock factor in response to heat shock. J. Biol. Chem. 2001;276:1889–1895. doi: 10.1074/jbc.M006042200. [DOI] [PubMed] [Google Scholar]
- HARGITAI J., LEWIS H., BOROS I., RACZ T., FISER A., KURUCZ I., BENJAMIN I., VIGH L., PENZES Z., CSERMELY P., LATCHMAN D.S. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem. Biophys. Res. Commun. 2003;307:689–695. doi: 10.1016/s0006-291x(03)01254-3. [DOI] [PubMed] [Google Scholar]
- HARGREAVES R., DAVID C.L., WHITESELL L., SKIBO E.B. Design of quinolinedione-based geldanamycin analogues. Bioorg. Med. Chem. Lett. 2003;13:3075–3078. doi: 10.1016/s0960-894x(03)00650-4. [DOI] [PubMed] [Google Scholar]
- HARTL F.U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- HEADS R.J., YELLON D.M., LATCHMAN D.S. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J. Mol. Cell. Cardiol. 1995;27:1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- HIRADE K., KOZAWA O., TANABE K., NIWA M., MATSUNO H., OISO Y., AKAMATSU S., ITO H., KATO K., KATAGIRI Y., UEMATSU T. Thrombin stimulates dissociation and induction of HSP27 via p38 MAPK in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H941–H948. doi: 10.1152/ajpheart.00060.2001. [DOI] [PubMed] [Google Scholar]
- HOLMBERG C.I., LEPPA S., ERIKSSON J.E., SISTONEN L. The phorbol ester 12-O-tetradecanoylphorbol 13-acetate enhances the heat-induced stress response. J. Biol. Chem. 1997;272:6792–6798. doi: 10.1074/jbc.272.10.6792. [DOI] [PubMed] [Google Scholar]
- HONGER T., JORGENSEN K., BILTONEN R.L., MOURITSEN O.G. Systematic relationship between phospholipase A2 activity and dynamic lipid bilayer microheterogeneity. Biochemistry. 1996;35:9003–9006. doi: 10.1021/bi960866a. [DOI] [PubMed] [Google Scholar]
- HOOPER P.L. Hot-tub therapy for type 2 diabetes mellitus. N. Engl. J. Med. 1999;341:924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- HOOPER P.L., HOOPER J.J. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol. Ther. 2005;7:204–208. doi: 10.1089/dia.2005.7.204. [DOI] [PubMed] [Google Scholar]
- HORVATH I., GLATZ A., VARVASOVSZKI V., TOROK Z., PALI T., BALOGH G., KOVACS E., NADASDI L., BENKO S., JOO F., VIGH L. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a ‘fluidity gene'. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUSE S.D., GUIDON P.T., JR, PERDRIZET G.A., REWINSKI M., KYRIAKOS R., BOCKMAN R.S., MISTRY T., GALLAGHER R.A., HIGHTOWER L.E. Effects of heat shock, stannous chloride, and gallium nitrate on the rat inflammatory response. Cell Stress Chaperones. 2001;6:164–171. doi: 10.1379/1466-1268(2001)006<0164:eohssc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU Y., MIVECHI N.F. HSF-1 interacts with Ral-binding protein 1 in a stress-responsive, multiprotein complex with HSP90 in vivo. J. Biol. Chem. 2003;278:17299–17306. doi: 10.1074/jbc.M300788200. [DOI] [PubMed] [Google Scholar]
- IKUINA Y., AMISHIRO N., MIYATA M., NARUMI H., OGAWA H., AKIYAMA T., SHIOTSU Y., AKINAGA S., MURAKATA C. Synthesis and antitumor activity of novel O-carbamoylmethyloxime derivatives of radicicol. J. Med. Chem. 2003;46:2534–2541. doi: 10.1021/jm030110r. [DOI] [PubMed] [Google Scholar]
- ITOH H., OGURA M., KOMATSUDA A., WAKUI H., MIURA A.B., TASHIMA Y. A novel chaperone-activity-reducing mechanism of the 90-kDa molecular chaperone HSP90. Biochem. J. 1999;343:697–703. [PMC free article] [PubMed] [Google Scholar]
