Abstract
The Golgi apparatus is a highly dynamic organelle whose organization is maintained by a proteinaceous matrix, cytoskeletal components, and inositol phospholipids. In mammalian cells, disassembly of the organelle occurs reversibly at the onset of mitosis and irreversibly during apoptosis. Several pharmacological agents including nocodazole, brefeldin A (BFA), and primary alcohols (1-butanol) induce reversible fragmentation of the Golgi apparatus. To dissect the mechanism of Golgi reassembly, rat NRK and GH3 cells were treated with 1-butanol, BFA, or nocodazole. During washout of 1-butanol, clathrin, a ubiquitous coat protein implicated in vesicle traffic at the trans-Golgi network and plasma membrane, and abundant clathrin coated vesicles were recruited to the region of nascent Golgi cisternae. Knockdown of endogenous clathrin heavy chain showed that the Golgi apparatus failed to reform efficiently after BFA or 1-butanol removal. Instead, upon 1-butanol washout, it maintained a compact, tight morphology. Our results suggest that clathrin is required to reassemble fragmented Golgi elements. In addition, we show that after butanol treatment the Golgi apparatus reforms via an initial compact intermediate structure that is subsequently remodeled into the characteristic interphase lace-like morphology and that reassembly requires clathrin.
INTRODUCTION
The Golgi apparatus is a polarized organelle that in interphase cells exists as a highly organized membranous network in the juxtanuclear region of the cell; it plays key roles in protein and lipid modification as well as cargo sorting. The structural organization of the Golgi apparatus is maintained by a family of high-molecular-weight proteins (e.g., GM130, GRASP65, Golgin-160, and p115), cytoskeletal components such as βIII-spectrin as well as by the microtubule cytoskeleton (Nakamura et al., 1995; Barr et al., 1997; Stankewich et al., 1998; Shorter and Warren, 1999; Thyberg and Moskalewski, 1999). Several lipids, including phosphatidic acid (PA), diacylglycerol, phosphatidylinositol 4-phosphate [PtdIns(4)P], phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] have also been implicated in maintaining Golgi structure and function (De Matteis et al., 2005). In mammalian cells, the Golgi apparatus has a characteristic lace-like morphology that disassembles into vesicles and tubules at the onset of mitosis and reforms in telophase after the equal distribution of Golgi-derived vesicles into daughter cells. In contrast, during apoptosis the organelle fragments irreversibly (Machamer, 2003). Two models have been proposed to explain the inheritance of the Golgi complex. One suggests that free mitotic tubulovesicular clusters (MGCs) are the unit of Golgi inheritance (Jokitalo et al., 2001; Axelsson and Warren, 2004), whereas a second model proposes that the Golgi is inherited through a merged endoplasmic reticulum (ER)/Golgi compartment (Zaal et al., 1999). Furthermore, Seemann et al. (2002) suggested that the Golgi matrix provides a scaffold for reformation of the membranous compartment, whereas others propose that the entire Golgi apparatus reforms de novo from MGCs and vesicles (Puri et al., 2004).
The trans-Golgi network (TGN) is the most distal sorting and processing station within the Golgi apparatus. Cargo sorting at this site is mediated by compartment-specific coat and adaptor proteins (Bonifacino and Lippincott-Schwartz, 2003; Gleeson et al., 2004). Whereas the COPI and -II coats participate in retrograde and anterograde trafficking events, respectively, between ER and cis-Golgi (Lee et al., 2004), clathrin mediates cargo selection and vesicle fission at the TGN, in the endosomal, lysosomal, and regulated secretory pathways, as well as endocytosis at the plasma membrane (Kirchhausen, 2000). The specificity of clathrin-coated vesicle (CCV) formation and targeting is determined by adaptors that localize to subcellular compartments via binding motifs in target proteins as well as through interactions with the lipid–protein environment. The monomeric adaptor AP180/CALM binds PtdIns(4,5)P2 at the plasma membrane via its (N-terminal) ANTH domain and promotes clathrin lattice formation through direct interaction of its C terminus with the clathrin heavy chain (CHC) and the tetrameric activator protein (AP)-2 complex (Ford et al., 2001). Clathrin function in TGN-endosome sorting is mediated in part by the small GTP binding ADP-ribosylation factor-1 (ARF-1), which binds membranes in its activated form (Donaldson and Klausner, 1994; Donaldson et al., 2005) and recruits AP-1 (Traub et al., 1993), GGA3 (Dell'Angelica et al., 2000), and EpsinR (Hirst et al., 2003) to sites of CCV formation. ARF-1 activation is BFA sensitive (Helms and Rothman, 1992) and plays a role in Golgi structure and function by regulating phospholipid composition and assembly of the β-III spectrin cytoskeleton (Godi et al., 1999; Jones et al., 2000; De Matteis and Morrow, 2001). AP-1 interacts with ARF-1 and PtdIns(4)P via motifs in the β1, γ, and μ1 subunits (Traub et al., 1993; Wang et al., 2003) and binds clathrin through its β1 hinge domain (Traub et al., 1995; Doray and Kornfeld, 2001). Epsin R is a novel ENTH domain-containing protein that is enriched in purified clathrin-coated vesicles and binds PtdIns(4)P. It was identified as a major interaction partner for the AP-1 γ-adaptin subunit and has been implicated in clathrin-mediated anterograde as well as retrograde trafficking between the endosomal and TGN compartments (Hirst et al., 2003; Mills et al., 2003; Saint-Pol et al., 2004).
Numerous pharmacological agents that reversibly perturb Golgi components have been used to study mechanisms involved in maintaining Golgi structure (Dinter and Berger, 1998). These effects are reversible and can be used as a model for the reformation of the Golgi apparatus after mitosis and physiological stress. The fungal metabolite BFA triggers rapid COPI dissociation and reversible redistribution of Golgi enzymes into the ER by inhibiting a guanine nucleotide exchange factor (GEF) of ARF-1 (Lippincott-Schwartz et al., 1989; Donaldson et al., 1992). It causes matrix proteins (GM130 and GRASP65) to disperse throughout the cytoplasm without merging with the ER. In addition, BFA triggers the TGN to fragment into vesicles and tubules, a process that disrupts protein sorting (Ladinsky and Howell, 1992; Reaves and Banting, 1992; Wagner et al., 1994). The Golgi complex is localized to the pericentriolar region of the cell by virtue of interactions with the microtubule cytoskeleton and minus-end–directed motors (Allan et al., 2002). Treatment of cells with nocodazole, a microtubule depolymerizing agent, causes the Golgi apparatus to break down into functional ministacks (Turner and Tartakoff, 1989) that become redistributed from the juxtanuclear region to random sites throughout the cytoplasm.
To investigate the mechanism of Golgi fragmentation, our laboratory has exploited the transphosphatidylation activity of phospholipase D (PLD) whereby in the presence of 1-butanol (1-BtOH), phosphatidylbutanol (PtdBtOH) is generated at the expense of total PA; we showed that diminished PA and PtdIns(4,5)P2 synthesis in vitro coincided with Golgi vesiculation in vivo (Siddhanta et al., 2000, 2003). Additionally, fragmentation of the Golgi apparatus correlated with phosphorylation of βIII-spectrin and its redistribution from Golgi membranes to the cytosol (Siddhanta et al., 2003). Given the role of structural proteins and phospholipids in mediating Golgi structure and function, we have now examined the kinetics of Golgi reassembly after its fragmentation in response to several pharmacological agents. Here, we demonstrate that unexpectedly, reassembly of the Golgi apparatus required the presence of the clathrin heavy chain. Furthermore, when CHC levels were decreased by use of a dominant-negative construct or small interfering RNA (siRNA), the reformation of the Golgi apparatus was delayed and instead remained in a “tight” structure that localized to the juxtanuclear region of the cells. We speculate that this structure corresponds to a physiological assembly intermediate required for the establishment of normal Golgi morphology.
