Abstract
β-Blockers are widely used in the treatment of cardiovascular diseases. However, their effects on HERG channels at comparable conditions remain to be defined. We investigated the direct acute effects of β-blockers on HERG current and the molecular basis of drug binding to HERG channels with mutations of putative common binding site (Y652A and F656C).
β-Blockers were selected based on the receptor subtype. Wild-type, Y652A and F656C mutants of HERG channel were stably expressed in HEK293 cells, and the current was recorded by using whole-cell patch-clamp technique (23°C).
Carvedilol (nonselective), propranolol (nonselective) and ICI 118551 (β2-selective) inhibited HERG current in a concentration-dependent manner (IC50 0.51, 3.9 and 9.2 μM, respectively). The IC50 value for carvedilol was a clinically relevant concentration. High metoprolol (β1-selective) concentrations were required for blockade (IC50 145 μM), and atenolol (β1-selective) did not inhibit the HERG current.
Inhibition of HERG current by carvedilol, propranolol and ICI 118551 was partially but significantly attenuated in Y652A and F656C mutant channels. Affinities of metoprolol to Y652A and F656C mutant channels were not different compared with the wild-type.
HERG current block by all β-blockers was not frequency-dependent.
Drug affinities to HERG channels were different in β-blockers. Our results provide additional strategies for clinical usage of β-blockers. Atenolol and metoprolol may be preferable for patients with type 1 and 2 long QT syndrome. Carvedilol has a class III antiarrhythmic effect, which may provide the rationale for a favourable clinical outcome compared with other β-blockers as suggested in the recent COMET (Carvedilol Or Metoprolol European Trial) substudy.
Keywords: β-Blockers, arrhythmia, ion channels, K+ channel, membrane currents, long QT syndrome, heart failure, HEK293 cells
Introduction
In human cardiac ventricular cells, the principal repolarising currents activated during the action potential plateau are the rapidly (IKr) and slowly (IKs) activating components of the delayed rectifier K+ current (Sanguinetti & Jurkiewicz, 1990; Carmeliet, 1993), with IKr encoded by human ether-a-go-go-related gene (HERG or KCNH2) (Curran et al., 1995; Sanguinetti et al., 1995; Trudeau et al., 1995). Pharmacological blockade of HERG currents exhibits antiarrhythmic effect by lengthening of the cardiac action potential duration such as the effects of class III antiarrhythmic drugs, but excessive prolongation of the action potential duration sometimes leads to life-threatening ventricular tachyarrhythmia, torsades de pointes.
β-Blockers are widely used in the treatment of cardiovascular diseases such as hypertension, coronary heart diseases, chronic heart failure and congenital long QT syndrome (LQT). In patients with congenital LQT, β-blockers are reported to be effective in suppressing life-threatening arrhythmia especially in LQT1 and LQT2 patients (Dorostkar et al., 1999; Moss et al., 2000; Schwartz et al., 2001; Shimizu et al., 2002). However, nonselective β-receptor antagonist, carvedilol, inhibits the HERG potassium current by direct action on the channels and prolongs the QT interval (Cheng et al., 1999; Karle et al., 2001). Propranolol overdose has also been reported to cause prolongation of QT interval (Farhangi & Sansone, 2003). Question arises as which type of β-blockers is the most suitable for the treatment of LQT. On the other hand, the recent COMET (Carvedilol Or Metoprolol European Trial) study suggested that carvedilol reduced the rate of sudden cardiac death compared with β1-receptor selective antagonist, metoprolol, in patients with severe heart failure (Poole-Wilson et al., 2003). Since the life-threatening arrhythmias are major cause of sudden cardiac death, differences in electrophysiological actions of carvedilol and metoprolol might be involved in the outcome of the study.
Although the electrophysiological effects of β-blockers have been studied in different species and expression systems, it is important to compare the electrophysiological activities of these drugs at comparable conditions (Dupuis et al., 2005). Interactions of drugs with HERG channels have been so far most often studied in human embryonic kidney 293 (HEK293) cells or Xenopus oocytes. However, HEK293 cells are more suitable for studying the drug affinity to HERG channels because the results correlate well with those observed in native cells and in clinical practice (Redfern et al., 2003).