- JAYAKUMAR J., SUZUKI K., SAMMUT I.A., SMOLENSKI R.T., KHAN M., LATIF N., ABUNASRA H., MURTUZA B., AMRANI M., YACOUB M.H. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia–reperfusion injury. Circulation. 2001;104:I303–I307. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- JEDNAKOVITS A., FERDINANDY P., JASZLITS L., BANYASZ T., MAGYAR J., SZIGLIGETI P., KORTVELY A., SZENTMIKLOSI J.A., NANASI P.P. In vivo and in vitro acute cardiovascular effects of bimoclomol. Gen. Pharmacol. 2000;34:363–369. doi: 10.1016/s0306-3623(01)00074-x. [DOI] [PubMed] [Google Scholar]
- JOLLY C., METZ A., GOVIN J., VIGNERON M., TURNER B.M., KHOCHBIN S., VOURC'H C. Stress-induced transcription of satellite III repeats. J. Cell Biol. 2004;164:25–33. doi: 10.1083/jcb.200306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURIVICH D.A., SISTONEN L., KROES R.A., MORIMOTO R.I. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- JURIVICH D.A., SISTONEN L., SARGE K.D., MORIMOTO R.I. Arachidonate is a potent modulator of human heat shock gene transcription. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2280–2284. doi: 10.1073/pnas.91.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABAKOV A.E., BUDAGOVA K.R., MALYUTINA Y.V., LATCHMAN D.S., CSERMELY P. Pharmacological attenuation of apoptosis in reoxygenated endothelial cells. Cell. Mol. Life Sci. 2005;61:3076–3086. doi: 10.1007/s00018-004-4204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALMAR B., BURNSTOCK G., VRBOVA G., URBANICS R., CSERMELY P., GREENSMITH L. Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats. Exp. Neurol. 2002;176:87–97. doi: 10.1006/exnr.2002.7945. [DOI] [PubMed] [Google Scholar]
- KALMAR B., GREENSMITH L., MALCANGIO M., MCMAHON S.B., CSERMELY P., BURNSTOCK G. The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. Exp. Neurol. 2003;184:636–647. doi: 10.1016/S0014-4886(03)00343-1. [DOI] [PubMed] [Google Scholar]
- KAMAL A., THAO L., SENSINTAFFAR J., ZHANG L., BOEHM M.F., FRITZ L.C., BURROWS F.J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- KATO K., ITO H., KAMEI K., IWAMOTO I. Selective stimulation of Hsp27 and alphaB-crystallin but not Hsp70 expression by p38 MAP kinase activation. Cell Stress Chaperones. 1999;4:94–101. [PMC free article] [PubMed] [Google Scholar]
- KAWAMURA H., OTSUKA T., MATSUNO H., NIWA M., MATSUI N., KATO K., UEMATSU T., KOZAWA O. Endothelin-1 stimulates heat shock protein 27 induction in osteoblasts: involvement of p38 MAP kinase. Am. J. Physiol. 1999;277:E1046–E1054. doi: 10.1152/ajpendo.1999.277.6.E1046. [DOI] [PubMed] [Google Scholar]
- KHAR A., ALI A.M., PARDHASARADHI B.V., VARALAKSHMI C.H., ANJUM R., KUMARI A.L. Induction of stress response renders human tumor cell lines resistant to curcumin-mediated apoptosis: role of reactive oxygen intermediates. Cell Stress Chaperones. 2001;6:368–376. doi: 10.1379/1466-1268(2001)006<0368:iosrrh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIANG J.G., KIANG S.C., JUANG Y.T., TSOKOS G.C. N(omega)-nitro-L-arginine inhibits inducible HSP-70 via Ca(2+), PKC, and PKA in human intestinal epithelial T84 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G415–G423. doi: 10.1152/ajpgi.00138.2001. [DOI] [PubMed] [Google Scholar]