MATERIALS AND METHODS
Cell Culture, Treatments, and Transfection
GH3 cells were grown in F-10 medium supplemented with 12.5% horse serum, 5% fetal calf serum (FCS) (Chen et al., 1997). NRK cells were grown in DMEM supplemented with 10% FCS, glutamine, and penicillin/streptomycin, at 37°C in 5% CO2. To induce fragmentation of the Golgi apparatus, cells were treated with 1% 1-BtOH, 30 μM nocodazole (Calbiochem, San Diego, CA) or 5 μg/ml BFA (Calbiochem) for the indicated times, and reassembly was induced by washout of the drug in complete tissue culture medium. FuGENE6 (Roche Diagnostics, Indianapolis, IN) and Oligofectamine (Invitrogen, Carlsbad, CA) were used for plasmid and siRNA transfection, respectively, according to the manufacturers' instructions.
Antibodies and cDNA Constructs
Mouse monoclonal antibody to mannosidase II (ManII; 53FC3) was provided by Dr. Brian Burke (University of Florida, Gainesville, FL); rabbit anti-clathrin was a gift from Dr. Sylvia Corvera (University of Massachusetts Medical School, Worcester, MA). The antibody to recombinant GRASP65 was a generous gift from Dr. Yanzhuang Wang (University of Michigan, Ann Arbor, MI). The rabbit anti-epsinR antibody used for Western blotting was from Dr. Margaret Robinson (University of Cambridge, Cambridge, United Kingdom). Rabbit anti-CI-Man6PR was a gift from Dr. Sharon Tooze (Cancer Research, London, United Kingdom). The X22 antibody was from Affinity Bioreagents (Golden, CO). Mouse monoclonal antibodies to clathrin heavy chain, γ-adaptin, α-adaptin, vti1b, vti1a, and syntaxin6 were purchased from BD Biosciences (San Jose, CA). The monoclonal M3A5 β-COP antibody was purchased from Abcam (Cambridge, MA). The rabbit anti-FLAG antibody (F-7425) was from Sigma-Aldrich (St. Louis, MO). Alexa-conjugated secondary antibodies were purchased from Invitrogen. Horseradish-peroxidase (HRP)-conjugated antibodies were from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom).
A mammalian expression plasmid (pTG192) encoding the clathrin binding domain (CBD) of AP180 was a kind gift from Dr. Lois Greene (Laboratory of Cell Biology, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD). The clathrin light chain (CLC-pDSRed) construct was a gift from Dr. James Keen (Thomas Jefferson University, Philadelphia, PA) and was used to establish stable NRK cells lines, by Geneticin (G-418) selection and fluorescence-activated cell sorting.
siRNA Knockdown
Several siRNAs were tested for potency to knock down the endogenous clathrin heavy chain in NRK cells. The siCHC2 was synthesized by QIAGEN (high-performance purity grade; 20-nmol scale) against the target sequence 5′-AACATCTCACTTGCTCAACGT-3′; the PDCHC sequence was purchased from Ambion (Austin, TX) as predesigned sequence ID 50717; the rchc2 sequence, which was used for most studies, was the equivalent of a CHC siRNA (hchc2) designed against the human sequence and described in Motley et al. (2003); the nonsilencing siRNA was purchased from Ambion. Cells were grown in the absence of antibiotics in 60-mm dishes and transfected when 30–50% confluent with 100 nM siRNA. siRNA–Oligofectamine complex formation was achieved at room temperature according to the manufacturer's instructions (Invitrogen). In some experiments cells were doubly transfected at 2-d intervals and assayed at 48 h after the second transfection.
Microscopy
Electron Microscopy (EM).
GH3 cells were grown on poly-lysine–coated glass coverslips and treated with or without 1% 1-BtOH. Samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer and postfixed with 1% osmium tetroxide followed by 1% uranyl acetate. The samples were then dehydrated through a series of graded ethanol concentrations and embedded in LX112 resin (LADD Research Industries, Burlington, VT). Ultrathin sections were cut on a Reichert Ultracut E, stained with uranyl acetate followed by lead citrate, and viewed on a 1200EX transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV (Siddhanta et al., 2003). For siRNA studies, NRK CLC-pDsRed cells were grown on 60-mm plastic dishes transfected with either control nonsilencing siRNA or rchc2 siRNA for 3 d and treated with 1% BtOH for 30 min followed by washout for 40 and 90 min. Cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pelleted, and processed for EM as described above.
Immunofluorescence Microscopy.
Cells were grown on poly-lysine–coated coverslips and fixed with 3% paraformaldehyde (PFA) for 20 min at room temperature. Coverslips were incubated with primary antibodies in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin, 1% FCS, and 0.2% saponin for 90 min at room temperature followed by incubation with Alexa-conjugated secondary antibodies for 1 h at room temperature and Hoechst 33342 (Sigma-Aldrich) for 10 min. Coverslips were mounted in n-propylgallate/50% glycerol and examined using an IX70 (Olympus America, Melville, NY) equipped with a SensiCam QE cooled charge-coupled device (CCD) camera or an AOBS laser scanning confocal microscope (Leica, Mannheim, Germany). Z-series were acquired in IPLab Spectrum (Scanalytics, Fairfax, VA) or Leica Confocal Software (Leica Microsystems, Heidelberg, Germany) through the depth of cells with a step size of 0.3 μm. CCD-acquired images were preprocessed for deconvolution using NIH Image and deconvolved with a Vaytek PowerHazeBuster (Fairfield, IA) followed by maximum pixel projection (ImageJ; http://rsb.info.nih.gov/ij/) and processing in Adobe Photoshop (Adobe Systems, Mountain View, CA) (Freyberg et al., 2001).
Quantitative Analysis
Z-series images were captured using a Leica confocal microscope with gain and offset levels adjusted to maintain submaximal pixel intensities. Images were then quantified using ImageJ. Regions of interest (ROIs) through the Z-series corresponding to the Golgi apparatus were generated by thresholding TGN38 staining, and ROIs corresponding to the cell were generated by thresholding of clathrin heavy chain staining. Clathrin levels in ROIs were quantified as the sum total of clathrin pixel intensities through the Z-series. ROI volume was determined by the total number of pixels in the ROIs through the Z-series. Clathrin concentration was defined as percentage of clathrin in the Golgi apparatus relative to total clathrin level in the cell normalized to the relative ratio of Golgi volume to cell volume. All measurements were normalized to cell volume (e.g., total clathrin pixels) to account for changes in cell size/shape during treatments.
Clathrin-coated Vesicle Isolation and Blotting
CCVs were isolated from 10- to 15-cm confluent plates of GH3 cells and prepared as described by Hirst et al. (2004) except that the cells were disrupted using a ball bearing homogenizer. The CCV fractions were resolved by SDS-PAGE followed by transfer onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA), which were probed with antibodies to clathrin heavy chain, γ-adaptin, α-adaptin, epsinR, vti1b, vti1a, syntaxin6, and CI-Man6PR, followed by the corresponding HRP-conjugated secondary antibodies (GE Healthcare).
Transferrin Uptake Assay
siRNA-transfected HeLa cells, grown on coverslips, were incubated in transferrin uptake buffer for 3 h at 37°C, followed by incubation with 50 μg/ml fluorescein-labeled human transferrin at either 37 or 4°C. Finally, cells were washed with ice-cold PBS, fixed with 3% PFA, and processed for immunofluorescence microscopy, as described above. Alternatively, to assay bulk fluid phase endocytosis, cells were incubated for 3 h at 37°C with 1 mg/ml lysine fixable fluorescein-labeled dextran (70,000 mol. wt.) in PBS buffer containing Ca2+ and Mg2+, followed by several washes with ice-cold PBS and PFA fixation (Liu et al., 1998).
RESULTS
Clathrin-coated Vesicles Are Recruited to the Reforming Golgi Apparatus
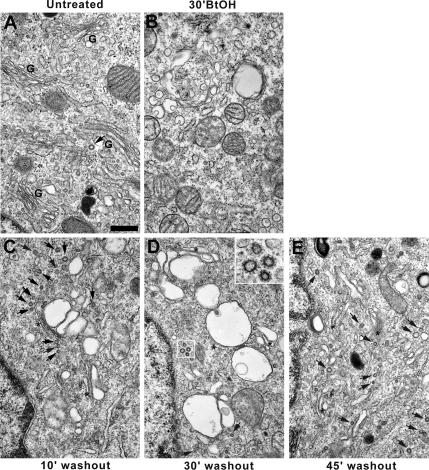
Previous work from our laboratory (Siddhanta et al., 2000, 2003) showed that brief treatment of rat pituitary GH3 cells with 1% 1-BtOH results in reversible fragmentation of the Golgi apparatus. To dissect the molecular basis of Golgi reformation, a time course of the morphological changes that occur during reassembly was performed. Control GH3 cells (Figure 1A) displayed a characteristic intact juxtanuclear Golgi apparatus composed of stacks of flattened cisternae and a low level of clathrin-coated vesicles. In contrast, BtOH-treated cells (Figure 1B) lacked an intact Golgi apparatus, but they were rich in Golgi-derived vesicles and tubules (Sweeney et al., 2002) and were virtually devoid of CCVs. However, during Golgi reformation after alcohol removal and concomitant with reassembly of Golgi stacks via large fusing structures, abundant CCVs were evident in the vicinity of nascent cisternae (Figure 1, C–E, inset, arrows).
