Abstract
Platelet secretion is critical to hemostasis. Release of granular cargo is mediated by soluble NSF attachment protein receptors (SNAREs), but despite consensus on t-SNAREs usage, it is unclear which Vesicle Associated Membrane Protein (VAMPs: synaptobrevin/VAMP-2, cellubrevin/VAMP-3, TI-VAMP/VAMP-7, and endobrevin/VAMP-8) is required. We demonstrate that VAMP-8 is required for release from dense core granules, alpha granules, and lysosomes. Platelets from VAMP-8−/− mice have a significant defect in agonist-induced secretion, though signaling, morphology, and cargo levels appear normal. In contrast, VAMP-2+/−, VAMP-3−/−, and VAMP-2+/−/VAMP-3−/− platelets showed no defect. Consistently, tetanus toxin had no effect on secretion from permeabilized mouse VAMP-3−/− platelets or human platelets, despite cleavage of VAMP-2 and/or -3. Tetanus toxin does block the residual release from permeabilized VAMP-8−/− platelets, suggesting a secondary role for VAMP-2 and/or -3. These data imply a ranked redundancy of v-SNARE usage in platelets and suggest that VAMP-8−/− mice will be a useful in vivo model to study platelet exocytosis in hemostasis and vascular inflammation.
INTRODUCTION
The primary function of platelets is to maintain vascular homeostasis. On vascular damage, platelets carry out a series of processes critical for proper hemostasis, one of which is the release of granule contents to promote clot formation (reviewed in Reed et al., 2000; Furie et al., 2001; Reed, 2002, 2004; Flaumenhaft, 2003). Platelets have three types of granules: dense core, alpha, and lysosomes. The dense core granules contain small molecules (e.g., ADP, ATP, serotonin [5-HT], calcium, and pyrophosphate) that are important for activation of other circulating platelets. Alpha granules contain polypeptides (e.g., P-selectin, fibrinogen, and von Willebrand factor) that are key to platelet adhesion, aggregation, and clot formation. Alpha granules are also the source for many of the cytokines (e.g., platelet factor IV [PF4]) released by activated platelets (Coppinger et al., 2004). Like other hematopoetic cells, platelets can release lysosomal enzymes (e.g., β-hexosaminidase and cathepsin D) that could be important for clot remodeling (Greenberg-Sepersky and Simons, 1985; Ciferri et al., 2000). Platelets also have an elaborate, invaginated membrane system called the open canalicular system (OCS), which serves as a conduit between the granules and the extra-platelet environment and is thought to be a reservoir of plasma membrane for platelet spreading (White and Escolar, 1991).
Platelet exocytosis involves both direct fusion between the granule membrane and the OCS/plasma membrane and compound fusion between granules (discussed in Flaumenhaft, 2003). This regulated exocytosis is mediated by a family of integral membrane proteins called soluble NSF attachment protein receptors (SNAREs) present on the vesicles or granules (v-SNAREs) or on the target membrane (t-SNAREs; reviewed in Ungar and Hughson, 2003). The v-SNAREs and the t-SNAREs form a heteromeric complex that spans the two bilayers and mediates membrane fusion and thus granule cargo release (Weber et al., 1998). Human platelets have the t-SNAREs syntaxin-2, -4, -7, and, -11 (Lemons et al., 1997; Chen et al., 2000a, 2000b; McRedmond et al., 2004) as well as SNAP-23 (Flaumenhaft et al., 1999), SNAP-25, and SNAP-29 (Polgar et al., 2003a). Flaumenhaft et al. (1999) demonstrated that syntaxin-4 and SNAP-23 are important for α-granule release. Subsequent studies showed that SNAP-23 is involved in all three release events, as is syntaxin-2 (Lemons et al., 1997; Chen et al., 2000a, 2000b). Syntaxin-4 is also involved in lysosome release (Chen et al., 2000b). The assignments for SNAP-23 were based on the use of inhibitory antibodies (and Fab fragments) and peptides in permeabilized cells. The role of syntaxin 4 was assigned using an inhibitory, isoform-specific mAb. Syntaxin-2's role was assessed using an inhibitory, isoform-specific polyclonal antibody.
Platelets contain several v-SNAREs: synaptobrevin/VAMP-2, cellubrevin/VAMP-3, endobrevin/VAMP-8 (Bernstein and Whiteheart, 1999; Flaumenhaft et al., 1999; Polgar et al., 2002; Schraw et al., 2003), and TI-VAMP/VAMP-7 reported here; however, their roles have been controversial. VAMP-3, first identified in human platelets by Bernstein and Whiteheart (1999), is highly homologous to VAMP-1 and -2 and is thus a member of the “brevin” family of v-SNAREs. Flaumenhaft et al. (1999) first reported a role for VAMP-3 in alpha granule secretion by showing that the tetanus toxin endopeptidase inhibited P-selectin exposure. This toxin cleaves VAMP-1, -2, and -3 (Schiavo et al., 2000), and because neither VAMP-1 nor -2 had been detected in platelets at that time, it was concluded that VAMP-3 was required for alpha granule release. Subsequent studies using an anti-VAMP-3 peptide antibody were consistent with a role for VAMP-3 (Feng et al., 2002). Using the cytoplasmic domains of VAMP-2, -3, and -8 as inhibitors, Polgar et al. (2002) reported that VAMP-3 was important for both alpha granule and dense core granule secretion. Though consistent as a whole, these experiments individually were not without caveats due to the specificity of tetanus toxin for VAMP-1, -2, and -3, the potential for steric hindrance by the antibodies, and the promiscuity of the v-SNARE cytoplasmic domains (Fasshauer et al., 1999; Yang et al., 1999; Brandhorst et al., 2006). Studies with transgenic mice showed that secretion from mouse platelets lacking VAMP-3 was normal (Schraw et al., 2003), if not enhanced (Polgar et al., 2003), suggesting that VAMP-3 is not absolutely required. During the analysis of VAMP-3−/− platelets, VAMP-2 was detected in both human and mouse platelets, further confusing the interpretation of previous data. VAMP-2 is the central v-SNARE for neurotransmitter release and insulin secretion from pancreatic beta cells (Randhawa et al., 2000; Schoch et al., 2001); therefore, it could be the v-SNARE required for platelet secretion. Alternatively, the other two v-SNAREs, VAMP-7 and/or -8 could be important for platelet granule release. The goal of the work presented here is to address these questions.
Tetanus toxin insensitive VAMP(TI-VAMP)/VAMP-7's involvement in the exocytosis was first identified in apical transport in epithelial cells and neurite growth (Galli et al., 1998; Martinez-Arca et al., 2000). It is also proposed that VAMP-7 is involved in late endosome-to-lysosome transport and heterotypic fusion (Advani et al., 1999). The N-terminal extension of VAMP-7, called the Longin domain, is not present in VAMP-1, -2, -3, or -8 (Rossi et al., 2004). This Longin domain has been implicated in membrane trafficking and in the regulation of SNARE complex formation (Martinez-Arca et al., 2003).
Endobrevin/VAMP-8 was originally identified as an endosomal v-SNARE involved in fusion between early and late endosomes (Wong et al., 1998; Antonin et al., 2000a). It can interact with syntaxin-7, syntaxin-8, and Vti1b to form an endosomal fusion complex (Antonin et al., 2000b). VAMP-8's role in mast cell degranulation is implied by its binding to syntaxin-4 and SNAP-23 (Paumet et al., 2000). VAMP-8 also couples with syntaxin-2 and is involved in the terminal step of cytokinesis in mammalian cells (Low et al., 2003). A major physiological role of VAMP-8 was revealed by the analysis of VAMP-8−/− mice (Wang et al., 2004). Secretion of zymogen granules from VAMP-8−/− pancreatic acinar cells was reduced when compared with wild type (WT). A role for VAMP-8 in platelet dense core granule secretion was suggested because inclusion of its cytoplasmic domain inhibited release from permeabilized platelets (Polgar et al., 2002). However, because of the potential for interactions with syntaxins and SNAP-23, this could be more an indicator of which t-SNAREs are required.