In addition to class III antiarrhythmic drugs, a remarkable array of structurally diverse therapeutic agents that cause acquired LQT are known to block HERG or IKr channels (Viskin, 1999; Roden, 2004; Tamargo et al., 2004). One possible explanation for this is that lack of a highly conserved amino-acid motif (proline-X-proline) in HERG S6 domains compared with other potassium channels may cause the HERG channel pore and its vestibule to be uniquely large. Another important explanation for this is that these drugs bind to a common drug receptor within the pore of HERG channels. Using alanine scanning mutagenesis of the pore-S6 region of HERG channel, aromatic amino-acid residues (Y652 and particularly F656) were reported to be key determinants of drug binding to HERG channels (Mitcheson et al., 2000). Therefore, it is useful to use these mutant channels to examine the direct action of drugs on HERG channels.
The aim of the present study was to investigate the direct effect of various β-blockers on cloned HERG potassium channels expressed heterologously in HEK293 cells and compare the relative potencies of HERG channels blockade. In addition, we investigated the molecular basis of drug binding to HERG channels with the S6 domain mutations (Y652A and F656C).
Methods
DNA constructs and transfection of HEK293 cells
HERG cDNA (GenBank Accession Number: U04270) was subcloned into BamHI/EcoRI sites of the pCDNA3 vector (Invitrogen, San Diego, CA, U.S.A.). HERG wild-type channels were stably expressed in HEK293 cell line as described previously (Zhou et al., 1998). HERG Y652A (tyrosine to alanine at position 652) and F656C (phenylalanine to cysteine at position 656) mutations were generated by site-directed mutagenesis of wild-type HERG cDNA as described previously (Kikuchi et al., 2005). HEK293 cells were transfected with this construct using the lipofectamine method (Invitrogen). Stably transfected cells generated through G418 antibiotic selection were subcloned to achieve a uniform HERG expression level. The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM: Life Technologies, Gaithersburg, MD, U.S.A.) supplemented with 10% foetal bovine serum, 100 U ml−1 penicillin and 100 μg streptomycin. For electrophysiological analysis, the cells were harvested from the culture dish by trypsinisation, washed with D-MEM, and stored in this medium at room temperature for later use. The cells in culture dishes were easily detached by trypsinisation and studied within 8 h of harvest.
Electrophysiological recordings
HERG channel current was recorded using the whole-cell patch-clamp technique at room temperature (23±1°C). The transfected cells were transferred to a bath mounted on the stage of an inverted microscope (Diaphot, Nikon, Japan). The bath was perfused with 4-(2-hydroxyethyl)-1-piperazine ethane sulphonic acid (HEPES)-buffered Tyrode solution containing (in mM) 137 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4). The volume of the bath was 0.6 ml and the external solution in the bath was almost completely exchanged within 1 min at a perfusion rate of 1.25 ml min−1. The internal pipette solution contained (in mM) 130 KCl, 1 MgCl2, 5 ethylene glycol bis (beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 Mg-ATP, and 10 HEPES (pH 7.2). The electrodes were constructed from borosilicate glass using a micropipette puller (P-87, Sutter Instrument Co., Novato, CA, U.S.A.) and heat-polished with a microforge (MF-83, Narishige, Tokyo, Japan). The final resistance of the electrode was 3–5 MΩ when filled with the pipette solution. Membrane currents were recorded with an Axopatch 200B amplifier (Axon Instruments, Union City, CA, U.S.A.) and digitised at 2 kHz with an analogue-to-digital converter (DigiData 1200B; Axon Instruments). The computer software (pCLAMP Ver. 8.1, Axon Instruments) was used to generate voltage clamp protocols, acquire data and analyse current traces.