Figure 1.
Reversible effects of 1-BtOH on Golgi structure. GH3 cells were either untreated (A) or treated for 30 min with 1% 1-BtOH (B–E) after which the alcohol-containing medium was replaced with normal medium for 10, 30, and 45 min (C, D, and E, respectively), and the cells were processed for electron microscopy. (A) Control Golgi apparatus (G) with few clathrin-coated vesicles (arrow). (B) Golgi membranes vesiculate completely in response to 1% 1-BtOH. (C–E) BtOH washout and sequential reassembly of Golgi cisternae (asterisks). Note abundant CCVs in the vicinity of the reforming Golgi apparatus (D, arrows and inset). Bar, 500 nm.
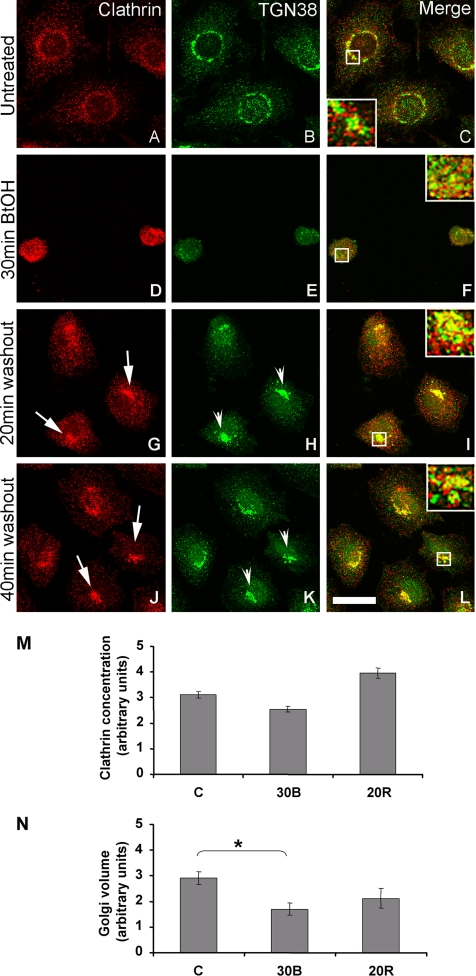
Similarly treated NRK cells also showed recruitment of clathrin to the region of the reforming Golgi apparatus (Figure 2 and Supplemental Figure 1). Untreated control NRK cells displayed punctate clathrin immunoreactivity that localized to the TGN-endosomal region of the cell. Both TGN38 (Figure 2) and ManII (Supplemental Figure 1) staining showed the characteristic lace-like juxtanuclear distribution; however, whereas Man II and clathrin displayed minimal colocalization (Supplemental Figure 1C), TGN38 overlapped with clathrin extensively (Figure 2C). As observed previously (Siddhanta et al., 2000), after 30 min of BtOH treatment, TGN38- and ManII-immunoreactive structures became highly vesiculated and were redistributed throughout the cell (Figure 2, D–F, and Supplemental Figure 1, D–F); furthermore, TGN38 maintained its colocalization with clathrin (Figure 2F), whereas ManII segregated in clathrin-negative structures (Supplemental Figure 1F). Throughout Golgi reassembly after alcohol removal, the Golgi apparatus assumed several distinct morphologies, most notably, a compact or tight intermediate that was accompanied by recruitment of clathrin to structures that possessed TGN38 immunoreactivity (Figure 2, G–L, insets). Significantly, by 90-min washout, ManII (Supplemental Figure 1, M–O) and TGN38 (data not shown) staining resumed the characteristic lace-like appearance, showing that the BtOH effect is completely reversible.
Figure 2.
Recruitment of clathrin to the reforming TGN in NRK cells. NRK cells were treated with 1-BtOH for 30 min followed by washout in complete medium for the indicated times. Cells were double labeled using antibodies specific for clathrin (red) and TGN38 (green). In control cells, clathrin localized to the TGN-endosomal region and displayed extensive colocalization with TGN38 (A–C). During BtOH treatment, TGN38 maintained its colocalization with clathrin (D–F, inset). After alcohol washout, TGN38 assumed an intermediate tight morphology (H and K, arrowheads) and colocalized with clathrin (G–L, arrows). All micrographs are projected Z-series images (see Materials and Methods). Overlap of single sections yielded identical results. Bar, 10 μm. (M and N) Quantitation of clathrin pixel intensity in Golgi-thresholded areas (see Materials and Methods). 30B, 30-min BtOH treatment; 20R, 20-min washout (recovery). Asterisk indicates the only statistically significant difference (p < 0.05, Student's t test).
To determine the level of clathrin recruitment to reforming Golgi structures and ensure that it was not a consequence of changes in cell shape or volume due to alcohol treatment, the Golgi clathrin concentration was quantified (Figure 2, M and N; see Materials and Methods). Thus, although there was a change in TGN volume at 30 min of BtOH treatment, our quantitative analysis showed that by 20 min of Golgi reformation (20R), the volume of the TGN in alcohol washout cells was not significantly different from control untreated cells (Figure 2N). Most importantly, this analysis confirmed our morphological data and showed an ∼30% increase in clathrin levels in TGN38-positive structures during alcohol washout (Figure 2M).
Clathrin Is Required for Golgi Reformation after BtOH Treatment
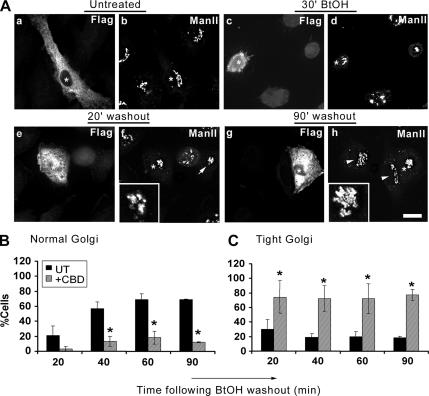
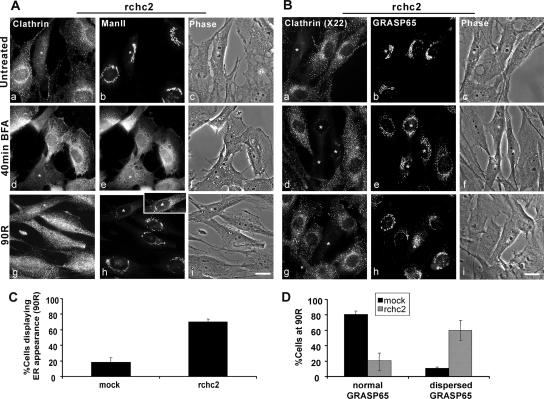
Our observation of CHC enrichment during Golgi reformation suggested a role for clathrin in the reassembly process; however, it did not exclude the possibility that clathrin could mediate vesicle release from the reforming TGN rather than organelle reassembly. To address the role of clathrin during Golgi reformation, we exploited the clathrin binding domain (CBD) of AP180. Other investigators (Zhao et al., 2001) have shown that its overexpression disrupts the cellular distribution of AP-2, AP-1, and TGN38/46 as well as transferrin endocytosis, whereas it has no effect on clathrin-independent markers such as the cis-Golgi GM130 and GRASP65 (our unpublished observations) and the medial-Golgi ManII (Figure 3A, a and b, and Supplemental Figure 2, A and C). In addition, overexpression of the AP180 CBD did not interfere with the redistribution of these markers in BtOH-treated cells (Figure 3A, c and d); however, it prevented ManII-containing structures from assuming the extended lace-like morphology characteristic of the normal Golgi apparatus (Figure 3A, e–h, insets). After BtOH washout, ManII-immunoreactive structures displayed a tight, compact juxtanuclear staining pattern that was evident in both transfected and untransfected cells, particularly at early time points during Golgi reformation (Figure 3A, e and f, inset and arrow, respectively). Interestingly, at 60 and 90 min during washout in cells expressing the AP180 clathrin binding domain, ManII staining remained tight and compact (Figure 3A, g and h, inset) whereas untransfected cells regained the normal lace-like Golgi appearance (Figure 3A, h, arrowheads).