In an attempt to resolve the ambiguities surrounding the role of VAMPs in platelet exocytosis, we used four transgenic knockout mouse strains combined with tetanus toxin light chain (TeNT LC) treatment to determine which VAMPs are required for exocytosis of platelet granules. Here we analyze platelets from VAMP-3−/−, VAMP-2+/−, and VAMP-2+/−/VAMP-3−/− mice and demonstrate that VAMP-2 and -3 are not essential for platelet secretion. Analysis of platelets from VAMP-8−/− mice indicates that this v-SNARE is the primary SNARE involved in platelet dense core granule, alpha granule, and lysosome secretion. Further experiments show that a TeNT LC–sensitive VAMP(s) can play a secondary, less-efficient role in the absence of VAMP-8. This ranked redundancy appears to be a general theme because it is also seen in chromaffin granule release (Borisovska et al., 2005). Based on our studies, the VAMP-8−/− mouse strain will provide a useful model to study the role of secretion in platelet functions in vivo.
MATERIALS AND METHODS
Antibodies and Reagents
The anti-synaptobrevin/VAMP-2 mAb (clone Cl69.1) and the anti-endobrevin/VAMP-8 polyclonal antibody were from Synaptic Systems (Gottingen, Germany). The anti-cellubrevin/VAMP-3 polyclonal antibody was described (Schraw et al., 2003). The anti-TI-VAMP/VAMP-7 mAb (clone TG158.2) was from Dr. Thierry Galli (INSERM, Paris, France). Polyclonal anti-syntaxin-2, -syntaxin-4, and -SNAP-23 antibodies were generated as described (Schraw et al., 2003). A monoclonal anti-mouse fibrinogen antibody was from Innovative Research (Southfield, MI). A polyclonal anti-β-actin was from Sigma (St. Louis, MO). The monoclonal anti-phospho-tyrosine antibody (clone 4G10) was from Upstate (Lake Placid, NY). FITC-conjugated anti-human P-selectin (CD62P, AK-4), and anti-mouse P-selectin antibodies were from BD PharMingen (San Jose, CA). Appropriate anti-immunoglobulin secondary antibodies coupled to alkaline phosphatase were from Sigma.
The pQE3-TeNT LC expression plasmid (from Dr. Phyllis Hanson, Washington University, St. Louis, MO, and originally generated by Dr. Heiner Niemann) was used to express His6-tagged TeNT LC in Escherichia coli. The recombinant protein was purified, dialyzed against phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, pH 7.4, PBS) with 1 mM DTT, 5% glycerol, and assayed using GST-VAMP-2 as substrate.
The synaptobrevin/VAMP-2+/− mice, on a C57Bl/6J background (Schoch et al., 2001), were from Dr. Thomas Südhof (University of Texas Southwestern, TX). The cellubrevin/VAMP-3−/− mice, on a mixed C57Bl/6J-129Sv background (Yang et al., 2001), were from Dr. Jeffery Pessin (State University of New York, Stonybrook, NY). VAMP-2+/−/VAMP-3−/− mice were generated by a cross of VAMP-2+/− and VAMP-3−/− mice. The endobrevin/VAMP-8−/− mice, on a mixed C57Bl/6J-129Sv background, were as described (Wang et al., 2004). All animal work was approved by the University of Kentucky IACUC. To lessen the effects of the mixed genetic backgrounds, littermate and age-matched controls were used for all experiments. Platelets were pooled from a number of individuals (4–10) with the same VAMP genotype to further dilute the effects of differences in genetic background.
Genotyping
The genotype of each mouse was determined by PCR using DNA from the tail tip. PCR analysis was carried out as outlined in Schoch et al. (2001) for VAMP-2 and in Wang et al. (2004) for VAMP-8. PCR analysis for VAMP-3 was carried out with the forward primers 3F (5′-cacaggcactctgttgcatt-3′), or Neo-NTR (5′-gagcagccgattgtctgttg-3′), and the reverse primer 6R (5′-ccacacaggctcctgatctt-3′).
Blood Collection
Mice were euthanatized by CO2 inhalation. Blood was collected from the right ventricle and was mixed with sodium citrate to a final concentration 0.38%. The citrated blood was mixed with an equal volume of PBS, pH 7.4. Platelet-rich plasma (PRP) was prepared by centrifugation at 250 × g for 10 min. After adding 10 ng/ml prostaglandin I2 (Sigma) for 5 min, the PRP was centrifuged at 500 × g for 15 min, and the platelet pellet was gently resuspended in HEPES/Tyrode's buffer (10 mM HEPES/NaOH, pH 7.4, 5.56 mM glucose, 137 mM NaCl, 12 mM NaHCO3, 2.7 mM KCl, 0.36 mM NaH2PO4, 1 mM MgCl2) in the presence of 3 μg/ml apyrase (Sigma) and 1 mM EGTA.
Measurement of Secretion from Intact Platelet
Washed platelets were labeled with 0.4 μCi/ml [3H]5-HT (Perkin-Elmer Cetus Life Sciences, Boston, MA) for 1 h at 37°C. After washing with HEPES/Tyrode's buffer (pH 7.4) in the presence of 3 μg/ml apyrase, the platelets were resuspended with HEPES/Tyrode's buffer (pH 7.4). Platelet concentrations were determined by hemocytometer and adjusted to 2.5 × 108/ml. A final concentration of 0.7 mM CaCl2 was added to the platelet suspension before stimulation.
For titration experiments, the indicated concentrations of thrombin (Chrono-Log, Havertown, PA) were added to stimulate platelets. The reactions were stopped by adding a twofold excess of hirudin (Sigma) and put on ice. For the time-course experiments, 0.05 U/ml thrombin was added for the indicated time periods, and 0.1 U/ml hirudin was added. When all reactions were finished, the samples were centrifuged at 13,800 × g for 1 min. The supernatants were recovered, and the pellets were lysed with an equal volume of lysis buffer (PBS, pH 7.4, 1% Triton X-100) for 1 h on ice. Equal volumes of the supernatant and the pellet were assayed for the three granule cargo markers: [3H]5-HT for dense core granules (DC), platelet factor IV (PF4) for alpha granules, and β-hexosaminidase for lysosomes as described previously (Schraw et al., 2003).
Streptolysin-O–permeablized Platelet Secretion Assay
The streptolysin-O (SLO)-permeabilized platelet secretion assay was described previously (Rutledge and Whiteheart, 2004). Banked human platelets (PRP) were obtained from Central Kentucky Blood Center (Lexington, KY). Mouse platelets were prepared as PRP. Twenty milliliters of PRP was labeled with 0.4 μCi/ml [3H]5-HT at 37°C for 45 min. After washing twice with Ca2+-free Tyrode's buffer (154 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5.6 mM d-glucose, 7 mM NaHCO3, 0.6 mM NaH2PO4, 5 mM sodium PIPES, pH 6.5, 5 mM EGTA, 0.35% BSA) in the presence of 3 μg/ml apyrase, the platelets were resuspended in buffer A (120 mM sodium glutamate, 5 mM potassium glutamate, 20 mM HEPES, pH 7.4, 2.5 mM EDTA, 2.5 mM EGTA, 3.15 mM MgCl2, 1 mM DTT). After adjusting the platelet concentration to 5 × 108/ml, 50 μl was mixed with 50 ml of buffer A containing 8 mM ATP, 1.6 U/ml SLO (Corgenix, Peterborough, United Kingdom) and the purified recombinant TeNT LC or equal volumes of PBS with boiled TeNT LC at RT for 10 min. The reaction was further incubated on ice for 30 min. The samples were then warmed to 25°C for 5 min and stimulated with calcium for 5 min. The reactions were stopped by centrifugation at 13,800 × g for 1 min to separate the supernatant from the pellet. The pellet samples were lysed with 100 μl lysis buffer for 1 h on ice. Assays for granule cargo are described above. For each data point, parallel samples were stopped with SDS sample buffer and subjected to Western blotting for the indicated proteins.
Platelet Aggregation and ATP Release
Platelet aggregation was measured using a model 460Vs Lumi-Dual aggregometer. Aggregation and ATP release traces were acquired using a model 810 Aggro/Link computer interface and Aggro/Link software (Chrono-Log). Mouse platelets were prepared as above and recalcified with 0.7 mM CaCl2. WT or VAMP-8−/− platelet suspensions (250 μl; 2.5 × 108/ml) were put into siliconized cuvettes and stirred for 5 min at 37°C at 800 rpm. Luciferase substrate/luciferase mixture (12.5 μl, Chrono-Log) was added, followed by the addition of the indicated agonists: thrombin, collagen (Chrono-Log), and A23187 (Calbiochem, La Jolla, CA).