- KIERAN D., KALMAR B., DICK J.R., RIDDOCH-CONTRERAS J., BURNSTOCK G., GREENSMITH L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- KIM D., KIM S.H., LI G.C. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem. Biophys. Res. Commun. 1999a;254:264–268. doi: 10.1006/bbrc.1998.9840. [DOI] [PubMed] [Google Scholar]
- KIM H.R., KANG H.S., KIM H.D. Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life. 1999b;48:429–433. doi: 10.1080/713803536. [DOI] [PubMed] [Google Scholar]
- KIM S.A., YOON J.H., LEE S.H., AHN S.G. Polo-like kinase 1 phosphorylates heat shock transcription factor 1 and mediates its nuclear translocation during heat stress. J. Biol. Chem. 2005;280:12653–12657. doi: 10.1074/jbc.M411908200. [DOI] [PubMed] [Google Scholar]
- KIM W.Y., HORBINSKI C., SIGURDSON W., HIGGINS D. Proteasome inhibitors suppress formation of polyglutamine-induced nuclear inclusions in cultured postmitotic neurons. J. Neurochem. 2004;91:1044–1056. doi: 10.1111/j.1471-4159.2004.02788.x. [DOI] [PubMed] [Google Scholar]
- KOZAWA O., TANABE K., ITO H., MATSUNO H., NIWA M., KATO K., UEMATSU T. Sphingosine 1-phosphate regulates heat shock protein 27 induction by a p38 MAP kinase-dependent mechanism in aortic smooth muscle cells. Exp. Cell Res. 1999;250:376–380. doi: 10.1006/excr.1999.4536. [DOI] [PubMed] [Google Scholar]
- KREUSCH A., HAN S., BRINKER A., ZHOU V., CHOI H.S., HE Y., LESLEY S.A., CALDWELL J., GU X.J. Crystal structures of human HSP90alpha-complexed with dihydroxyphenylpyrazoles. Bioorg. Med. Chem. Lett. 2005;15:1475–1478. doi: 10.1016/j.bmcl.2004.12.087. [DOI] [PubMed] [Google Scholar]
- KUDUK S.D., HARRIS T.C., ZHENG F.F., SEPP-LORENZINO L., OUERFELLI Q., ROSEN N., DANISHEFSKY S.J. Synthesis and evaluation of geldanamycin-testosterone hybrids. Bioorg. Med. Chem. Lett. 2000;10:1303–1306. doi: 10.1016/s0960-894x(00)00208-0. [DOI] [PubMed] [Google Scholar]
- KUDUK S.D., ZHENG F.F., SEPP-LORENZINO L., ROSEN N., DANISHEFSKY S.J. Synthesis and evaluation of geldanamycin-estradiol hybrids. Bioorg. Med. Chem. Lett. 1999;9:1233–1238. doi: 10.1016/s0960-894x(99)00185-7. [DOI] [PubMed] [Google Scholar]
- KUNIMOTO S., MUROFUSHI W., KAI H., ISHIDA Y., UCHIYAMA A., KOBAYASHI T., KOBAYASHI S., MUROFUSHI H., MURAKAMI-MUROFUSHI K. Steryl glucoside is a lipid mediator in stress-responsive signal transduction. Cell Struct. Funct. 2002;27:157–162. doi: 10.1247/csf.27.157. [DOI] [PubMed] [Google Scholar]
- KUNIMOTO S., MUROFUSHI W., YAMATSU I., HASEGAWA Y., SASAKI N., KOBAYASHI S., KOBAYASHI T., MUROFUSHI H., MURAKAMI-MUROFUSHI K. Cholesteryl glucoside-induced protection against gastric ulcer. Cell Struct. Funct. 2003;28:179–186. doi: 10.1247/csf.28.179. [DOI] [PubMed] [Google Scholar]
- KUPATT C., DESSY C., HINKEL R., RAAKE P., DANEAU G., BOUZIN C., BOEKSTEGERS P., FERON O. Heat shock protein 90 transfection reduces ischemia–reperfusion-induced myocardial dysfunction via reciprocal endothelial No synthase serine 1177 phosphorylation and threonine 495 dephosphorylation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1435–1441. doi: 10.1161/01.ATV.0000134300.87476.d1. [DOI] [PubMed] [Google Scholar]