Figure 3.
Overexpression of the AP180 clathrin binding domain: effects on the reformation of the Golgi apparatus. (A) NRK cells were transiently transfected with cDNA encoding the FLAG-tagged CBD of AP180. Transfected cells were detected using a polyclonal anti-FLAG antibody, and the Golgi apparatus was visualized with an anti-ManII antibody. Untreated, ManII displays a characteristic lace-like distribution and juxtanuclear localization in both transfected (A, a, asterisk) and untransfected cells (A, b, asterisk) and a fragmented morphology in treated cells (30′ BtOH; d). During washout, ManII reforms with an initial tight intermediate morphology (20′ washout; f) in both transfected (asterisk and inset) and untransfected cells (arrow). At later times (90′ washout; h), the Golgi apparatus regains its normal morphology in untransfected cells (arrowheads); however, it maintains a tight intermediate conformation in transfected cells (asterisks and inset). (B and C) Quantitation of Golgi morphologies during BtOH washout. Golgi morphologies were defined as normal or tight intermediate and were counted by two independent observers by scoring untransfected and transfected cells from three different experiments. (B) Frequency of normal morphology in untransfected (black bars) or transfected cells (gray bars) at the indicated times after BtOH removal. (C) Frequency of the tight structure in untransfected (black bars) or transfected cells (gray bars). Asterisks indicate statistically significant differences from untransfected cells (p < 0.05, Student's t test). Bar, 10 μm.
To further delineate the role of clathrin in the reassembly of the Golgi apparatus, we quantified the morphologies of Golgi reformation in the absence and presence of the AP180 clathrin binding domain by scoring the different ManII-immunoreactive structures (Figure 3, B and C). The lace-like, extended structure localized to the juxtanuclear region of the cell was scored as “intact”; the extensive array of dispersed vesicles as a consequence of alcohol treatment was designated “fragmented,” and the intermediate Golgi morphology apparent during transition, from fragmented to intact, was defined as “tight.” Untreated cells displayed similar Golgi morphology profiles in the absence or presence of the AP180 clathrin binding domain (Figure 3A, a and b, and Supplemental Figure 2, A and C). Consistent with our qualitative analysis, after BtOH washout ∼70–80% of untransfected cells regained a lace-like Golgi morphology by 60 min; in contrast, only 10–20% of cells expressing the CBD had a normal Golgi appearance, even at 90-min washout (Figure 3B). Strikingly, at 90 min after alcohol removal, these cells maintained the tight, compact Golgi morphology (Figure 3C and Supplemental Figure 2B). Kinetic analysis showed that in the presence of the CBD the tight morphology persisted, whereas the normal, lace-like Golgi configuration failed to accumulate significantly (Supplemental Figure 2D), suggesting that expression of AP180 CBD inhibited the transition from the intermediate-to-normal Golgi morphology. In addition, this result is consistent with a regulatory role for clathrin in the initial membrane assembly into the intermediate structure, because in CBD-transfected cells the tight intermediate occurred more rapidly than in untransfected cells (Supplemental Figure 2D; compare −CBD with +CBD).
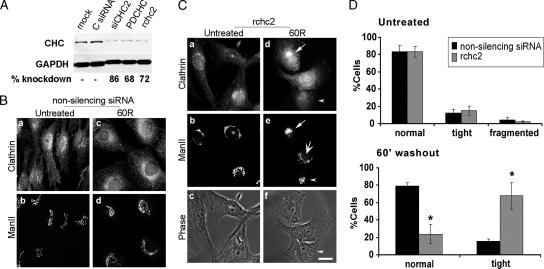
These results suggested that one function of clathrin is in the reassembly of the Golgi apparatus after its fragmentation. To examine this idea directly, we used siRNA to knock down the clathrin heavy chain specifically (Figure 4). NRK cells were transfected with a nonsilencing siRNA or a series of CHC siRNAs for various times, and the optimal conditions for CHC knockdown were determined (Figure 4A). The rchc2 sequence was used in subsequent experiments, and we used the previously described homologous human sequence (Motley et al., 2003) for transferrin endocytosis studies in HeLa cells (Supplemental Figure 7B). We reasoned that if CHC were required for Golgi reassembly, then its knockdown would slow or inhibit Golgi reformation. Knockdown of the clathrin heavy chain did not interfere with Golgi structure in control cells (Figure 4C, a and b, asterisk; D, untreated) nor did it prevent Golgi vesiculation in response to BtOH (data not shown). In agreement with the AP180 clathrin binding domain data, at 60 min after BtOH washout, cells transfected with CHC siRNA failed to remodel the tight ManII morphology into the normal, lace-like Golgi appearance (Figure 4C, d and e, arrowhead). However, because at this time point Golgi reformation is not complete, i.e., a subset of the untransfected cells display a tight intermediate and corresponding clathrin recruitment (Figure 4C, d and e, arrow), we performed statistical analysis to measure the frequency of normal and intermediate ManII-positive structures in the control and CHC siRNA-transfected populations. Consistently, ∼70% of the rchc2-transfected cells remained trapped in the tight morphology, whereas only 15% of the control-transfected cells displayed this intermediate structure at 60 min postwashout (Figure 4D, 60′ washout). Untransfected cells regained a normal Golgi with a frequency of ∼80% compared with ∼25% in the rchc2 sample (Figure 4D, 60′ washout). However, the rabbit anti-clathrin antibody displayed background staining in some siRNA-transfected cells (Figure 4C, a and d), which although less intense and more diffuse than the bone fide clathrin signal, could have suggested that clathrin knockdown was inefficient. In addition, these cells lacked any background clathrin localization to the perinuclear region or the plasma membrane. To exclude false positives, cells from the same experiment were also stained with the monoclonal anti-clathrin antibody X22, which has little or no background staining (Supplemental Figure 3). Not only did this facilitate the unambiguous identification of clathrin knockdown cells but also demonstrated that clathrin knockdown was efficient and the absence of CHC delayed formation of the Golgi ribbon (Supplemental Figure 3). It is noteworthy that in these cells the time required for complete Golgi reformation was 135 min, approximately twice that of cells transfected with nonsilencing siRNA (Figure 4B; data not shown).
Figure 4.
Diminished CHC protein leads to persistence of the compact Golgi structure. (A) NRK cells were either mock-transfected or treated with a control, nonsilencing siRNA (C siRNA) or with three different specific CHC siRNAs for 3 d. Ten micrograms of total homogenate was resolved by SDS-PAGE followed by Western blotting by using mouse anti-clathrin and mouse anti-GAPDH, the latter as a loading control. The bands were quantified with Quantity One (Bio-Rad, Hercules, CA), and CHC knockdown levels are shown below the blots. NRK cells were transfected with control nonsilencing siRNA (B) or CHC siRNA (rchc2) (C) for 3 d, after which they were either untreated or treated with 1% 1-BtOH for 30 min followed by washout in normal medium for 60 min (30 min BtOH; 60R). Both samples were processed for immunofluorescence with antibodies to ManII and clathrin. Arrow (d and e), clathrin recruitment and corresponding Golgi tight intermediate in untransfected cell; also, note fully reformed Golgi in normal cell (large arrow; e); arrowhead, tight Golgi morphology in rchc2-transfected cell (d–f); asterisk, clathrin knockdown cell (a–c); note that the polyclonal anti-clathrin antibody used here displayed nonspecific staining in CHC knockdown cells (for knockdown efficiency, see Supplemental Figure 3). Clathrin knockdown cells were identified as cells displaying low, background levels of staining and no specific subcellular localization. Bar, 20 μm. (D) Quantitation of Golgi morphologies: ∼150 normal or CHC knockdown cells in each of four experiments were scored by two independent observers. Asterisks indicate statistically significant differences from untransfected cells (p < 0.05, Student's t test). Bar, 10 μm.