Flow Cytometry
SLO permeablization of human platelet was described above. Permeablized human platelet suspension, 200 μl, was stimulated with calcium for 5 min and then fixed with 2% formaldehyde/PBS for 30 min at RT. Platelets were washed with PBS and stained with FITC-conjugated, anti-human P-selectin antibody for 30 min followed by further washing with PBS. Flow cytometry was carried out with a Becton and Dickinson FACScalibur (BD Biosciences, San Jose, CA), and data were analyzed using CellQuest/Prosoftware (BD Biosciences). Intact mouse platelets were prepared and stimulated as described above. P-selectin exposure was measured by flow cytometry using an anti-mouse P-selectin antibody.
Intracellular Calcium Measurement in Platelets
Intraplatelet calcium was measured using Fura-2/AM as described in Ohlmann et al. (2004). Mouse platelets (4.0 × 108/ml) were labeled with 12.5 μM Fura-2/AM/0.2% Pluronic F-127 (Invitrogen, Carlsbad, CA) for 45 min at 37°C. After washing, the platelets were resuspended in HEPES/Tyrode's buffer (pH 7.4) without apyrase. After adjusting platelet concentrations to 5.0 × 107/ml and adding 0.7 mM CaCl2, 1-ml samples were added to siliconized cuvettes and stimulated with 0.05 U/ml thrombin with constant stirring. Fluorescence was analyzed by excitation at 340 nm and 380 nm, and emission was measured at 509 nm using a model LS55 Luminescence Spectrometer (Perkin-Elmer Cetus). The ratio of emissions was calculated simultaneously using FL WinLab4.0 software (Perkin-Elmer Cetus) and used to calculate free calcium levels.
Electron Microscopy
Platelets (2.5 × 108/ml) were either held as resting with 10 ng/ml prostaglandin I2 or stimulated with 0.1 U/ml thrombin for 2 min in the presence of 0.7 mM CaCl2. The reaction was stopped with 6% glutaradehyde (Electron Microscopy Sciences, Ft. Washington, PA) in 0.2 M Sorenson's buffer (16.2 mg/ml KH2PO4, 3.76 mg/ml Na2HPO4, pH 8.0). The samples were fixed for 1 h, washed twice, and osmicated with 1% OsO4 for 30 min at RT. After washes in H2O, the platelets were dehydrated in a series of ethyl alcohol solutions. The platelets were incubated with three changes of propylene oxide and infiltrated overnight in a 1:1 mixture of propylene oxide and Epon 12 resin. Samples were embedded in Epon 12 resin at 50°C. Polymerized blocks were sectioned, mounted on copper grids, and stained with lead citrate and uranyl acetate. Samples were examined using a Philips Tecnai 12 transmission electron microscope (FEI, Hillsboro, OR), and images were recovered using Gatan Digitalmicrograph software (Pleasanton, CA).
Western Blotting Analysis
Washed platelets (1.0 × 1010/ml) were solublized with 2× SDS-PAGE sample buffer. The proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA). Immunodecorated proteins were visualized with enhanced chemifluorescent substrate (Amersham Biosciences, Piscataway, NJ). Images were obtained with a Typhoon 9400 phosphoimager and quantified with ImageQuant 5.2 software (Amersham Biosciences).
RESULTS
v-SNAREs Present in Mouse Platelets and Confirmation of Knockout Mouse Strains
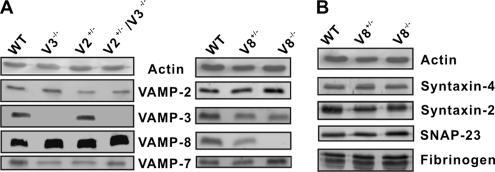
Before this report, three v-SNAREs (VAMP-2, -3, and -8) had been detected in both human and mouse platelets (Bernstein and Whiteheart, 1999; Polgar et al., 2002; Schraw et al., 2003). To dissect the roles of these VAMPs, we gathered or generated transgenic knockout mouse lines lacking one or two of the v-SNAREs: VAMP-2+/− (V2+/−), VAMP-3−/− (V3−/−), VAMP-2+/−/VAMP-3−/− (V2+/−/V3−/−), and VAMP-8−/− (V8−/−; VAMP-2−/− is perinatal lethal; Schoch et al., 2001). Initial Western blotting experiments confirmed the v-SNARE deletion and determined if any compensatory expression occurred. Actin was used as a loading control for this comparison. In Figure 1A, VAMP-2 levels decreased by ∼60% in V2+/− and in V2+/−/V3−/− platelets compared with WT. Similarly, VAMP-3 was undetectable in V3−/− and V2+/−/V3−/− platelets but slightly increased in V2+/− platelets (∼15%). VAMP-8 was increased by ∼30% in V2+/−, V3−/− and in V2+/−/V3−/− mouse strains compared with WT. VAMP-2 was increased by ∼20% in V8−/− platelets, but VAMP-3 was not obviously changed. These results are consistent with previous data (Borisovska et al., 2005) and suggest that there is some reciprocal regulation of v-SNARE expression between VAMP-2, -3, and -8 in the platelet-producing megakaryocytes.
Figure 1.
The secretory machinery in platelets from the VAMP knockout mouse strains. Platelet equivalents (5.0 × 107 per lane) were loaded and analyzed by Western blotting with antibodies to the indicated proteins. (A) The v-SNARE levels in platelets from the different transgenic mouse strains (wild type: WT; VAMP-2+/−: V2+/−; VAMP-3−/−: V3−/−; VAMP-2+/−/VAMP-3−/−: V2+/−/V3−/−; VAMP-8+/−: V8+/−; VAMP-8−/−: V8−/−). (B) Western blots for the indicated t-SNARE proteins and cargo proteins in WT, V8+/−, V8−/− platelets.
In the course of this analysis, we identified a fourth v-SNARE, TI-VAMP/VAMP-7, which was present in both mouse (Figure 1A) and human platelets (data not shown). Contrary to the apparent compensatory expression of VAMP-2, -3, and -8, VAMP-7 levels were not altered in the platelets from the other strains.
Previous studies examined the levels of secretory machinery components in platelets from VAMP-3−/− mice and showed that there were no significant alterations when compared with WT (Schraw et al., 2003). A similar characterization of VAMP-8−/− platelets is shown in Figure 1B. There were no significant differences in syntaxin-2, -4, or SNAP-23 levels when WT platelets were compared with VAMP-8+/− or VAMP-8−/− platelets. These data indicate that the deletion of this v-SNARE does not affect the expression of those t-SNAREs. Cargo levels were normal in the null platelets. Platelets from the VAMP-8−/− mice had normal levels of fibrinogen (Figure 1B), PF4, and β-hexosaminidase (data not shown). [3H]5-HT–labeling experiments showed that VAMP-8−/− platelets incorporated normal levels of serotonin (data not shown). These data show that deletion of VAMP-8 does not adversely affect the levels of the core SNARE proteins nor does it affect the biogenesis of the three platelet granules (see Figure 6 for morphology analysis).
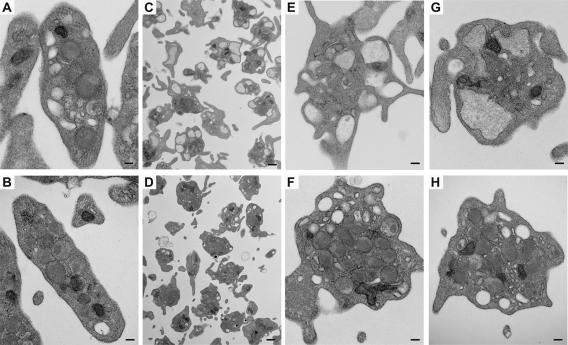
Figure 6.
Ultrastructural analysis of VAMP-8−/− platelets. Platelets were prepared and adjusted to 2.5 × 108/ml. After adding 0.7 mM CaCl2, the samples were either maintained in resting state (A and B) or activated with 0.1 U/ml thrombin for 2 min (C–H). The samples were fixed and analyzed by transmission electron microscopy. (A, C, E, and G) Wild-type platelets; (B, D, F, and H) VAMP-8−/− platelets. Scale bars, 1 μm for C and D, 200 nm for others.