Chemicals
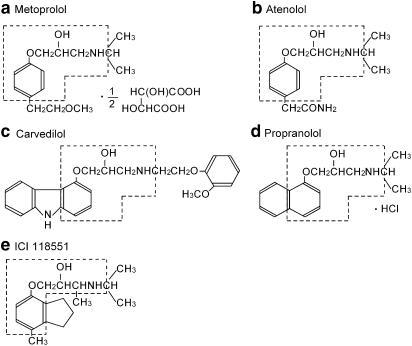
β-Blockers were selected based on their receptor subtype. Propranolol (nonselective β-blocker), atenolol (selective β-blocker) and ICI 118551 (selective β2-blocker) were purchased from Sigma-Aldrich Company (St Louis, MO, U.S.A.). Metoprolol (a selective β-blocker) was purchased from MP Biomedicals (Germany). These drugs were dissolved in distilled water to prepare a stock solution. Carvedilol, a nonselective β-blocker with α-blocking action, was kindly provided by Daiichi Pharmaceutical Co. (Tokyo, Japan) and was dissolved in dimethyl sulphoxide (DMSO) to prepare a stock solution (100 mM). The final concentration of the drug was prepared by diluting the stock solution with Tyrode solution. The highest concentration of DMSO used in the present study was 0.1%. In preliminary experiments, the amplitudes of HERG tail current expressed in HEK293 cells did not change significantly after 3 min of DMSO application at 0.1% (n=4). The chemical structures of β-blockers used in the present study are shown in Figure 1. The molecular weight, β-receptor selectivity (Smith & Teitler, 1999) and therapeutic plasma concentrations of β-blockers used in the present study are summarised in Table 1.
Figure 1.
Structure of β-blockers.
Table 1.
Molecular weight, β-selectivity and therapeutic plasma concentrations of β-blockers
| β-Blockers | Molecular weight | IC50 for β1 (nM)a | IC50 for β2 (nM)a | Selectivity (β1/β2)a | Therapeutic concentration (μM) |
|---|---|---|---|---|---|
| Metoprolol | 342.4 | 204±24 | 1227±270 | 6.0 | 0.40±0.07b |
| Atenolol | 266.3 | 1520±110 | 8600±1360 | 5.7 | 1.64±0.14b |
| Carvedilol | 406.5 | 0.32±0.06 | 0.18±0.04 | 0.6 | 0.1–0.6c |
| Propranolol | 295.8 | 3.6±0.3 | 1.1±0.2 | 0.3 | 0.145±0.07b |
| ICI 118551 | 313.9 | 148±1–9 | 1.48±1–2 | 0.01 | — |
Smith & Teitler (1999).
Data based on the information provided by manufacturer.
McPhillips et al. (1988).
Statistical analysis
Data are expressed as mean±s.e.m., unless otherwise noted. Where applicable, n represents the number of cells studied. Statistical significance was analysed using a two-tailed Student's t-test. P-values <0.05 were considered statistically significant. Curve fitting was performed using multiple nonlinear least-squares regression analysis (pCLAMP Ver. 8.1, Axon Instruments or Sigma Plot Ver. 7.0, SPSS Science, Chicago, IL, U.S.A.).
Results
Effects of β-blockers on wild-type HERG channels expressed in HEK293 cells
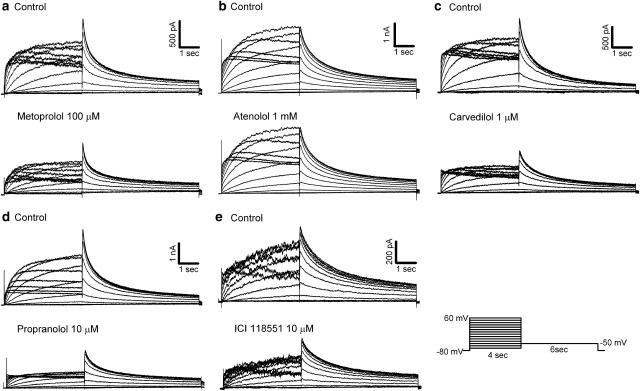
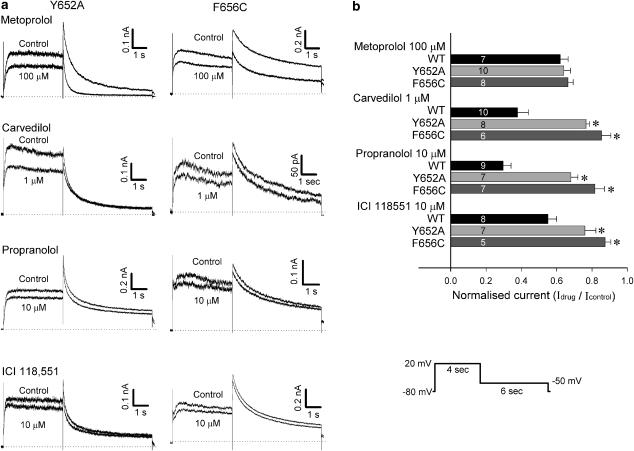
Representative current recordings in control and 3 min after application of β-blockers in the same cell are shown in Figure 2. HERG current was elicited from a holding potential of −80 mV by 4-s long depolarising steps to between −70 and 60 mV applied in 10 mV increments every 15 s. Tail current was recorded with a step pulse to −50 mV for 6 s. Carvedilol, propranolol, and ICI 118551 reduced HERG current amplitude during the depolarising step as well as tail current at relatively low concentrations. High concentrations of metoprolol were required to reduce HERG current. However, atenolol did not produce noticeable inhibition of HERG current when used at concentrations up to 1 mM.