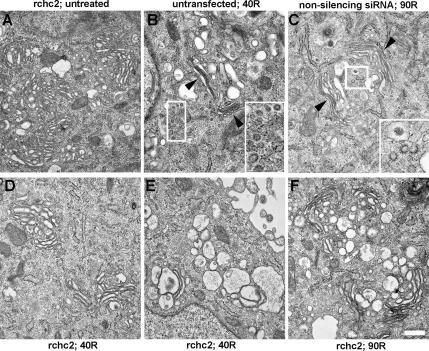
We attempted to identify the tight intermediate morphology by using ultrastructural analysis of siRNA-transfected NRK cells stably expressing CLC-pDsRed; use of this cell line enabled us to readily assess clathrin knockdown efficiency by light microscopy because CHC knockdown results in low CLC protein levels (Hinrichsen et al., 2003; Huang et al., 2004). Thus, cells were transfected with nonsilencing or rchc2 siRNA for 3 d, which resulted in ∼60–70% of the cells exhibiting reduced CLC-Red fluorescence and ∼73% CHC protein knockdown via Western blotting. Aliquots of these same cells were used for EM analysis (Figure 5). Transfection of the rchc2 sequence had no effect on Golgi morphology per se or on its breakdown during BtOH treatment (c.f. Figures 5A and 1). Most cells displayed the control Golgi morphology that consisted of thin, stacked cisternae. During washout, untransfected cells or those transfected with the nonsilencing siRNA (Figure 5B) had reforming Golgi cisternae similar to those seen in GH3 cells (Figure 1), i.e., enlarged vesicles as well as flattened stacks consisting of thin, closely apposed cisternae and abundant clathrin-coated vesicles (Figure 5B, arrowheads and insets). Presumably because of the transient nature of the putative intermediate, a single structure corresponding to “a definitive tight intermediate” was not obvious in the rchc2-transfected sample. However, a common feature was apparent, i.e., abundant, highly dilated, swollen cisternae and large vesicular profiles (Figure 5, D and E). In contrast to the morphology displayed by the control-transfected sample (Figure 5B), few organized, flattened stacks were present, and few if any CCVs were observed in the vicinity of the intermediate. It is noteworthy that these images are consistent with our data on changes in the volume of ManII-positive medial-Golgi structures, which transiently increased ∼2.5-fold during alcohol washout (Supplemental Figure 1P). Significantly, untransfected cells recovered a normal medial-Golgi volume by 40–60 min after washout (Supplemental Figure 1P, 40R). Importantly the dilated structures persisted even after 90-min washout in the rchc2-treated sample, whereas in control-transfected cells normal Golgi stacks were prevalent at this time point (Figure 5, C and F). Given that CHC knockdown efficiency averaged 70–75%, it was possible, but unlikely, that these images were derived from nontransfected cells. Indeed, several points suggest that these images correspond to the compact reforming Golgi intermediate. First, the frequency with which we observed the dilated structures was far greater in cells transfected with CHC siRNAs than in untransfected or control siRNA-transfected cells. Second, no clathrin-coated membranes and few, if any, CCVs were observed. Third, flattened, stacked cisternae were largely absent (Figure 5, compare B and C with E and F). Finally, these structures were evident after alcohol treatment and washout of untransfected GH3 cells where they are surrounded by abundant CCVs (Figure 1).
Figure 5.
Ultrastructural analysis of the tight intermediate. Stably transfected NRK-CLC-pDsRed cells were treated with nonsilencing siRNA (C) or rchc2 siRNA (A, D, E, and F) for 3 d and analyzed for the efficiency of CLC knockdown by light microscopy (60–70% of total cells) and by Western blotting for decrease in endogenous CHC levels (∼70% diminished CHC protein). Cells were either left untreated (A) or were treated with 1% 1-BtOH for 30 min followed by washout in normal medium for either 40 min (B, D, and E) or 90 min (C and F). (B) Untransfected cell. Arrowheads, flattened Golgi stacks. Note that the untransfected cell (B) displays flattened cisternae and abundant CCVs, whereas at the same time point (D and E), rchc2 containing cells show dilated cisternal structures and complete absence of CCVs. Bar, 500 nm.
Clathrin Is Required for Golgi Reassembly after BFA Treatment
Clathrin heavy chain knockdown also prevented ManII from regaining its normal Golgi distribution after BFA treatment (Figure 6); however, it had a minimal effect on Golgi ribbon reformation during nocodazole removal (Figure 7). In the absence or presence of the CHC siRNA and in response to BFA treatment, ManII redistributed to the ER (Figure 6A, d and e, and Supplemental Figure 6B). Whereas at 90 min after BFA washout ManII displayed a normal juxtanuclear distribution in mock-transfected cells (Supplemental Figure 4A, j), it either failed to exit the ER or was retained in a pre-Golgi compartment (possibly vesiculotubular clusters [VTCs]) in clathrin-depleted cells at this time point (Figure 6A, g and h, asterisk, inset). Quantification showed that the frequency of ManII ER-like distribution at 90-min washout was approximately 3 times higher in CHC siRNA-transfected than mock-transfected cells (Figure 6C). We suggest that traffic to the Golgi was inhibited not because CHC is needed for exit from post-ER compartments but rather because of the absence of an “acceptor” Golgi apparatus, whose assembly after BFA washout required clathrin. To test this idea directly, we used the matrix golgin GRASP65 as a marker for Golgi cisternae because some matrix components redistribute to peripheral punctate structures rather than the ER upon treatment with BFA alone (Puri and Linstedt, 2003). Analysis of GRASP65 morphology in clathrin knockdown cells suggested that at 90-min after BFA removal, 60% of the cells failed to regain the normal GRASP65 morphology compared with <20% in mock-treated cells (Figure 6D). Together, these data suggested that clathrin was responsible for reforming Golgi cisternae rather than mediating post-ER transport.
Figure 6.
Clathrin heavy chain knockdown prevents Golgi reassembly after BFA treatment. NRK cells transfected with rchc2 siRNA for 3.5 d were untreated or treated with 5 μg/ml BFA for 40 min (40 min BFA), followed by 90 min washout (90R) after which Golgi morphology was determined using either a mouse monoclonal anti-ManII and rabbit anti-clathrin antibody (A) or a rabbit anti-GRASP65 antibody in conjunction with the monoclonal anti-CHC, X22 antibody (B). BFA-treated cells: (A) ManII redistributes into the ER in rchc2-transfected or untransfected cells (40 min BFA; e) but fails to regain its juxtanuclear localization in rchc2 containing cells (90R; h); inset, because of its lower fluorescence intensity than the surrounding cells, the levels of the CHC knockdown cell were adjusted to reveal ER-like staining. (B) GRASP65 redistributes independently of the ER and maintains a loose juxtanuclear localization in rchc2-transfected and untransfected cells (40 min BFA; e); however, it does not regain its lace-like structure (90R; h). Asterisks indicate siRNA-transfected cells. Bar, 20 μm. (C) Golgi morphologies were quantified by counting cells that displayed either Golgi or ER-like localization of ManII in nonsilencing siRNA or rchc2-treated samples at 90-min washout (90R). The results were expressed as the average percentage of cells displaying ER distribution. (D) GRASP65-positive Golgi morphologies were defined as normal and dispersed and quantified as in C. The frequency of each morphology in mock- and rchc2-transfected cells at 90-min washout (90R) is expressed as the percentage of cells displaying each phenotype. Cells were scored by two independent observers from three experiments.
Figure 7.
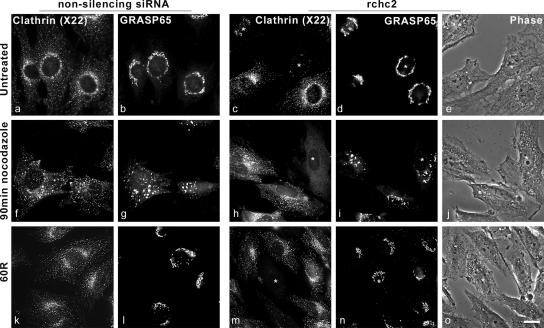
CHC knockdown minimally affects Golgi reformation in response to nocodazole treatment. NRK cells were transfected with either control nonsilencing siRNA or with CHC siRNA (rchc2) for 3 d, after which they were untreated (a–e) or treated with 30 μM nocodazole for 90 min (f–j). Nocodazole treatment triggered GRASP65 redistribution into peripherally distributed immunoreactive puncta in cells with nonsilencing or rchc2 siRNA (g and i). The medium was replaced (k–o), after which clathrin and GRASP65 were localized using mouse anti-CHC antibody (X22) and rabbit anti-GRASP65 antibody. In rchc2-transfected cells, GRASP65 regained its normal morphology less efficiently than in untransfected cells (n; asterisk) or cells transfected with nonsilencing siRNA (l). Asterisk indicates siRNA-transfected cells. Bar, 20 μm.