VAMP-2 and -3 Are Not Required for Platelet Secretion
Previous studies supported a role for VAMP-3 and potentially VAMP-8 in platelet exocytosis, specifically alpha and dense core granule release (Flaumenhaft et al., 1999; Feng et al., 2002; Polgar et al., 2002). These results were not consistent with the data, which showed no secretion defect in platelets from VAMP-3−/− mice (Schraw et al., 2003). In fact, there was a slight increase in the rate of release from VAMP-3−/− platelets (Supplementary Figure 1 and Polgar et al., 2003). Given that tetanus toxin can cleave both VAMP-2 and -3, it seemed possible that VAMP-2 could mediate platelet exocytosis, thus potentially explaining the effect of tetanus toxin on alpha granule release (Flaumenhaft et al., 1999). Initial experiments were performed to examine thrombin-induced secretion from VAMP-2+/− and VAMP-2+/−/VAMP-3−/− platelets (Supplementary Figures 2 and 3). In Supplementary Figure 2, there was no apparent difference in secretion from WT and VAMP-2+/− platelets. Neither the extent of release in response to thrombin nor the time course of release (0.05 U/ml thrombin) differed between the two platelet preparations. This result suggests that the deletion of one VAMP-2 allele (∼60% reduction in protein) is insufficient to affect secretion from dense core granules, alpha granules, or lysosomes. VAMP-3 has been reported to play a redundant role for VAMP-2 in large dense core granule secretion from chromaffin cells (Borisovska et al., 2005); therefore, we compared the secretion from VAMP-2+/− and VAMP-2+/−/VAMP-3−/− platelets. In Supplementary Figure 3, there were no significant differences between the thrombin response curves or kinetics of release. Taken together, these data suggest that either the genetic manipulations are not sufficient to cause a defect or that VAMP-2 and -3 are not strictly required for secretion from mouse platelets.
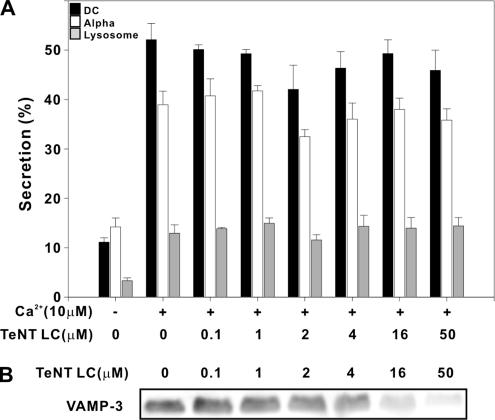
Previous studies showed that the VAMP-1, -2, and -3–specific tetanus toxin endopeptidase inhibits P-selectin exposure on permeabilized human platelets (Flaumenhaft et al., 1999). We took a similar tact using a recombinant version of the catalytic TeNT LC in a permeabilized platelet secretion assay. In Figure 2, increasing concentrations of TeNT LC (0.1–50 μM) led to a significant decrease in VAMP-3 (Figure 2B) but failed to significantly affect calcium (10 μM)-stimulated release (Figure 2A) of soluble cargo from dense core granules (■), alpha granule (□), or lysosomes (▩). A similar analysis of permeabilized, TeNT LC-treated (25 μM) human platelets failed to show an effect on P-selectin exposure, as measured by flow cytometry using FITC-conjugated anti-human P-selectin antibody (Supplementary Figure 4). It should be noted that our experiments used higher levels of TeNT LC (>25-fold more) than the intact tetanus toxin used previously (Flaumenhaft et al., 1999). The increased concentrations required for cleavage of VAMP-3 in our studies may suggest differences in platelet permeabilization or toxin specific activity. Regardless of this difference, we did not detect any significant effects of the TeNT LC on platelet secretion. Given the fact that VAMP-3 is more abundant in human platelets than is VAMP-2, we assume that the cleavage of VAMP-3 is indicative of the effect of the toxin on VAMP-2 (the recombinant toxin does cleave VAMP-2; see Materials and Methods). Unfortunately, this could not be addressed directly because the anti-VAMP-2 antibody does not yield a robust signal when used to detect VAMP-2 in human platelets. These data suggest that though TeNT LC cleaves VAMP-3 (and presumably VAMP-2) in the permeabilized platelets, secretion is unaffected.
Figure 2.
Tetanus toxin light chain does not affect secretion from permeabilized human platelets. SLO-permeablized human platelets and secretion assays were performed as described. Aliquots of permeabilized human platelets (50 μl; 5 × 108/ml) were incubated with increasing concentrations of tetanus toxin light chain. Platelet secretion was stimulated with 10 μM CaCl2. Release of [3H]5-HT from dense core granules (■), of PF4 from alpha granules (□), and of β-hexosaminidase from lysosomes (▩) was measured and percent secretion was calculated. Data points represent the average of triplicate measurements and the SD is indicated (A). A separate set of samples was probed by Western blotting for VAMP-3 (B).
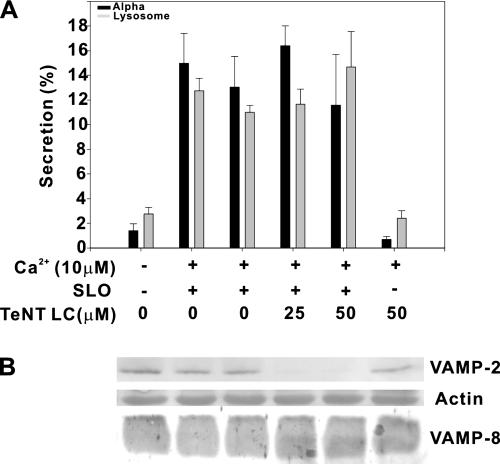
In a perhaps more definitive experiment, permeabilized platelets from VAMP-3−/− mice were treated with TeNT LC (Figure 3 and also see Figure 8). VAMP-2 was completely cleaved in the presence of TeNT LC, whereas VAMP-8, a toxin-insensitive v-SNARE (Wong et al., 1998) was unaffected (Figure 3B). Secretion of PF4 and β-hexosaminidase from the permeabilized mouse platelets was clearly calcium-dependent but was not affected by toxin treatment (Figure 3A). These data demonstrate that TeNT LC does cleave VAMP-2 in situ. Because VAMP-3−/− platelets were used and VAMP-2 was cleaved by TeNT LC, calcium-stimulated release in the permeabilized platelet assay system does not appear to require VAMP-2 or -3.
Figure 3.
Tetanus toxin light chain does not affect secretion from permeabilized mouse platelets lacking cellubrevin/VAMP-3. Mouse platelets were prepared from VAMP-3−/− animals, permeabilized, and treated with tetanus toxin light chain. Measurement of secretion (A) from alpha granules (■) and lysosomes (▩) was as in Figure 2. A separated set of samples was fractionated by SDS-PAGE and transferred to PVDF membrane. The membrane was stained with Ponceau S red for actin and probed by Western blotting for VAMP-2 and -8 (B).
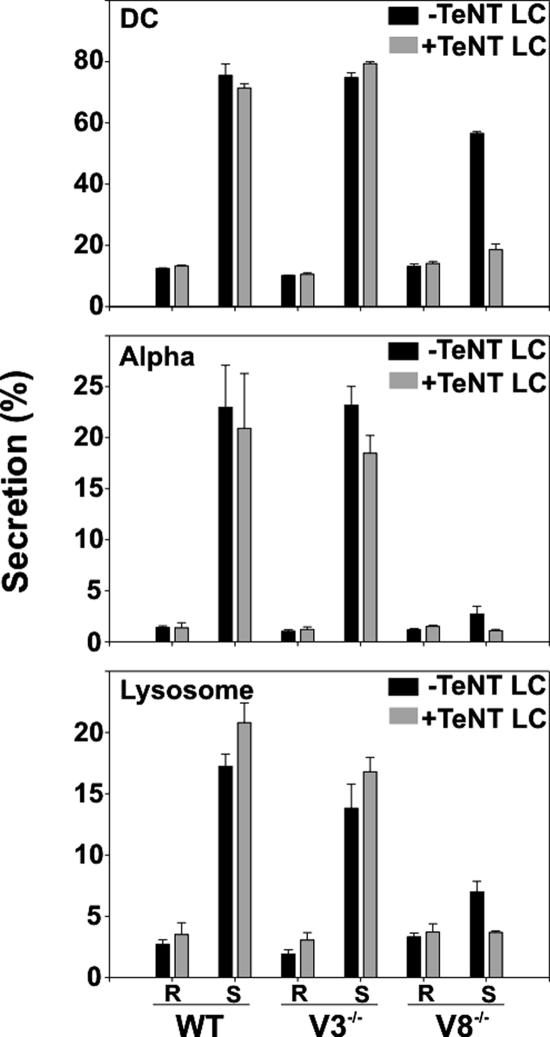
Figure 8.