Figure 2.
Effects of β-blockers on HERG current. HERG currents in control (top traces) and in the presence of β-blockers (bottom traces) at indicated concentrations were recorded using the pulse protocol shown at bottom right: (a) 100 μM metoprolol, (b) 1 mM atenolol, (c) 1 μM carvedilol, (d) 10 μM propranolol and (e) 10 μM ICI 118551.
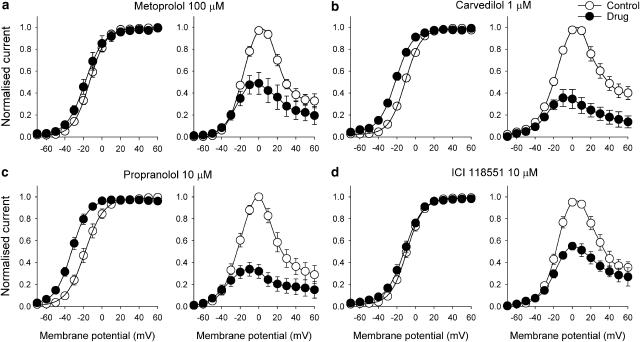
The effects of metoprolol, carvedilol, propranolol and ICI 118551 on the HERG current–voltage (I–V) relationship are shown in Figure 3. The averaged normalised I–V relationships for HERG current measured at the end of the depolarising steps and for tail current are shown. The control HERG current during the depolarising steps was maximal at about 0–10 mV, with tail current fully activated following steps to 10–20 mV. Metoprolol (100 μM), carvedilol (1 μM), propranolol (10 μM) and ICI 118551 (10 μM) reduced the steady-state current amplitude to 48.9±10% (n=5), 34.6±8.6% (n=4), 32.1±6.4% (n=5) and 55.0±3.5% (n=7) of the control at 0 mV, respectively, and the peak tail current amplitude to 54.3±6.1% (n=5), 41.1±8.3% (n=4), 44.3±2.1% (n=5) and 61.6±3.8% (n=7) of the control at 20 mV, respectively. Voltage-dependence of activation (Figure 3) was also evaluated by plotting normalised tail current as a function of voltage. Normalised data were fitted with a Boltzmann function:
where Imax is maximum amplitude, V1/2 and κ are half-activation voltage and the slope factor, respectively. After application of the test drugs, the half-maximal activation voltages significantly shifted in a negative direction compared with the control (metoprolol −13.2±1.5 to −17.0±3.8 mV, P<0.05, n=5; carvedilol −11.0±1.3 to −21.3±1.8 mV, P<0.05, n=4; propranolol −15.1±2.4 to −29.5±4.9 mV, P<0.05, n=5; ICI 118551 −9.1±1.7 to −12.1±2.1 mV, P<0.05, n=7).
Figure 3.