Nocodazole causes microtubule depolymerization resulting in scattering of the Golgi apparatus to peripheral sites in the cell and the generation of so-called ministacks (Cole et al., 1996; Storrie et al., 1998; Drecktrah and Brown, 1999). We therefore determined whether clathrin was required for Golgi reassembly after recovery from nocodazole treatment (Figure 7). In untransfected cells or those transfected with control nonsilencing siRNA or rchc2 siRNA and those that were treated with nocodazole, both GRASP65 and ManII redistributed into peripherally dispersed punctate structures corresponding to Golgi ministacks (Figure 7 and Supplemental Figure 6A). In contrast to BtOH or BFA washout, however, after nocodazole removal, GRASP65 recovered its control juxtanuclear distribution similarly in CHC knockdown cells and those transfected with a nonsilencing siRNA (Figure 7, m and n, asterisk).
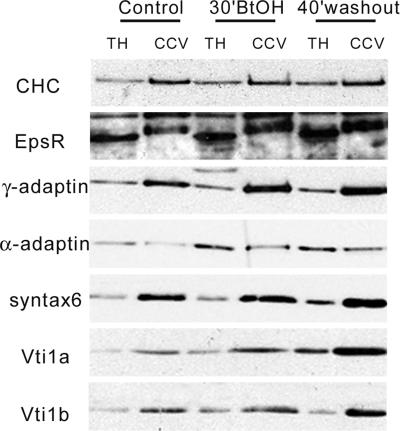
To further characterize the CCVs present during Golgi reformation, vesicles were isolated from control, BtOH-treated, and washout cells (Figure 8). The COPI subunit β-COP was absent (Supplemental Figure 5), suggesting minimal contamination of the CCV preparation with nonclathrin-coated vesicles. As expected, CHC was enriched in the CCV fraction compared with the total homogenate as were Man6PR (Supplemental Figure 5), γ-adaptin, and the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins Vti1a and Vti1b (Figure 8). In contrast, although present in these vesicles, the AP-2 plasma membrane adaptor subunit α-adaptin was not enriched significantly in the CCV fraction, consistent with a model whereby AP-2 participates in the assembly of the clathrin coat at the plasma membrane but does not segregate on free CCVs (Rappoport et al., 2003, 2005). It is noteworthy that the distribution of Vti1a, Vti1b, epsinR, and γ-adaptin was enhanced in the CCV fraction after alcohol washout (Figure 8) when abundant CCVs were evident morphologically. Furthermore, the yield of CCVs from control, treated, and washout cells was very similar, suggesting that its Golgi recruitment resulted from relocation of existing CHC rather than increased synthesis. These data suggest that Golgi disassembly is not a random process but results in differential protein segregation, which occurs in response to BtOH treatment. Additionally, our findings imply that Golgi reformation is regulated by clathrin auxiliary proteins that may mediate vesicle recognition as well as SNAREs that could facilitate fusion.
Figure 8.
Characterization of isolated clathrin-coated vesicles. CCVs were isolated from control, BtOH-treated (30′BtOH), and 40′ washout GH3 cells as described previously (Hirst et al., 2004). Ten micrograms of protein from total homogenates (TH) or the CCV fraction (CCV) were analyzed by SDS-PAGE, transferred to PVDF membranes, and probed with antibodies to the indicated proteins.
DISCUSSION
Clathrin Is Recruited to the Reforming Golgi Cisternae
Although the effects of depleting TGN-associated proteins have not been studied extensively, clathrin heavy chain knockdown was shown to result in failure of coated pit formation at the plasma membrane as well as subsequent inhibition of transferrin endocytosis (Motley et al., 2003). Interestingly, after BtOH treatment, Golgi reassembly was accompanied by recruitment of abundant clathrin-coated vesicles to transitional distended cisternae and large fusing vesicles that with time regained their control appearance (Figure 1). Clathrin was increased ∼30% in TGN38 positive structures after BtOH washout compared with untreated cells. In addition, reformation of both the medial- and TGN compartments coincided with clathrin recruitment to a transiently lived, compact Golgi structure, which we speculate corresponds to a putative assembly intermediate (see below).
CHC Is Required for Normal Golgi Reassembly
It was possible that the appearance of CCVs in the juxtanuclear region was a consequence of increased anterograde vesicle traffic from reforming Golgi cisternae rather than a requirement for reassembly of the organelle. Alternatively, CCVs could function in mediating Golgi reformation by acting as a nucleating site for organelle reassembly. To distinguish between these possibilities, we used two independent experimental strategies to inhibit clathrin function: overexpression of the clathrin binding domain of AP180, which sequesters the endogenous CHC, and siRNA-mediated CHC knockdown. Both approaches yielded identical results and showed that depletion of CHC significantly delayed reformation of the normal Golgi complex. Instead, the Golgi apparatus assumed a tight appearance, first noted in untransfected cells, which failed to be reorganized into the ribbon-like control structure (Figures 3 and 4). This intermediate was evident in ∼35% of untransfected cells at 20 min after BtOH washout and in <20% of cells at later times (Figure 3 and Supplemental Figure 2). It might be expected that if this structure constituted an assembly intermediate, then more cells would posses a tight Golgi morphology, particularly at early times. However, our data demonstrate that the intermediate is highly dynamic and very short-lived, and that it is likely this led to an underestimate of the fraction of cells exhibiting the compact Golgi appearance. Most significantly, the tight intermediate was present in ∼70–80% of clathrin knockdown cells, even at 90 min after BtOH washout. In addition, clathrin knockdown did not disrupt Golgi structure in untreated cells (Figures 3–7). Together, these results suggest that clathrin depletion caused a kinetic block that dramatically slowed the efficiency of Golgi reassembly rather than being absolutely required for Golgi structure.
During BtOH washout, in the absence of clathrin, EM showed that the tight intermediate consisted of swollen cisternae and distended vesicular profiles similar to those of untransfected or control siRNA-transfected cells (Figures 1, C, D, and E, and 5). Furthermore, in the absence of endogenous CHC levels, the dilated vesicles and cisternae persisted at late time points, whereas in control siRNA-transfected cells the Golgi apparatus recovered its normal appearance (Figure 5, C and F), suggesting that clathrin may play a role in Golgi membrane remodeling. Interestingly, our data suggest that in contrast to the TGN, the volume of the medial-compartment increased ∼2.5-fold during reassembly (Figure 2 and Supplemental Figure 1), and it is possible that a subset of the enlarged vesicular profiles may correspond to the medial-Golgi; however, immunogold experiments are required to test this idea. Based on the foregoing observations, we speculate that the TGN and the medial-compartments may be reformed independently of each other by clathrin-mediated mechanisms and that these compartments are likely to be reassembled at different rates, with the TGN regaining its normal morphology most rapidly.
In addition, clathrin was necessary for Golgi reformation after BFA treatment, although the mechanism of organelle disassembly is very different from that in BtOH or nocodazole-treated cells. Interestingly, in the absence of clathrin, the matrix component GRASP65 did not regain its juxtanuclear localization, and ManII failed to exit the ER or VTC compartment (Figure 6). These data suggest that reassembly of the Golgi cisternae mediated by clathrin is likely to be a prerequisite for post-ER exit of Golgi-resident enzymes (i.e., ManII). Consistent with these observations, we observed no colocalization of ManII and clathrin during BFA washout (Supplemental Figure 6B). If such a model were correct, it proposes a tight signaling feedback between the integrity of the Golgi apparatus and budding of nascent COPII vesicles from ER exit sites or transport from VTCs. This model would be consistent with several observations demonstrating that the maintenance of Golgi structure is dependent on Golgi protein recycling via the ER and very recent data showing that increased levels of Golgi proteins in the ER led to enhanced COPII assembly at exit sites (Miles et al., 2001; Ward et al., 2001; Puri and Linstedt, 2003; Guo and Linstedt, 2006). Alternatively, in the absence of a Golgi acceptor compartment, ER-derived vesicles may accumulate in the cytoplasm because they have no target membrane with which to fuse.