A tetanus toxin–sensitive v-SNARE(s) is responsible for the residual secretion in the absence of endobrevin/VAMP-8. Wild-type (WT), VAMP-3−/− (V3−/−), and VAMP-8−/− (V8−/−) platelets were prepared and permeabilized. Aliquots (50 μl; 5 × 108/ml) were incubated with 25 μM tetanus toxin light chain (+TeNT LC, ▩) or PBS (−TeNT LC, ■). The samples were then either maintained in resting state (R) or stimulated with 10 μM CaCl2 (S). Release of [3H]5-HT from dense core granules (DC), PF4 from alpha granules (Alpha), and β-hexosaminidase from lysosomes (Lysosome) were measured, and percent secretion was calculated as described. Data represent the mean values of triplicate measurements, and the SD is indicated.
In summary, the above experiments using human platelets and platelets from different mouse strains fail to show a strict requirement for VAMP-2 or -3. Secretion is normal in intact platelets when VAMP-2 is genetically reduced and VAMP-3 is genetically ablated. Secretion from permeabilized cells is unaffected when VAMP-2 is cleaved in platelets lacking VAMP-3. These data strongly argue that these two v-SNAREs are not essential for the platelet release reaction. These data do not however exclude a role for these two v-SNAREs and could be consistent with them playing a supporting role in the platelet release reaction.
Platelet Aggregation and ATP Release are Defective in VAMP-8−/− Platelets
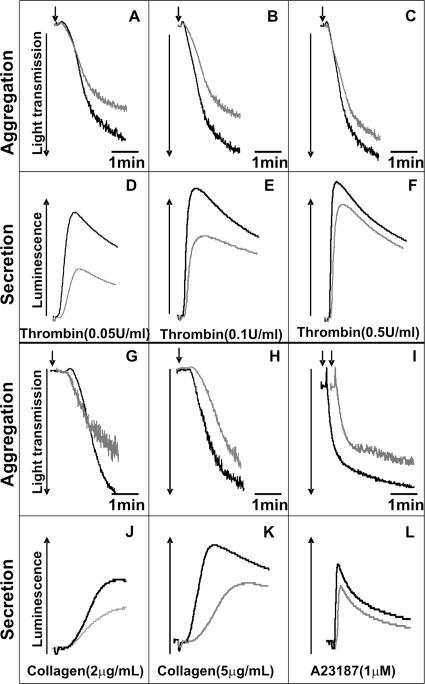
The above data suggest that, contrary to some reports (Flaumenhaft et al., 1999; Feng et al., 2002; Polgar et al., 2002), but in agreement with others (Schraw et al., 2003), VAMP-2 and -3 are not required for platelet secretion. The question remains as to which of the remaining v-SNAREs is required for platelet exocytosis. To address this question we analyzed VAMP-8−/− platelets. Figure 4, A–C, show the aggregation traces for washed mouse platelets (WT, black traces; VAMP-8−/−, gray traces) stimulated with thrombin. At 0.05 and 0.1 U/ml thrombin, there was a significant defect in aggregation seen in the VAMP-8−/− platelets. Consistently, in Figure 4, D and E, there were significant defects in ATP release from dense core granules as measured with luciferin/luciferase. This defect in release was also seen when P-selectin exposure was measured (data not shown), suggesting that both dense core and alpha granule release are defective in VAMP-8−/− platelets. The aggregation and secretion defects were almost overcome when thrombin levels were increased to 0.5 U/ml (Figure 4, C and F). A similar defect in aggregation and ATP release was observed when collagen was used as agonist (Figure 4, G, H, J, and K). As with thrombin, the defect is lessened by increasing agonist concentrations but not completely overcome. The secretion defect was less obvious when A23187 was used as agonist (Figure 4, I and L), which is consistent with the robust stimulatory effect of the ionophore. With all three stimulators, higher levels of activation can at least partially overcome the VAMP-8−/− defect in secretion, suggesting that the loss of VAMP-8 can be compensated, albeit inefficiently.
Figure 4.
Aggregation and secretion of ATP is defective in endobrevin/VAMP-8−/− platelets. Wild-type (WT, black traces) and VAMP-8 null (V8−/−, gray traces) mouse platelets were prepared and after adding 0.7 mM CaCl2, and aliquots (2.5 × 108/ml) were stimulated with indicated concentrations of thrombin (A–F), collagen (G, H, J, and K) or A23187 (I and L). Platelet aggregation (A–C and G–I) and ATP release (D–F and J–L) were measured with constant stirring.
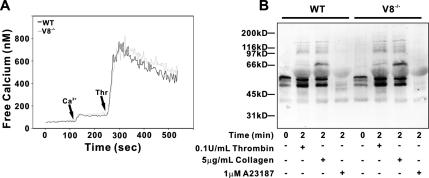
An explanation for the observed secretion defect is that the signaling cascades in the VAMP-8−/− platelets cannot fully initiate secretion. One signaling event, key to platelet secretion, is the agonist-induced increase in intraplatelet calcium. To examine whether this was defective in VAMP-8−/− platelets, WT and null cells were labeled with the calcium probe, Fura-2/AM. Figure 5A shows that the thrombin (0.05 U/ml)-induced increases in intraplatelet calcium in WT and VAMP-8−/− platelets are nearly identical. We additionally examined the degree of total platelet protein tyrosine phosphorylation in response to thrombin, collagen, and A23187 stimulation. As shown in Figure 5B, there were no significant differences in the array or intensities of the phospho-tyrosine–containing proteins present in activated WT versus VAMP-8−/− platelets. These data suggest that the signaling cascades initiated in activated VAMP-8−/− platelets do not grossly differ from that in the WT platelets.
Figure 5.
Calcium mobilization and tyrosine kinase signaling are unaffected in endobrevin/VAMP-8−/− platelets. (A) Wild-type (WT, black traces) and VAMP-8−/− platelets (V8−/−, gray traces) were prepared, and intracellular free calcium levels were measured with Fura-2. The arrows indicate the times at which extracellular calcium and thrombin were added. Each trace represents the average of three. (B) WT or VAMP-8−/− platelets were incubated with the indicated agonist for 2 min at RT. The reactions were stopped and the samples were analyzed by Western blotting using an anti-phosphotyrosine antibody.
Electron Microscopy of Thrombin-stimulated WT and VAMP-8−/− Platelets
It is possible that the defect is due to a failure of the cytoskeletal rearrangements that occur during platelet activation and are important for secretion (Flaumenhaft et al., 2005). If actin cytoskeletal changes do occur normally, one would predict that VAMP-8−/− platelets would form filopodia and centralize their granules. However, they would not fuse their granules with the plasma membrane, and thus granules would still be visible. The expected morphological phenotype would be similar to what Lemons et al. (2000) observed when they inhibited platelet secretion with an anti-SNAP-23 antibody. Resting and thrombin-stimulated platelets from WT and VAMP-8−/− animals were examined by transmission electron microscopy. As shown in Figure 6, A and B, resting WT and VAMP-8−/− platelets have normal discoid shapes with similar arrays of granules, mitochondria, microtubular networks, and OCS. Resting WT and VAMP-8−/− platelets contained similar numbers of granules (WT: 6.8 ± 2.9 granules/platelet, n = 52; VAMP-8−/−: 6.9 ± 2.4 granules/platelet, n = 62). When stimulated with thrombin (0.1 U/ml; 2 min), WT platelets showed a more irregular appearance with protruding filopodia and a generalized lack of granules (Figure 6, C, E, and F). This appearance is consistent with thrombin-induced cytoskeletal rearrangements and secretion of granular contents. Thrombin-stimulated VAMP-8−/− platelets showed a distinctively different morphology. Most of the VAMP-8−/− platelets (Figure 6, D, G, and H) had filopodia, suggesting agonist-induced cytoskeletal rearrangements. Under these conditions, 29% (48/166) of the WT platelets and 74% (198/266) of the VAMP-8−/− platelets contained identifiable granules. The granules were pushed to the middle of the cells, but they were still present suggesting that their fusion with the plasma membrane was defective. This is consistent with a lack of secretion seen previously (Lemons et al., 2000) and also indicates that the thrombin-initiated signaling required for cytoskeletal rearrangements is still intact in the VAMP-8−/− platelets.