Current–voltage relationship for HERG channels and blockade by β-blockers. Normalised I–V relationships for peak tail currents (left) and currents measured at the end of depolarising steps (right) in the control and presence of β-blockers. Peak tail currents were normalised to their respective maximum current amplitude (control and drugs) to illustrate changes in half-maximal activation voltages: (a) 100 μM metoprolol (n=5), (b) 1 μM carvedilol (n=4), (c) 10 μM propranolol (n=5), (d) 10 μM ICI 118551 (n=7). Data are mean±s.e.m.
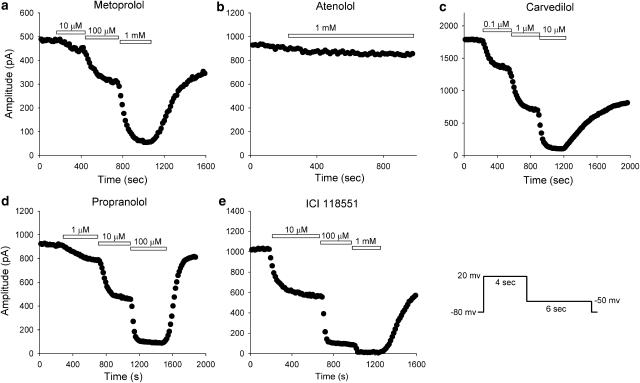
Figure 4 shows the effects of β-blockers on the HERG peak tail current during a drug wash-in protocol at various drug concentrations. HERG current was elicited from a holding potential of −80 mV by a 4-s depolarising step to 20 mV followed by a repolarising step to −50 mV for 6 s, and the protocol was applied every 15 s. After obtaining the control record, β-blockers were applied to the bath. Application of metoprolol, carvedilol, propranolol and ICI 118551 resulted in a rapid HERG channel blockade to reach a nearly steady-state level within 5 min. The blocking effects were reversed gradually during washout (45–88%). Atenolol at 1 mM did not induce marked reduction of the HERG tail current.
Figure 4.
Time course of β-blocker-induced HERG tail current inhibition. HERG currents were elicited every 15 s by the pulse protocol shown at bottom right. Metoprolol (a), carvedilol (c), propranolol (d) and ICI 118551 (e) inhibited HERG tail current in a concentration-dependent manner and reached nearly a steady-state level within 5 min at each concentration. A partial recovery of current was observed after drug washout. (b) Atenolol at 1 mM did not significantly inhibit HERG tail current.
The concentration–response relationship of drug block was assessed by changes in the HERG peak tail current amplitude at 5 min after application of the drug. Currents in the presence of varying concentrations of β-blockers were normalised to the control current amplitude and plotted as a function of drug concentration. The concentration-response curves were obtained by using the Hill equation:
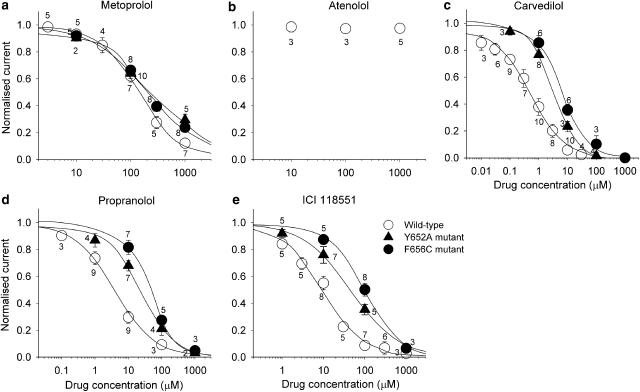
where D is the drug concentration, IC50 is the drug concentration for 50% block, and n is the Hill coefficient. Metoprolol, carvedilol, propranolol and ICI 118551 reduced HERG current amplitude in a concentration-dependent manner but atenolol did not inhibit HERG channels for up to 1 mM (Figure 5). The IC50 value and Hill coefficient are summarised in Table 2.
Figure 5.
Concentration–response relationships for peak tail current in HERG wild-type, Y652A and F656C mutant channels. Currents in the presence of varying concentrations of β-blockers were normalised to the control amplitude and plotted as a function of drug concentration: (a) metoprolol, (b) atenolol, (c) carvedilol, (d) propranolol, (e) ICI 118551. Data are mean±s.e.m. Numbers shown near the data points indicate the number of cells tested. The effects of atenolol on Y652A and F656C mutant channels were not tested. Solid lines represent fits with a Hill equation (see text for detail).