- KURTHY M., MOGYOROSI T., NAGY K., KUKORELLI T., JEDNAKOVITS A., TALOSI L., BIRO K. Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann. N.Y. Acad. Sci. 2002;967:482–489. doi: 10.1111/j.1749-6632.2002.tb04306.x. [DOI] [PubMed] [Google Scholar]
- LATCHMAN D.S. Heat shock proteins and cardiac protection. Cardiovasc. Res. 2001;51:637–646. doi: 10.1016/s0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- LEE Y.S., MARCU M.G., NECKERS L. Quantum chemical calculations and mutational analysis suggest heat shock protein 90 catalyzes trans–cis isomerization of geldanamycin. Chem. Biol. 2004;11:991–998. doi: 10.1016/j.chembiol.2004.05.010. [DOI] [PubMed] [Google Scholar]
- MALONEY A., CLARKE P.A., WORKMAN P. Genes and proteins governing the cellular sensitivity to HSP90 inhibitors: a mechanistic perspective. Curr. Cancer Drug Targets. 2003;3:331–341. doi: 10.2174/1568009033481822. [DOI] [PubMed] [Google Scholar]
- MANJILI M.H., WANG X.Y., MACDONALD I.J., ARNOUK H., YANG G.Y., PRITCHARD M.T., SUBJECK J.R. Cancer immunotherapy and heat-shock proteins: promises and challenges. Expert Opin. Biol. Ther. 2004;4:363–373. doi: 10.1517/14712598.4.3.363. [DOI] [PubMed] [Google Scholar]
- MARCU M.G., CHADLI A., BOUHOUCHE I., CATELLI M., NECKERS L.M. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- MORIMOTO R.I. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- MORIMOTO R.I. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7. [DOI] [PubMed] [Google Scholar]
- MORRIS S.D., CUMMING D.V.E., LATCHMAN D.S., YELLON D.M. Specific iduction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. J. Clin. Invest. 1996;97:706–712. doi: 10.1172/JCI118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULTHOFF G. Activation of natural killer cells by heat shock protein 70. Int. J. Hyperthermia. 2002;18:576–585. doi: 10.1080/0265673021000017109. [DOI] [PubMed] [Google Scholar]
- MULTHOFF G., BOTZLER C., JENNEN L., SCHMIDT J., ELLWART J., ISSELS R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J. Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- MUNSTER P.N., BASSO A., SOLIT D., NORTON L., ROSEN N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. Clin. Cancer Res. 2001;7:2228–2236. [PubMed] [Google Scholar]
- MUNSTER P.N., MARCHION D.C., BASSO A.D., ROSEN N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res. 2002;62:3132–3137. [PubMed] [Google Scholar]
- MURAKOSHI H., IINO R., KOBAYASHI T., FUJIWARA T., OHSHIMA C., YOSHIMURA A., KUSUMI A. Single-molecule imaging analysis of Ras activation in living cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAYAMA S., JONO H., SUZAKI H., SAKAI K., TSURUYA E., YAMATSU I., ISOHAMA Y., MIYATA T., KAI H. Carbenoxolone, a new inducer of heat shock protein 70. Life Sci. 2001;69:2867–2873. doi: 10.1016/s0024-3205(01)01362-5. [DOI] [PubMed] [Google Scholar]
- NANASI P.P., JEDNAKOVITS A. Multilateral in vivo and in vitro protective effects of the novel heat shock protein coinducer, bimoclomol: results of preclinical studies. Cardiovasc. Drug Rev. 2001;19:133–151. doi: 10.1111/j.1527-3466.2001.tb00060.x. [DOI] [PubMed] [Google Scholar]
- NAYEEM M.A., ELLIOTT G.T., SHAH M.R., HASTILLO-HESS S.L., KUKREJA R.C. Monophosphoryl lipid A protects adult rat cardiac myocytes with induction of the 72-kD heat shock protein: a cellular model of pharmacologic preconditioning. J. Mol. Cell Cardiol. 1997;29:2305–2310. doi: 10.1006/jmcc.1997.0452. [DOI] [PubMed] [Google Scholar]