In contrast to BtOH- or BFA-treated cells, clathrin was minimally required for reformation of the Golgi ribbon from nocodazole-generated ministacks (Figure 7). This result was consistent with our colocalization studies of nocodazole washout that showed modest recruitment of clathrin to Golgi (ManII) structures (Supplemental Figure 6A, arrowheads). It might be argued that CHC would be required for Golgi reformation regardless of the reagent used to fragment the organelle. However, given that the mechanisms of Golgi fragmentation are very different, the intermediate morphologies and requirements are not expected to be universal. In this context, nocodazole results in disassembly of the Golgi from the juxtanuclear ribbon to peripheral large puncta and transmembrane proteins redistribute slowly to the ER over a prolonged period (3–6 h) where ministacks form at exit sites (Cole et al., 1996; Storrie et al., 1998). Most importantly, in our experiments cells were treated with nocodazole for only 90 min before washout; at this time, the dispersed Golgi still maintains its stack-like structure (Storrie et al., 1998); therefore, it is unlikely that there is significant protein missorting and the stacks maintain their distribution of resident molecules. Consequently, in this case, ribbon reassembly may only involve low levels of lateral membrane fusion and modest local reorganization of Golgi residents that may be mediated by clathrin. This observation is consistent with our hypothesis that clathrin is required for sorting-dependent events that mediate massive rearrangement of Golgi content during reassembly.
Kinetics of Golgi Reassembly
The rapid formation of the intermediate in the absence of clathrin (Supplemental Figure 2D) suggests that under normal conditions CHC may play a regulatory role in the initial membrane aggregation, such as mediating return of Golgi proteins from for example, the endosomal compartment (data not shown) or ordered/sequential fusion of free vesicles. Our data are consistent with a model in which clathrin mediates cisternal reorganization. Thus, in the absence of clathrin, unregulated, rapid membrane aggregation may give rise to a functionally aberrant although morphologically similar structure that cannot be remodeled efficiently. Together, these results suggest that clathrin is required for initial membrane aggregation in the pericentrosomal region of the cells and for remodeling from the tight intermediate into the lace-like structure characteristic of the interphase Golgi apparatus.
A Model for Golgi Biogenesis
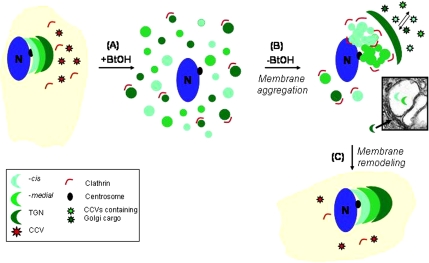
In our experiments, the highest level of Golgi vesiculation was achieved by treating cells with 1-BtOH, and the extent of vesiculation was similar to that observed in mitosis (Sutterlin et al., 2002). However, it is unclear how reassembly of the highly organized Golgi apparatus is achieved from randomly distributed vesicles. We propose a model (Figure 9) whereby clathrin/CCVs in combination with TGN adaptors and SNAREs (data not shown) may regulate differential targeting and sorting of Golgi components. In this context, it is noteworthy that abundant clathrin-coated buds were evident mostly at the rims of reforming cisternae and were absent from intracisternal structures (Figures 1 and 5). Based on these images of clathrin budding vesicles/tubules, we speculate that one function of clathrin might be to remove misorted cargo molecules from the reforming, highly dilated Golgi cisternae, which results in “shrinkage” of cisternae to their normal morphology. Thus, in the absence of CHC, sorting is abrogated or attenuated leading to the appearance of the compact intermediate that cannot proceed efficiently to the normal morphology because of “trapped” cargo. It is noteworthy that although the swollen morphology may be caused by an inhibition of clathrin mediated anterograde sorting, this is not equivalent to basal clathrin-mediated sorting, because this phenotype was absent from CHC siRNA-transfected cells that were not treated with BtOH. We suggest that these CCVs containing Golgi resident proteins are required for the orchestrated reassembly of cisternae from random Golgi-derived vesicles, by virtue of their differential SNARE and adaptor composition. Currently, experiments are in progress to characterize these vesicles and to further delineate their role in mediating Golgi reassembly.
Figure 9.
A model for Golgi reformation. In response to BtOH, the Golgi apparatus fragments into a heterogeneous population of vesicles (A), a subset of which may redistribute into the endosomal compartment. After BtOH washout, clathrin is recruited to these vesicles, which accumulate in the juxtanuclear region of the cell, to facilitate formation of the intermediate structure (B). The tight intermediate is remodeled by a clathrin-dependent mechanism that may involve sorting of Golgi resident proteins into their respective compartments and/or removal of nonresident proteins via basal and reformation-specific anterograde transport (C). EM inset from Figure 1C.
Finally, our results are relevant to a role for clathrin during mitosis; we (Radulescu and Shields, unpublished data) and others have observed the presence of clathrin in the mitotic spindle and spindle poles of multiple cell lines (Okamoto et al., 2000; Royle et al., 2005) and early mouse embryos (Maro et al., 1985). In addition, Royle et al., 2005 showed that disruption of clathrin causes defects in chromosome segregation. Furthermore, during Golgi reformation in mitosis, Golgi marker proteins have been detected on the mitotic spindle (Sutterlin et al., 2005) and during reassembly assume an initial morphology reminiscent of the tight intermediate (our unpublished observations). Consequently, we are now investigating the role of clathrin in Golgi reformation upon exit from mitosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Som-Ming Leung and Michael Cammer for help with quantitative immunofluorescence microscopy and Frank Macaluso and Leslie Gunther for help with EM. We thank Dr. Duncan Wilson for critically reading the manuscript and Dr. Christian Riebeling and Shaeri Mukherjee for helpful suggestions. We thank Drs. Silvia Corvera, Margaret Robinson, Brian Burke, and Sharon Tooze for generous gifts of antibodies; Dr. Lois Green for the pTG192 plasmid; and Dr. James Keen for the CLC-pDsRed construct. A.E.R. thanks Sunny Gupta and Dr. Violeta Chitu for valuable discussions throughout the duration of this project. This work was supported by National Institutes of Health Grants DK-21860 and a pilot and feasibility study from the Diabetes Research Training Center 5P60 DK-20541 (to D.S.). A.E.R. was supported, in part, by National Institutes of Health Training Grant T32GM-07491.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0532) on October 25, 2006.