Thrombin-induced Platelet Secretion Is Defective in VAMP-8−/− Platelets
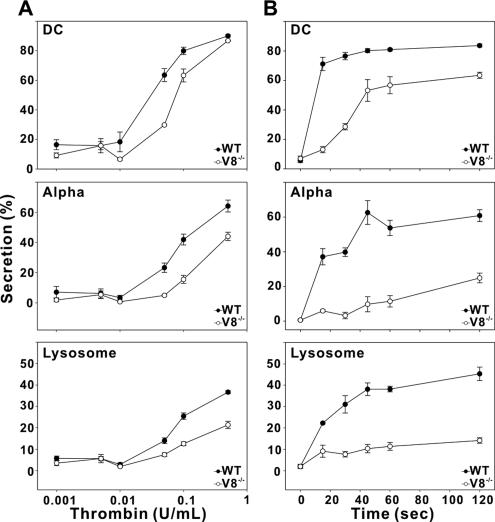
To confirm the secretion defect in VAMP-8−/− platelets, we examined release from all three granules in agonist-titration and time-course experiments. Thrombin was chosen as the agonist because hirudin, a potent inhibitor, can be used to stop the reactions rapidly, thus improving the resolution of time-course studies. In Figure 7B, the time course of serotonin secretion from dense core granules was clearly impaired in the VAMP-8−/− platelets when 0.05 U/ml thrombin was used. In WT platelets, dense core granule secretion was relatively fast, arriving at its plateau by 15 s. The final percent release was ∼80%. However, dense core granule secretion from VAMP-8−/− platelets was much slower and did not reach its plateau until 45 s. The final percent release was also less (∼60%). The alpha granule and lysosome release were more affected in the VAMP-8−/− platelets. Alpha granule secretion from WT platelets arrived at its plateau in 1–2 min (60% of total PF4 released from alpha granule), whereas alpha granule secretion from VAMP-8−/− platelets started slowly and gradually increased to only 20% by 2 min. This result suggests that platelet alpha granule secretion is inhibited by more than 70% in VAMP-8−/− platelets. Similar results were observed for lysosome secretion. Thrombin-induced lysosome secretion in VAMP-8−/− platelets was inhibited by ∼70% compared with the WT platelet (40% for WT vs. 10% for VAMP-8−/− platelet at 2-min secretion). These data show that VAMP-8−/− platelets have a profound defect in the kinetics of release from all three granule stores. In Figure 7A, a thrombin titration experiment was performed using 1 min as the incubation time. Secretion from each of the three granules showed the expected dose-dependence. Interestingly, the defect in secretion from VAMP-8−/− platelets was less obvious as the concentration of thrombin increased. This was especially true for release from dense core granules, which reached almost WT levels at the highest stimulation levels. This shows that although VAMP-8−/− platelets are defective in secretion at moderate levels of agonist, the defect can be overridden by increasing stimulation. This result is consistent with the data in Figure 4 and suggests that stimulatory signal strength may affect the core mechanisms of the membrane fusion process leading to exocytosis.
Figure 7.
Thrombin-induced secretion is defective in endobrevin/VAMP-8−/− platelets. Platelets from wild-type (WT, •) and VAMP-8−/− (V8−/−, ○) mice were prepared as described. After adding 0.7 mM CaCl2, aliquots (50 μl; 2.5 × 108/ml) were incubated with the indicated concentration of thrombin at RT for 1 min (A) or 0.05 U/ml thrombin for the indicated time points (B). Reactions were stopped with hirudin. Release of [3H]5-HT from dense core granules (DC), PF4 from alpha granules (Alpha), and β-hexosaminidase from lysosomes (Lysosome) were measured, and percent secretion was calculated. Each data point represents the mean values of triplicate samples, and the SD is indicated.
Despite using littermate controls and platelets pooled from different animals of the same VAMP genotype (generally 4–10), the knockout transgenic mouse strains used are on a mixed background of C57Bl/6J and 129Sv; therefore, it is possible that variations in genetic background could account for the secretion defects in VAMP-8−/− platelets. To establish the statistical significance of the secretion defect, data from four different VAMP-8+/+ strains (i.e., VAMP-3−/−, VAMP-2+/−, VAMP-2+/−/VAMP-3−/−, and WT; 21–26 measurements) were pooled, averaged, and compared with that from the VAMP-8−/− strain (3–6 measurements). Analysis of the secretion time course is shown in Supplementary Figure 5. Two features are apparent from the data: First, the VAMP-8+/+ strains show a remarkable consistency and reproducibility in their response with a small SEM for each time point. Second, the statistical significance between VAMP-8+/+ and VAMP-8−/− was generally p < 0.0001 for each time point as determined by Student's t test. These data show that any variation in strain background due to the C57Bl/6J-129Sv mixture is less significant that the deletion of the VAMP-8 gene.
In summary, VAMP-8 appears to be required for secretion from all three granular stores. Such a requirement is not apparent for VAMP-2 and/or -3 when VAMP-8 is present. However, because the secretion defect in VAMP-8−/− platelets is not complete under all conditions, one might concluded that in the absence of VAMP-8, another VAMP(s) can promote cargo release in a less efficient manner, i.e., slower and requiring more stimulation.
Hierarchy of VAMP Usage in Platelet Exocytosis
Given that the defect in platelet exocytosis was significant but not complete, we conclude that VAMP-8 is the primary v-SNARE for rapid secretion and that another VAMP(s) (e.g., VAMP-2 and/or -3) could fill the void, albeit with lower efficacy. Attempts to address this point with knockout transgenic mice proved difficult because the VAMP-3−/−/VAMP-8−/− genotype appears to be embryonic lethal (Barber and Whiteheart, unpublished results). Given this, we used permeabilized VAMP-8−/− mouse platelets and treated them with TeNT LC. In Figure 8, release from permeabilized WT mouse platelets was unaffected by TeNT LC treatment. Consistent with previous data, there was no significant difference in secretion from any of the three granular stores when VAMP-2 and -3 were cleaved by the toxin. There was also no significant, TeNT LC-induced decrease in release from permeabilized VAMP-3−/− platelets, again, consistent with previous experiments. Calcium-induced secretion from permeabilized VAMP-8−/− platelets was significantly lower than secretion from WT or VAMP-3−/− cells. This result demonstrates that VAMP-8 is required for secretion from all three granules and that the secretion defect seen in intact cells (Figures 4 and 7) is not caused by an insufficient release of ADP (dense core granule cargo), which is an important secondary agonist. Interestingly, the residual release seen from permeabilized VAMP-8−/− platelets was significantly reduced by TeNT LC treatment. These data strongly suggest that VAMP-8 is the dominant v-SNARE required for platelet secretion and further indicate that a tetanus toxin-sensitive VAMP (either VAMP-2 or -3) can inefficiently promote fusion in its absence. This ranked redundancy of v-SNARE usage is similar to the relationship between VAMP-2 and VAMP-3 in chromaffin granule release (Borisovska et al., 2005).
DISCUSSION
In this article, we attempt to clarify the identity of the v-SNARE molecules required for platelet exocytosis. By examining platelets lacking certain VAMPs and through the use of a VAMP-specific endopeptidase, we show that VAMP-2 and VAMP-3 are not required for platelet exocytosis under normal circumstances. VAMP-7 also does not appear to play a role because inclusion of its inhibitory Longin domain (Prombona et al., 1995; Filippini et al., 2001; Martinez-Arca et al., 2003) or antibodies to the Longin domain do not affect secretion from permeabilized platelets (up to 70 μM of Longin domain and 1 mg/ml antibody; Ren and Whiteheart, unpublished results). The phenotype of the VAMP-8−/− platelets, however, suggests that this v-SNARE is required for platelet exocytosis from dense core, alpha granules, and lysosomes. However, this does not identify the specific fusion step(s) mediated by VAMP-8, because it could be involved in either granule-plasma membrane fusion, granule–granule (compound) fusion, or some trafficking step(s) during platelet biogenesis. It should be noted that of the four VAMP-deletion strains tested only VAMP-8−/− mice had a statistically significant platelet secretion defect. Based on the analysis of calcium and tyrosine kinase signaling, it does not appear that the defect in VAMP-8−/− platelets is due to aberrant activation. Morphological analysis supports this contention because stimulated VAMP-8−/− platelets showed activation-dependent cytoskeleton rearrangements but reduced membrane fusion. Though significant, the defect in secretion from VAMP-8−/− platelets is not absolute and some residual release is seen; especially when more robust activation conditions are used. This residual secretion is sensitive to tetanus toxin treatment, suggesting a supplementary role for VAMP-2 and/or -3. This ranked redundancy of v-SNARE usage has been seen in other systems. In summary, the data in this article do support the conclusion that VAMP-8 is the primary v-SNARE required for all three release events in an activated platelet.