- NECKERS L., NECKERS K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics – an update. Expert Opin. Emerg. Drugs. 2005;10:137–149. doi: 10.1517/14728214.10.1.137. [DOI] [PubMed] [Google Scholar]
- NEGRE-AMINOU P., VAN LEEUWEN R.E., VAN THIEL G.C., VAN DEN I.J., DE JONG W.W., QUINLAN R.A., COHEN L.H. Differential effect of simvastatin on activation of Rac(1) vs activation of the heat shock protein 27-mediated pathway upon oxidative stress, in human smooth muscle cells. Biochem. Pharmacol. 2002;64:1483–1491. doi: 10.1016/s0006-2952(02)01388-6. [DOI] [PubMed] [Google Scholar]
- OKI Y., YOUNES A. Heat shock protein-based cancer vaccines. Expert Rev. Vaccines. 2004;3:403–411. doi: 10.1586/14760584.3.4.403. [DOI] [PubMed] [Google Scholar]
- OOIE T., TAKAHASHI N., SAIKAWA T., NAWATA T., ARIKAWA M., YAMANAKA K., HARA M., SHIMADA T., SAKATA T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- PAWELEC G., BARNETT Y., FORSEY R., FRASCA D., GLOBERSON A., MCLEOD J., CARUSO C., FRANCESCHI C., FULOP T., GUPTA S., MARIANI E., MOCCHEGIANI E., SOLANA R. T cells and aging. Front Biosci. 2002;7:d1056–d1183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- PERDRIZET G.A., KANEKO H., BUCKLEY T.M., FISHMAN M.S., PLEAU M., BOW L., SCHWEIZER R.T. Heat shock and recovery protects renal allografts from warm ischemic injury and enhances HSP72 production. Transplant. Proc. 1993;25:1670–1673. [PubMed] [Google Scholar]
- PRATT W.B., TOFT D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- PRODROMOU C., ROE S.M., O'BRIEN R., LADBURY J.E., PIPER P.W., PEARL L.H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- PUSKAS L.G., NAGY Z.B., GIRICZ Z., ONODY A., CSONKA C., KITAJKA K., HACKLER L., JR, ZVARA A., FERDINANDY P. Cholesterol diet-induced hyperlipidemia influences gene expression pattern of rat hearts: a DNA microarray study. FEBS Lett. 2004;562:99–104. doi: 10.1016/S0014-5793(04)00189-9. [DOI] [PubMed] [Google Scholar]
- RAKONCZAY Z., JR, IVANYI B., VARGA I., BOROS I., JEDNAKOVITS A., NEMETH I., LONOVICS J., TAKACS T. Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats. Free Radic. Biol. Med. 2002;32:1283–1292. doi: 10.1016/s0891-5849(02)00833-x. [DOI] [PubMed] [Google Scholar]
- RANFORD J.C., HENDERSON B. Chaperonins in disease: mechanisms, models, and treatments. Mol. Pathol. 2002;55:209–213. doi: 10.1136/mp.55.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN H., MUSCH M.W., KOJIMA K., BOONE D., MA A., CHANG E.B. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631–639. doi: 10.1053/gast.2001.27028. [DOI] [PubMed] [Google Scholar]
- ROE S.M., PRODROMOU C., O'BRIEN R., LADBURY J.E., PIPER P.W., PEARL L.H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- ROSSI A., ELIA G., SANTORO M.G. 2-Cyclopenten-1-one, a new inducer of heat shock protein 70 with antiviral activity. J. Biol. Chem. 1996;271:32192–32196. doi: 10.1074/jbc.271.50.32192. [DOI] [PubMed] [Google Scholar]
- SAMPLES B.L., POOL G.L., LUMB R.H. Polyunsaturated fatty acids enhance the heat induced stress response in rainbow trout (Oncorhynchus mykiss) leukocytes. Comp Biochem. Physiol. B Biochem. Mol. Biol. 1999;123:389–397. doi: 10.1016/s0305-0491(99)00083-8. [DOI] [PubMed] [Google Scholar]