REFERENCES
- Allan V. J., Thompson H. M., McNiven M. A. Motoring around the Golgi. Nat. Cell Biol. 2002;4:E236–E242. doi: 10.1038/ncb1002-e236. [DOI] [PubMed] [Google Scholar]
- Axelsson M. A., Warren G. Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze. Mol. Biol. Cell. 2004;15:1843–1852. doi: 10.1091/mbc.E03-07-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F. A., Puype M., Vandekerckhove J., Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- Chen Y. G., Siddhanta A., Austin C. D., Hammond S. M., Sung T. C., Frohman M. A., Morris A. J., Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J. Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N. B., Sciaky N., Marotta A., Song J., Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M. A., Di Campli A., Godi A. The role of the phosphoinositides at the Golgi complex. Biochim. Biophys. Acta. 2005;1744:396–405. doi: 10.1016/j.bbamcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- De Matteis M. A., Morrow J. S. ADP-ribosylation factor (ARF) as regulator of spectrin assembly at Golgi complex. Methods Enzymol. 2001;329:405–416. doi: 10.1016/s0076-6879(01)29101-0. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Puertollano R., Mullins C., Aguilar R. C., Vargas J. D., Hartnell L. M., Bonifacino J. S. GGAs: a family of ADP ribosylation factor–binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter A., Berger E. G. Golgi-disturbing agents. Histochem. Cell Biol. 1998;109:571–590. doi: 10.1007/s004180050256. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Honda A., Weigert R. Multiple activities for Arf1 at the Golgi complex. Biochim. Biophys. Acta. 2005;1744:364–373. doi: 10.1016/j.bbamcr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Klausner R. D. ARF: a key regulatory switch in membrane traffic and organelle structure. Curr. Opin. Cell Biol. 1994;6:527–532. doi: 10.1016/0955-0674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Doray B., Kornfeld S. Gamma subunit of the AP-1 adaptor complex binds clathrin: implications for cooperative binding in coated vesicle assembly. Mol. Biol. Cell. 2001;12:1925–1935. doi: 10.1091/mbc.12.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D., Brown W. J. Phospholipase A(2) antagonists inhibit nocodazole-induced Golgi ministack formation: evidence of an ER intermediate and constitutive cycling. Mol. Biol. Cell. 1999;10:4021–4032. doi: 10.1091/mbc.10.12.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. G., Pearse B. M., Higgins M. K., Vallis Y., Owen D. J., Gibson A., Hopkins C. R., Evans P. R., McMahon H. T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Freyberg Z., Sweeney D., Siddhanta A., Bourgoin S., Frohman M., Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol. Biol. Cell. 2001;12:943–955. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P. A., Lock J. G., Luke M. R., Stow J. L. Domains of the TGN: coats, tethers and G proteins. Traffic. 2004;5:315–326. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Godi A., Santone I., Pertile P., Marra P., Di Tullio G., Luini A., Corda D., De Matteis M. A. ADP-ribosylation factor regulates spectrin skeleton assembly on the Golgi complex by stimulating phosphatidylinositol 4,5-bisphosphate synthesis. Biochem. Soc. Trans. 1999;27:638–642. doi: 10.1042/bst0270638. [DOI] [PubMed] [Google Scholar]
- Guo Y., Linstedt A. D. COPII-Golgi protein interactions regulate COPII coat assembly and Golgi size. J. Cell Biol. 2006;174:53–63. doi: 10.1083/jcb.200604058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- Hirst J., Miller S. E., Taylor M. J., von Mollard G. F., Robinson M. S. EpsinR is an adaptor for the SNARE protein Vti1b. Mol. Biol. Cell. 2004;15:5593–5602. doi: 10.1091/mbc.E04-06-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Motley A., Harasaki K., Peak Chew S. Y., Robinson M. S. EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol. Biol. Cell. 2003;14:625–641. doi: 10.1091/mbc.E02-09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Jokitalo E., Cabrera-Poch N., Warren G., Shima D. T. Golgi clusters and vesicles mediate mitotic inheritance independently of the endoplasmic reticulum. J. Cell Biol. 2001;154:317–330. doi: 10.1083/jcb.200104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. H., Morris J. B., Morgan C. P., Kondo H., Irvine R. F., Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annu. Rev. Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Ladinsky M. S., Howell K. E. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by brefeldin A. Eur. J. Cell Biol. 1992;59:92–105. [PubMed] [Google Scholar]
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. H., Marks M. S., Brodsky F. M. A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J. Cell Biol. 1998;140:1023–1037. doi: 10.1083/jcb.140.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E. Golgi disassembly in apoptosis: cause or effect? Trends Cell Biol. 2003;13:279–281. doi: 10.1016/s0962-8924(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Maro B., Johnson M. H., Pickering S. J., Louvard D. Changes in the distribution of membranous organelles during mouse early development. J. Embryol. Exp. Morphol. 1985;90:287–309. [PubMed] [Google Scholar]
- Miles S., McManus H., Forsten K. E., Storrie B. Evidence that the entire Golgi apparatus cycles in interphase HeLa cells: sensitivity of Golgi matrix proteins to an ER exit block. J. Cell Biol. 2001;155:543–555. doi: 10.1083/jcb.200103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills I. G., Praefcke G. J., Vallis Y., Peter B. J., Olesen L. E., Gallop J. L., Butler P. J., Evans P. R., McMahon H. T. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 2003;160:213–222. doi: 10.1083/jcb.200208023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto C. T., McKinney J., Jeng Y. Y. Clathrin in mitotic spindles. Am. J. Physiol. 2000;279:C369–C374. doi: 10.1152/ajpcell.2000.279.2.C369. [DOI] [PubMed] [Google Scholar]
- Puri S., Linstedt A. D. Capacity of the Golgi apparatus for biogenesis from the endoplasmic reticulum. Mol. Biol. Cell. 2003;14:5011–5018. doi: 10.1091/mbc.E03-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S., Telfer H., Velliste M., Murphy R. F., Linstedt A. D. Dispersal of Golgi matrix proteins during mitotic Golgi disassembly. J. Cell Sci. 2004;117:451–456. doi: 10.1242/jcs.00863. [DOI] [PubMed] [Google Scholar]
- Rappoport J. Z., Benmerah A., Simon S. M. Analysis of the AP-2 adaptor complex and cargo during clathrin-mediated endocytosis. Traffic. 2005;6:539–547. doi: 10.1111/j.1600-0854.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport J. Z., Taha B. W., Lemeer S., Benmerah A., Simon S. M. The AP-2 complex is excluded from the dynamic population of plasma membrane-associated clathrin. J. Biol. Chem. 2003;278:47357–47360. doi: 10.1074/jbc.C300390200. [DOI] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle S. J., Bright N. A., Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature. 2005;434:1152–1157. doi: 10.1038/nature03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Pol A., Yelamos B., Amessou M., Mills I. G., Dugast M., Tenza D., Schu P., Antony C., McMahon H. T., Lamaze C., Johannes L. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev. Cell. 2004;6:525–538. doi: 10.1016/s1534-5807(04)00100-5. [DOI] [PubMed] [Google Scholar]
- Seemann J., Pypaert M., Taguchi T., Malsam J., Warren G. Partitioning of the matrix fraction of the Golgi apparatus during mitosis in animal cells. Science. 2002;295:848–851. doi: 10.1126/science.1068064. [DOI] [PubMed] [Google Scholar]
- Shorter J., Warren G. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J. Cell Biol. 1999;146:57–70. doi: 10.1083/jcb.146.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhanta A., Backer J. M., Shields D. Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J. Biol. Chem. 2000;275:12023–12031. doi: 10.1074/jbc.275.16.12023. [DOI] [PubMed] [Google Scholar]
- Siddhanta A., Radulescu A., Stankewich M. C., Morrow J. S., Shields D. Fragmentation of the Golgi apparatus. A role for beta III spectrin and synthesis of phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2003;278:1957–1965. doi: 10.1074/jbc.M209137200. [DOI] [PubMed] [Google Scholar]
- Stankewich M. C., Tse W. T., Peters L. L., Ch'ng Y., John K. M., Stabach P. R., Devarajan P., Morrow J. S., Lux S. E. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc. Natl. Acad. Sci. USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., White J., Rottger S., Stelzer E. H., Suganuma T., Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin C., Hsu P., Mallabiabarrena A., Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. doi: 10.1016/s0092-8674(02)00720-1. [DOI] [PubMed] [Google Scholar]
- Sutterlin C., Polishchuk R., Pecot M., Malhotra V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol. Biol. Cell. 2005;16:3211–3222. doi: 10.1091/mbc.E04-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney D. A., Siddhanta A., Shields D. Fragmentation and re-assembly of the Golgi apparatus in vitro. A requirement for phosphatidic acid and phosphatidylinositol 4,5-bisphosphate synthesis. J. Biol. Chem. 2002;277:3030–3039. doi: 10.1074/jbc.M104639200. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- Traub L. M., Kornfeld S., Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- Traub L. M., Ostrom J. A., Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J. Cell Biol. 1989;109:2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Rajasekaran A. K., Hanzel D. K., Mayor S., Rodriguez-Boulan E. Brefeldin A causes structural and functional alterations of the trans-Golgi network of MDCK cells. J. Cell Sci. 1994;107:933–943. doi: 10.1242/jcs.107.4.933. [DOI] [PubMed] [Google Scholar]
- Wang Y. J., Wang J., Sun H. Q., Martinez M., Sun Y. X., Macia E., Kirchhausen T., Albanesi J. P., Roth M. G., Yin H. L. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- Ward T. H., Polishchuk R. S., Caplan S., Hirschberg K., Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal K. J., et al. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]
- Zhao X., Greener T., Al-Hasani H., Cushman S. W., Eisenberg E., Greene L. E. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J. Cell Sci. 2001;114:353–365. doi: 10.1242/jcs.114.2.353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.