Table 2.
Drug affinities to wild type, Y652A and F656C HERG channels
| β-Blockers | Wild-type (WT) | Y652A | F656C | |||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | Hill Co. | IC50 (μM) | Hill Co. | ΔIC50 Y652A | IC50 (μM) | Hill Co. | ΔIC50 F656C | |
| Metoprolol | 145 | 1.1 | 266 | 0.7 | 1.8 | 207 | 0.8 | 1.4 |
| Carvedilol | 0.51 | 0.8 | 3.4 | 1.1 | 6.7 | 5.5 | 0.9 | 10.8 |
| Propranolol | 3.9 | 0.8 | 24.3 | 0.8 | 6.2 | 40.4 | 1.0 | 10.3 |
| ICI 118551 | 9.2 | 0.9 | 45.6 | 0.8 | 5.0 | 98.1 | 1.0 | 10.7 |
Hill Co.=Hill coefficient, ΔIC50 Y652A=IC50 Y652A/IC50 WT, ΔIC50 F656C=IC50 F656C/IC50 WT.
Attenuation of HERG current blockade by carvedilol, propranolol and ICI 118551 in Y652A and F656C mutant channels
Next, we assessed the molecular basis of drug binding to HERG channels using the HERG Y652A and F656C mutant channels. Figure 6a shows representative current recordings in control conditions and 5 min after application of β-blockers in Y652A and F656C mutant channels. Compared with the wild-type, HERG channels blockade by carvedilol, propranolol and ICI 118551, but not metoprolol, was partially but significantly attenuated in both the Y652A and the F656C mutant channels (Figure 6b). We also assessed the concentration–response relationships of the drug block by measuring the peak tail current amplitude of Y652A and F656C mutant current. Current reduction was evaluated at 5 min after application of the drug, similar to the protocol used in the wild-type HERG channels. The results of the concentration-response relationships were plotted simultaneously with those of wild-type channels (Figure 5). The IC50 values and Hill coefficients for Y652A and F656C mutant channels, and the relative IC50 values compared with wild-type (ΔIC50) are summarised in Table 2.
Figure 6.
Y652A and F656C mutant channels attenuated HERG channel blockade by carvedilol, propranolol and ICI 118551. (a) HERG currents in control and in the presence of β-blockers at the indicated concentrations elicited by using the pulse protocol shown at the bottom right. (b) Comparison of HERG current inhibition by β-blockers with wild-type (WT), Y652A and F656C mutant channels. HERG currents evaluated at peak tail current amplitude in the presence of β-blockers were normalised to respective control values. Data are mean±s.e.m. Numbers shown in parenthesis indicate the number of cells tested. *P<0.05, compared with the wild-type.
Frequency dependence of HERG current block
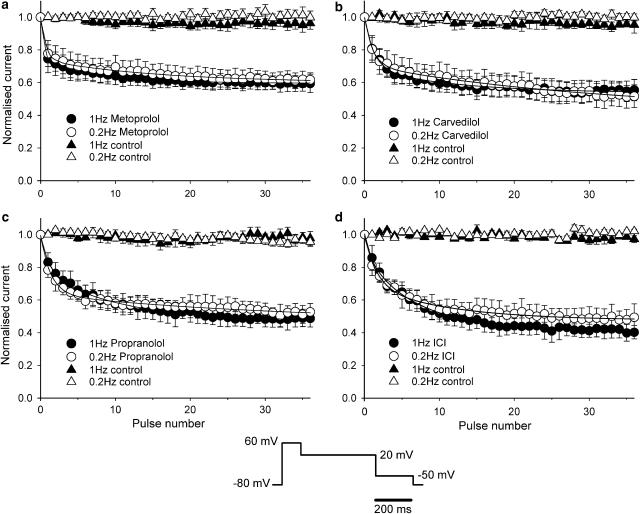
Finally, we investigated the frequency dependence of HERG current block by applying 35 repetitive pulses at 0.2 and 1 Hz, after holding the cell at −80 mV for 5 min during wash-in of 100 μM metoprolol, 1 μM carvedilol, 10 μM propranolol and 10 μM ICI 118551. HERG current was first rapidly activated by a 100 ms step to 60 mV, which was followed by a 400 ms step to 20 mV and a 200 ms step to −50 mV (see bottom of Figure 7). HERG current was measured as the peak tail current amplitude during the step pulse at −50 mV, and was normalised to the control current measured prior to drug exposure. For control conditions, the HERG current amplitude during the pulse train did not change significantly (<5%). Following exposure to drugs, application of the pulse train at either 0.2 or 1.0 Hz decreased current amplitude. The time course of HERG current block was fit with double exponential function:
where t is the pulse number; τf and τs are time constants of fast and slow components; Af and As are fractional amplitudes of fast and slow components. The time constants of the fast and slow components of the time course of block, and the amount of steady-state block were not significantly different between the 0.2 and 1.0 Hz frequencies (Table 3). The lack of frequency-dependent block can be interpreted as the result of fast onset of open channel block and slow unblocking.