- SAUSVILLE E.A.Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters. Commentary re: P. Munster et al., Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner Clin. Cancer Res. 200172228–2236., 2155–2158 [PubMed] [Google Scholar]
- SCHULTE T.W., AN W.G., NECKERS L.M. Geldanamycin-induced destabilization of Raf-1 involves the proteasome. Biochem. Biophys. Res. Commun. 1997;239:655–659. doi: 10.1006/bbrc.1997.7527. [DOI] [PubMed] [Google Scholar]
- SCHULTE T.W., NECKERS L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- SHIGAPOVA N., TOROK Z., BALOGH G., GOLOUBINOFF P., VIGH L., HORVATH I. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2005;328:1216–1223. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- SITTLER A., LURZ R., LUEDER G., PRILLER J., LEHRACH H., HAYER-HARTL M.K., HARTL F.U., WANKER E.E. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum. Mol. Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- SNOECKX L.H., CORNELUSSEN R.N., VAN NIEUWENHOVEN F.A., RENEMAN R.S., VAN DER VUSSE G.J. Heat shock proteins and cardiovascular pathophysiology. Physiol. Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461. [DOI] [PubMed] [Google Scholar]
- SOGA S., KOZAWA T., NARUMI H., AKINAGA S., IRIE K., MATSUMOTO K., SHARMA S.V., NAKANO H., MIZUKAMI T., HARA M. Radicicol leads to selective depletion of Raf kinase and disrupts K-Ras-activated aberrant signaling pathway. J. Biol. Chem. 1998;273:822–828. doi: 10.1074/jbc.273.2.822. [DOI] [PubMed] [Google Scholar]
- SOTI C., CSERMELY P. Ageing and molecular chaperones. Exp. Gerontol. 2003;38:1037–1040. doi: 10.1016/s0531-5565(03)00185-2. [DOI] [PubMed] [Google Scholar]
- SOTI C., RACZ A., CSERMELY P. A nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90. N-terminal nucleotide binding unmasks a C-terminal binding pocket. J. Biol. Chem. 2002;277:7066–7075. doi: 10.1074/jbc.M105568200. [DOI] [PubMed] [Google Scholar]
- SOTI C., VERMES A., HAYSTEAD T.A., CSERMELY P. Comparative analysis of the ATP-binding sites of Hsp90 by nucleotide affinity cleavage: a distinct nucleotide specificity of the C-terminal ATP-binding site. Eur. J. Biochem. 2003;270:2421–2428. doi: 10.1046/j.1432-1033.2003.03610.x. [DOI] [PubMed] [Google Scholar]
- SREEDHAR A.S., CSERMELY P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol. Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- SREEDHAR A.S., MIHALY K., PATO B., SCHNAIDER T., STETAK A., KIS-PETIK K., FIDY J., SIMONICS T., MARAZ A., CSERMELY P. Hsp90 inhibition accelerates cell lysis. Anti-Hsp90 ribozyme reveals a complex mechanism of Hsp90 inhibitors involving both superoxide- and Hsp90-dependent events. J. Biol. Chem. 2003;278:35231–35240. doi: 10.1074/jbc.M301371200. [DOI] [PubMed] [Google Scholar]
- SREEDHAR A.S., NARDAI G., CSERMELY P. Enhancement of complement-induced cell lysis: a novel mechanism for the anticancer effects of Hsp90 inhibitors. Immunol. Lett. 2004a;92:157–161. doi: 10.1016/j.imlet.2003.11.025. [DOI] [PubMed] [Google Scholar]