Previous Analysis of Platelet v-SNAREs
Past analysis of the platelet v-SNAREs has been equivocal for several reasons. Initial experiments by Flaumenhaft et al. (1999) showed that alpha granule release was sensitive to a commercial preparation of whole tetanus toxin (both heavy and light chain). It is unclear why our results differ from these experiments; perhaps it is due to a difference in toxin preparations. However, taken at face value, the data from Flaumenhaft et al. (1999) would be consistent with a role for either VAMP-2 or -3. Feng et al. (2002) showed that an anti-peptide antibody directed to the N-terminus of VAMP-3 could inhibit α-granule release. Unfortunately, these studies were not done with Fab fragments to eliminate nonspecific steric effects; therefore, interpretation of the inhibitory effect is limited. At best, these data show that VAMP-3 is “close” to the required secretory machinery. In separate studies, Polgar et al. (2002) showed that the cytoplasmic domains of VAMP-3 and -8 could inhibit release from dense core and alpha granules. Because the cytoplasmic domains of several SNAREs are promiscuous in their associations in vitro (Fasshauer et al., 1999; Yang et al., 1999; Brandhorst et al., 2006), it is difficult to determine if these inhibitory effects are illustrative of a v-SNARE's role or of the t-SNAREs heterodimer's binding preferences. Therefore, the data of Polgar et al. (2002) could be more indicative of the t-SNAREs involved. However, based on all these data, it was generally thought that VAMP-3 was important for platelet secretion. The data of Schraw et al. (2003) (also Supplementary Figure 1 and Polgar et al., 2003b) upset that conclusion by demonstrating that platelets lacking VAMP-3 had no defect in exocytosis. On the basis of the data presented in this article, we contend that the previous studies are equivocal because alternative interpretations of their meaning (presented above) were more accurate. The commercial preparation of whole tetanus toxin was perhaps not specific in its effects; the anti-peptide antibody sterically blocked release; and the cytoplasmic v-SNARE domains were promiscuously interacting. Our data clearly show that only VAMP-8, and not VAMP-2 or -3, is required for rapid and optimal platelet exocytosis.
Ranked Redundancy of v-SNARE Usage as a Common Theme
Our data show that VAMP-8 is the primary v-SNARE required for platelet exocytosis, and of the mouse strains tested, only VAMP-8−/− mice have a platelet secretion defect. This use of a primary v-SNARE is similar to what was shown in chromaffin cells (Borisovska et al., 2005). In that case, knockout of the primary v-SNARE (VAMP-2) had a profound effect but did not reduce secretion to zero. Interestingly, the residual release could be accounted for by the action of a secondary v-SNARE (VAMP-3). VAMP-3–mediated release was not as efficient as WT nor was it as extensive, but it did occur. Also interesting was the fact that the deletion of VAMP-3 did not affect the release event when VAMP-2 was present. Therefore, VAMP-3 does not take a primary role but instead can function at reduced efficiencies in the absence of the primary v-SNARE. Combining the chromaffin cell and platelet data, it would seem that VAMP-3 could play the role of “jack-of-all-trades” through its localization to the granules and its ability to pair with a wide range of t-SNAREs heterodimers. This type of redundancy in the secretory machinery may be a common aspect of regulated exocytosis though its true functional implications are not yet clear. We cannot, however, definitively assign VAMP-3 as the secondary v-SNARE for platelet exocytosis; experiments are underway to address this point.
This concept of ranked redundancy is consistent with previous reports that certain SNARE complexes are more fusogenic than others. Rothman and colleagues have shown that only certain combinations of SNARE mediate proteoliposome fusion in vitro (Parlati et al., 2002; Varlamov et al., 2004). This would suggest that some combinations are optimal for mediating secretion and thus are primarily used. Other combinations may be possible in the absence of the primary SNAREs, but these are expected to be less efficient in mediating secretion. Whether our findings in platelets and those in chromaffin cells are a true biological redundancy or just an oddity of experiments using knockout mice remains to be determined.
Utility of the VAMP-8−/− Mice
The importance of platelet secretion has long been recognized. The bleeding diathesis of Hermansky-Pudlak syndrome patients (who lack dense core granules) and Gray platelet syndrome patients (who lack alpha granules) is quite clear (Reed, 2002). It is also increasingly apparent that activated platelets secrete a number of cytokines and growth factors that play a role in inflammatory responses and tissue damage repair processes (Coppinger et al., 2004). The fact that VAMP-8−/− platelets have a secretion defect will make these animals a useful model system to examine the in vivo roles of platelet exocytosis. As a model, they are unique because the defect is in the secretion machinery and membrane fusion, not granule biogenesis or platelet signaling.
One potential implication of the work presented here is that v-SNARE usage may be tied to the degree of platelet activation. Under low to medial levels of stimulation, VAMP-8−/− platelets have a clear secretion defect. However, with increasing stimulation, a secondary v-SNARE (VAMP-2 and/or -3) can mediate release. This finding might prove useful in designing anti-platelet therapeutics for the treatment of hyper-thrombotic states. One might predict that VAMP-8-directed inhibitors would have significant effects on platelet secretion and subsequent thrombus formation under basal conditions where vessel damage is limited but would not grossly affect platelet function upon a larger injury of a blood vessel when higher concentrations of agonist are present. With the VAMP-8−/− mice, this scenario can be directly tested in vivo.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Thomas Südhof and Dr. Jeffery Pessin for the synaptobrevin/VAMP-2+/− and cellubrevin/VAMP-3−/− mice, respectively. We thank Dr. Theirry Galli for his gifts of reagents. We are indebted to the Central Kentucky Blood Center and to members of the Whiteheart Laboratory, especially Dr. Elena Matveeva and Dr. Todd Schraw, for critical reading of the manuscript. We thank Dr. Bill Dean for assistance in measuring intraplatelet calcium and Dr. Bruce Maley and Ms. Mary Gail Engels for their assistance with the electron microscopy. There is no conflict of interest to declare for any of the authors. This work was supported by National Heart, Lung, and Blood Institute (National Institutes of Health, Bethesda, MD) (to S.W.W.) and by the Ohio Valley Affiliate of the American Heart Association (to G.L.C., Q.R., and W.C.).
Abbreviations used:
- VAMP
vesicle associate membrane protein
- TeNT LC
tetanus toxin light chain
- TI-VAMP
tetanus toxin insensitive VAMP
- WT
wild type
- RT
room temperature (∼25°C)
- PF4
platelet factor IV
- OCS
open canalicular system
- 5-HT
5 hydroxy-tryptophan (serotonin)
- SLO
streptolysin O
- PRP
platelet-rich plasma
- DC
dense core.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0785 on October 25, 2006.