Figure 7.
Inhibition of HERG tail current by β-blockers was not frequency-dependent. After the cell was held at −80 mV for 5 min during exposure to 100 μM metoprolol (a), 1 μM carvedilol (b), 10 μM propranolol (c) and 10 μM ICI 11855 (d), 35 repetitive pulses (see protocol at centre bottom of the figure) were applied at 1 and 0.2 Hz. Solid lines represent fits to averaged data with double exponential function (see text for detail). Fitting parameters are summarised in Table 3.
Table 3.
Parameters of the time course of HERG current block by β-blockers
| β-Blockers | 0.2 Hz | 1 Hz | ||||||
|---|---|---|---|---|---|---|---|---|
| τf (pulses−1) | τs (pulses−1) | Base | n | τf (pulses−1) | τs (pulses−1) | Base | n | |
| Metoprolol | 0.55±0.14 | 13.7±2.4 | 0.56±0.05 | 4 | 0.35±0.11 | 9.8±1.8 | 0.59±0.04 | 4 |
| Carvedilol | 1.00±0.10 | 17.7±5.1 | 0.52±0.07 | 4 | 1.18±0.13 | 14.4±2.6 | 0.54±0.06 | 4 |
| Propranolol | 0.92±0.16 | 9.5±3.4 | 0.52±0.04 | 4 | 1.64±0.55 | 10.8±1.2 | 0.47±0.04 | 4 |
| ICI 118551 | 0.61±0.09 | 9.2±2.8 | 0.48±0.06 | 4 | 0.61±0.07 | 7.5±0.5 | 0.41±0.04 | 4 |
τf and τs, time constants of fast and slow components.
Discussion
In the present study, we examined the direct effect of β-blockers on HERG channels and the molecular basis of drug binding to the channel using HERG Y652A and F656C mutant channels. Our major findings were: (1) propranolol, carvedilol and ICI 118551 inhibited HERG current in a concentration-dependent manner. (2) High concentrations of metoprolol were required for blockade, and atenolol did not significantly inhibit HERG current. (3) Y652A and F656C mutations partially attenuated the affinity for propranolol, carvedilol and ICI 118551 but did not alter the affinity for metoprolol.
Effects of β-blockers on HERG channels
Carvedilol has been reported to inhibit HERG or IKr current. Cheng et al. (1999) reported that carvedilol inhibited IKr, L-type ICa, Ito and IKs in rabbit ventricular myocytes with IC50 values of 0.35, 3.59, 3.34 and 12.54 μM, respectively. It was important that IKr was the most sensitive to carvedilol and that the action potential duration was actually prolonged. The IC50 value for IKr blockade was very close to that determined in the present study (0.51 μM). Carvedilol has been reported also to inhibit HERG channels expressed in Xenopus oocytes by acting directly on the channels (Karle et al., 2001). Although the IC50 value in the oocyte expression system (10.4 μM) was much higher than those in native cardiac myocytes and HEK293 cells, the difference was attributed to the viteline membrane and yolk of oocytes, which required up to 30-fold higher concentrations for drug effects (Madeja et al., 1997). Taken together, carvedilol blocks HERG current at the range of therapeutic concentrations in clinical use (0.1–0.6 μM) (McPhillips et al., 1988), and has class III antiarrhythmic effect.