- SREEDHAR A.S., SOTI C., CSERMELY P. Inhibition of Hsp90: a new strategy for inhibiting protein kinases. Biochim. Biophys. Acta. 2004b;1697:233–242. doi: 10.1016/j.bbapap.2003.11.027. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA P. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- STEBBINS C.E., RUSSO A.A., SCHNEIDER C., ROSEN N., HARTL F.U., PAVLETICH N.P. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- SUN L., CHANG J., KIRCHHOFF S.R., KNOWLTON A.A. Activation of HSF and selective increase in heat-shock proteins by acute dexamethasone treatment. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1091–H1097. doi: 10.1152/ajpheart.2000.278.4.H1091. [DOI] [PubMed] [Google Scholar]
- SUPKO J.G., HICKMAN R.L., GREVER M.R., MALSPEIS L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother. Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- THIRUMALAI D., LORIMER G.H. Chaperonin-mediated protein folding. Annu. Rev. Biophys. Biomol. Struct. 2001;30:245–269. doi: 10.1146/annurev.biophys.30.1.245. [DOI] [PubMed] [Google Scholar]
- TONG P., THOMAS T., BERRISH T., HUMPHRISS D., BARRIOCANAL L., STEWART M., WALKER M., WILKINSON R., ALBERTI K.G. Cell membrane dynamics and insulin resistance in non-insulin-dependent diabetes mellitus. Lancet. 1995;345:357–358. doi: 10.1016/s0140-6736(95)90343-7. [DOI] [PubMed] [Google Scholar]
- TOROK Z., GOLOUBINOFF P., HORVATH I., TSVETKOVA N.M., GLATZ A., BALOGH G., VARVASOVSZKI V., LOS D.A., VIERLING E., CROWE J.H., VIGH L. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3098–3103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOROK Z., HORVATH I., GOLOUBINOFF P., KOVACS E., GLATZ A., BALOGH G., VIGH L. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOROK Z., TSVETKOVA N.M., BALOGH G., HORVATH I., NAGY E., PENZES Z., HARGITAI J., BENSAUDE O., CSERMELY P., CROWE J.H., MARESCA B., VIGH L. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3131–3136. doi: 10.1073/pnas.0438003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSVETKOVA N.M., HORVATH I., TOROK Z., WOLKERS W.F., BALOGI Z., SHIGAPOVA N., CROWE L.M., TABLIN F., VIERLING E., CROWE J.H., VIGH L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN KLOMPENBURG W., NILSSON I., VON HEIJNE G., DE KRUIJFF B. Anionic phospholipids are determinants of membrane protein topology. EMBO J. 1997;16:4261–4266. doi: 10.1093/emboj/16.14.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASILEVSKAYA I.A., RAKITINA T.V., O'DWYER P.J. Geldanamycin and its 17-allylamino-17-demethoxy analogue antagonize the action of Cisplatin in human colon adenocarcinoma cells: differential caspase activation as a basis for interaction. Cancer Res. 2003;63:3241–3246. [PubMed] [Google Scholar]
- VIGH L., MARESCA B.Dual role of membranes in heat stress: as thermosensors modulate the expression of stress genes and, by interacting with stress proteins, re-organize their own lipid order and functionality Cell and Molecular Responses to Stress 2002Amsterdam: Elsevier; 173–188.ed. Storey, K.B. & Story, J.M. pp [Google Scholar]