REFERENCES
- Advani R. J., Yang B., Prekeris R., Lee K. C., Klumperman J., Scheller R. H. VAMP-7 mediates vesicular transport from endosomes to lysosomes. J. Cell Biol. 1999;146:765–776. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W., Holroyd C., Fasshauer D., Pabst S., Von Mollard G. F., Jahn R. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 2000a;19:6453–6464. doi: 10.1093/emboj/19.23.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W., Holroyd C., Tikkanen R., Honing S., Jahn R. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol. Biol. Cell. 2000b;11:3289–3298. doi: 10.1091/mbc.11.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A. M., Whiteheart S. W. Identification of a cellubrevin/vesicle associated membrane protein 3 homologue in human platelets. Blood. 1999;93:571–579. [PubMed] [Google Scholar]
- Borisovska M., Zhao Y., Tsytsyura Y., Glyvuk N., Takamori S., Matti U., Rettig J., Sudhof T., Bruns D. v-SNAREs control exocytosis of vesicles from priming to fusion. EMBO J. 2005;24:2114–2126. doi: 10.1038/sj.emboj.7600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst D., Zwilling D., Rizzoli S. O., Lippert U., Lang T., Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc. Natl. Acad. Sci. USA. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Bernstein A. M., Lemons P. P., Whiteheart S. W. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000a;95:921–929. [PubMed] [Google Scholar]
- Chen D., Lemons P. P., Schraw T., Whiteheart S. W. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000b;96:1782–1788. [PubMed] [Google Scholar]
- Ciferri S., Emiliani C., Guglielmini G., Orlacchio A., Nenci G. G., Gresele P. Platelets release their lysosomal content in vivo in humans upon activation. Thromb. Haemost. 2000;83:157–164. [PubMed] [Google Scholar]
- Coppinger J. A., Cagney G., Toomey S., Kislinger T., Belton O., McRedmond J. P., Cahill D. J., Emili A., Fitzgerald D. J., Maguire P. B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- Fasshauer D., Antonin W., Margittai M., Pabst S., Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- Feng D., Crane K., Rozenvayn N., Dvorak A. M., Flaumenhaft R. Subcellular distribution of 3 functional platelet SNARE proteins: human cellubrevin, SNAP-23, and syntaxin 2. Blood. 2002;99:4006–4014. doi: 10.1182/blood.v99.11.4006. [DOI] [PubMed] [Google Scholar]
- Filippini F., Rossi V., Galli T., Budillon A., D'Urso M., D'Esposito M. Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem. Sci. 2001;26:407–409. doi: 10.1016/s0968-0004(01)01861-8. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R. Molecular basis of platelet granule secretion. Arterioscler. Thromb. Vasc. Biol. 2003;23:1152–1160. doi: 10.1161/01.ATV.0000075965.88456.48. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Croce K., Chen E., Furie B., Furie B. C. Proteins of the exocytotic core complex mediate platelet alpha-granule secretion. Roles of vesicle-associated membrane protein, SNAP-23, and syntaxin 4. J. Biol. Chem. 1999;274:2492–2501. doi: 10.1074/jbc.274.4.2492. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Dilks J. R., Rozenvayn N., Monahan-Earley R. A., Feng D., Dvorak A. M. The actin cytoskeleton differentially regulates platelet alpha-granule and dense-granule secretion. Blood. 2005;105:3879–3887. doi: 10.1182/blood-2004-04-1392. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C., Flaumenhaft R. A journey with platelet P-selectin: the molecular basis of granule secretion, signalling and cell adhesion. Thromb. Haemost. 2001;86:214–221. [PubMed] [Google Scholar]
- Galli T., Zahraoui A., Vaidyanathan V. V., Raposo G., Tian J. M., Karin M., Niemann H., Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg-Sepersky S. M., Simons E. R. Release of a fluorescent probe as an indicator of lysosomal granule secretion by thrombin-stimulated human platelets. Anal. Biochem. 1985;147:57–62. doi: 10.1016/0003-2697(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Lemons P. P., Chen D., Bernstein A. M., Bennett M. K., Whiteheart S. W. Regulated secretion in platelets: identification of elements of the platelet exocytosis machinery. Blood. 1997;90:1490–1500. [PubMed] [Google Scholar]
- Lemons P. P., Chen D., Whiteheart S. W. Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochem. Biophys. Res. Commun. 2000;267:875–880. doi: 10.1006/bbrc.1999.2039. [DOI] [PubMed] [Google Scholar]
- Low S. H., Li X., Miura M., Kudo N., Quinones B., Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev. Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S., Alberts P., Zahraoui A., Louvard D., Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 2000;149:889–900. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S., et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl. Acad. Sci. USA. 2003;100:9011–9016. doi: 10.1073/pnas.1431910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRedmond J. P., Park S. D., Reilly D. F., Coppinger J. A., Maguire P. B., Shields D. C., Fitzgerald D. J. Integration of proteomics and genomics in platelets: a profile of platelet proteins and platelet-specific genes. Mol. Cell Proteomics. 2004;3:133–144. doi: 10.1074/mcp.M300063-MCP200. [DOI] [PubMed] [Google Scholar]
- Ohlmann P., Hechle B., Cazenave J. P., Gachet C. Measurement and manipulation of [Ca2+]i in suspensions of platelets and cell cultures. Methods Mol. Biol. 2004;273:229–250. doi: 10.1385/1-59259-783-1:229. [DOI] [PubMed] [Google Scholar]
- Parlati F., Varlamov O., Paz K., McNew J. A., Hurtado D., Sollner T. H., Rothman J. E. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumet F., Le Mao J., Martin S., Galli T., David B., Blank U., Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J. Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- Polgar J., Chung S. H., Reed G. L. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- Polgar J., Lane W. S., Chung S. H., Houng A. K., Reed G. L. Phosphorylation of SNAP-23 in activated human platelets. J. Biol. Chem. 2003a;278:44369–44376. doi: 10.1074/jbc.M307864200. [DOI] [PubMed] [Google Scholar]
- Polgar J., Pan F., Cote N. A., Reed G. L. Deletion of the SNARE gene VAMP3 shortens bleeding time in vivo and enhances platelet secretion in vitro. Circulation. 2003;108(17 Suppl):IV-229. [Google Scholar]
- Prombona A., Tabler M., Providaki M., Tsagris M. Structure and expression of LeMA-1, a tomato protein belonging to the SEC18-PAS1-CDC48-TBP-1 protein family of putative Mg(2+)-dependent ATPases. Plant Mol. Biol. 1995;27:1109–1118. doi: 10.1007/BF00020884. [DOI] [PubMed] [Google Scholar]
- Randhawa V. K., et al. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol. Biol. Cell. 2000;11:2403–2417. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed G. San Diego, CA: Academic Press; 2002. Platelet Secretion. [Google Scholar]
- Reed G. L. Platelet secretory mechanisms. Semin. Thromb. Hemost. 2004;30:441–450. doi: 10.1055/s-2004-833479. [DOI] [PubMed] [Google Scholar]
- Reed G. L., Fitzgerald M. L., Polgar J. Molecular mechanisms of platelet exocytosis: insights into the “secrete” life of thrombocytes. Blood. 2000;96:3334–3342. [PubMed] [Google Scholar]
- Rossi V., Banfield D. K., Vacca M., Dietrich L. E., Ungermann C., D'Esposito M., Galli T., Filippini F. Longins and their longin domains: regulated SNAREs and multifunctional SNARE regulators. Trends Biochem. Sci. 2004;29:682–688. doi: 10.1016/j.tibs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rutledge T. W., Whiteheart S. W. Studies of secretion using permeabilized platelets. Methods Mol. Biol. 2004;272:109–120. doi: 10.1385/1-59259-782-3:109. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Matteoli M., Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Schoch S., Deak F., Konigstorfer A., Mozhayeva M., Sara Y., Sudhof T. C., Kavalali E. T. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schraw T. D., Rutledge T. W., Crawford G. L., Bernstein A. M., Kalen A. L., Pessin J. E., Whiteheart S. W. Granule stores from cellubrevin/VAMP-3 null mouse platelets exhibit normal stimulus-induced release. Blood. 2003;102:1716–1722. doi: 10.1182/blood-2003-01-0331. [DOI] [PubMed] [Google Scholar]
- Ungar D., Hughson F. M. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 2003;19:493–517. doi: 10.1146/annurev.cellbio.19.110701.155609. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Langosch D. Functions of SNAREs in intracellular membrane fusion and lipid bilayer mixing. J. Cell Sci. 2005;118:3819–3828. doi: 10.1242/jcs.02561. [DOI] [PubMed] [Google Scholar]
- Varlamov O., et al. i-SNAREs: inhibitory SNAREs that fine-tune the specificity of membrane fusion. J. Cell Biol. 2004;164:79–88. doi: 10.1083/jcb.200307066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Ng C. P., Lu L., Atlashkin V., Zhang W., Seet L. F., Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev. Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Sollner T. H., Rothman J. E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- White J. G., Escolar G. The blood platelet open canalicular system: a two-way street. Eur. J. Cell Biol. 1991;56:233–242. [PubMed] [Google Scholar]
- Wong S. H., Zhang T., Xu Y., Subramaniam V. N., Griffiths G., Hong W. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol. Biol. Cell. 1998;9:1549–1563. doi: 10.1091/mbc.9.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Gonzalez L., Jr, Prekeris R., Steegmaier M., Advani R. J., Scheller R. H. SNARE interactions are not selective. Implications for membrane fusion specificity. J. Biol. Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- Yang C., Mora S., Ryder J. W., Coker K. J., Hansen P., Allen L. A., Pessin J. E. VAMP3 null mice display normal constitutive, insulin- and exercise- regulated vesicle trafficking. Mol. Cell. Biol. 2001;21:1573–1580. doi: 10.1128/MCB.21.5.1573-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.