- VIGH L., LITERATI PN., HORVATH I., TÖRÖK Z., BALOGH G., GLATZ A., KOVACS E., BOROS I., FERDINANDY P., FARKAS B., JASZLITS L., JEDNAKOVITS A., KORANYI L., MARESCA B. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat. Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- VIGH L., MARESCA B., HARWOOD J.L. Does the membrane's physical state control the expression of heat shock and other genes. Trends Biochem. Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- VILENCHIK M., SOLIT D., BASSO A., HUEZO H., LUCAS B., HE H., ROSEN N., SPAMPINATO C., MODRICH P., CHIOSIS G. Targeting wide-range oncogenic transformation via PU24FCl, a specific inhibitor of tumor Hsp90. Chem. Biol. 2004;11:787–797. doi: 10.1016/j.chembiol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- WANG X., GRAMMATIKAKIS N., SIGANOU A., CALDERWOOD S.K. Regulation of molecular chaperone gene transcription involves the serine phosphorylation, 14-3-3 epsilon binding, and cytoplasmic sequestration of heat shock factor 1. Mol. Cell. Biol. 2003;23:6013–6026. doi: 10.1128/MCB.23.17.6013-6026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARRICK J.M., CHAN H.Y., GRAY-BOARD CHAI Y., PAULSON H.L., BONINI N.M. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- WHITESELL L., MIMNAUGH E.G., DE COSTA B., MYERS C.E., NECKERS L.M. Inhibition of heat shock protein HSP90–pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICK G., GRUBECK-LOEBENSTEIN B. Immunity and aging. Dev. Comp. Immunol. 1997;21:455–460. doi: 10.1016/s0145-305x(97)00025-6. [DOI] [PubMed] [Google Scholar]
- WORKMAN P. Auditing the pharmacological accounts for Hsp90 molecular chaperone inhibitors: unfolding the relationship between pharmacokinetics and pharmacodynamics. Mol. Cancer Ther. 2003;2:131–138. [PubMed] [Google Scholar]
- WORKMAN P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;206:149–157. doi: 10.1016/j.canlet.2003.08.032. [DOI] [PubMed] [Google Scholar]
- XU Q., SCHETT G., LI C., HU Y., WICK G. Mechanical stress-induced heat shock protein 70 expression in vascular smooth muscle cells is regulated by Rac and Ras small G proteins but not mitogen-activated protein kinases. Circ. Res. 2000;86:1122–1128. doi: 10.1161/01.res.86.11.1122. [DOI] [PubMed] [Google Scholar]
- YAN D., SAITO K., OHMI Y., FUJIE N., OHTSUKA K. Paeoniflorin, a novel heat shock protein-inducing compound. Cell Stress Chaperones. 2004;9:378–389. doi: 10.1379/CSC-51R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Z.Q., GENG X., SOLIT D., PRATILAS C.A., ROSEN N., DANISHEFSKY S.J. New efficient synthesis of resorcinylic macrolides via ynolides: establishment of cycloproparadicicol as synthetically feasible preclinical anticancer agent based on Hsp90 as the target. J. Am. Chem. Soc. 2004;126:7881–7889. doi: 10.1021/ja0484348. [DOI] [PubMed] [Google Scholar]
- YASUDA H., SHICHINOHE H., KURODA S., ISHIKAWA T., IWASAKI Y. Neuroprotective effect of a heat shock protein inducer, geranylgeranylacetone in permanent focal cerebral ischemia. Brain Res. 2005;1032:176–182. doi: 10.1016/j.brainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- YENARI M.A., FINK S.L., SUN G.H., CHANG L.K., PATEL M.K., KUNIS D.M., ONLEY D., HO D.Y., SAPOLSKY R.M., STEINBERG G.K. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann. Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- YU X., GUO Z.S., MARCU M.G., NECKERS L., NGUYEN D.M., CHEN G.A., SCHRUMP D.S. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J. Natl. Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- ZHAO R., DAVEY M., HSU Y.C., KAPLANEK P., TONG A., PARSONS A.B., KROGAN N., CAGNEY G., MAI D., GREENBLATT J., BOONE C., EMILI A., HOURY W.A. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]