At high dose, propranolol was reported to prolong QT interval (Farhangi & Sansone, 2003). More recently, propranolol has been reported to inhibit HERG channels expressed in Chinese hamster ovary cells with an IC50 value of 9.9±1.3 μM (Yao et al., 2005). The present study also suggested that propranolol has an inhibitory effect on HERG current by binding to the putative common binding site. However, the IC50 value (3.9 μM) was higher than the therapeutic concentration (Cmax: 0.145±0.07 μM/20 mg p.o., mean±s.d., n=10, based on the information provided by the manufacturer). Thus, propranolol might have a less powerful effect on QT interval at a clinically relevant concentration as reported previously (Linker et al., 1992; Shimizu & Antzelevitch, 1998; Shimizu et al., 2002).
In contrast to carvedilol, atenolol and metoprolol did not show significant effects on HERG currents at least at therapeutic concentrations (Cmax: atenolol: 1.64±0.14 μM/50 mg p.o., n=12; metoprolol 0.40±0.07 μM/120 mg p.o., n=12, based on the information provided by the manufacturer). The β1-selective antagonists have a single ring compound in the common structure of β-blockers, while nonselective and β2-receptor selective antagonists have other ring compounds in addition to the common structure (Figure 1). These structural differences may contribute to the drug affinity to HERG channels.
The structural requirements for the drug binding site in HERG channels have been studied recently in detail (Mitcheson et al., 2000; Kamiya et al., 2001; Milnes et al., 2003; Sanchez-Chapula et al., 2003; Scholz et al., 2003; Perry et al., 2004). It was demonstrated that aromatic amino-acid residues (Y652 and particularly F656) located in the S6 domain are the most important molecular determinants of drug binding (Mitcheson et al., 2000). In the present study, Y652A and F656C mutant channels attenuated HERG current blockade by carvedilol, propranolol and ICI 118551, suggesting that the binding of these β-blockers involves a putative drug receptor within the pore-S6 region of the HERG channel. However, inhibition of HERG channels by most drugs tested to date was abolished or markedly attenuated by F656 mutant channels except fluvoxamine and dronedarone (Milnes et al., 2003; Ridley et al., 2004). In the present study, the drug affinities of carvedilol, propranolol and ICI 118551 were about five- to 11-fold less to the Y652A and F656C mutant channels than to the wild-type channels. Considered together, in addition to Y652 and F656, other amino-acid residues might be also involved in the molecular determinant of drug binding. Metoprolol decreased HERG current in a dose-dependent manner, but Y652A and F656C mutant channels did not significantly attenuate the HERG current blockade. Since high dose of metoprolol was required to block HERG current, the drug-channel interaction might be considered nonspecific although the involvement of other amino-acid residues could not be excluded.
Clinical implications
In the COMET study (Poole-Wilson et al., 2003) and its subanalysis (Remme et al., 2004), carvedilol reduced the mortality rate and sudden cardiac death rate more effectively than metoprolol. Although the mechanisms for this favourable clinical outcome for patients treated with carvedilol are still unknown, it is likely to be multifactorial involving the blockade of β- and α-adrenergic receptors, a greater anti-ischemic effect, inhibition of apoptosis, an antioxidant action, free-radical scavenging, and/or electrophysiological effect. In the present study, we compared the class III antiarrhythmic effects of multiple β-blockers by estimating HERG channel blocking activity. The class III antiarrhythmic effects were significantly potent for carvedilol compared with other β-blockers. Carvedilol might provide favourable outcome via class III antiarrhythmic effects, in the context of β-blockade, in chronic heart failure patients. Thus, our results seem to provide a hypothetical molecular explanation for the favourable outcome in carvedilol treated patients in the COMET study.
Mutations in KCNQ1 (or KVLQT1) are responsible for defects in the slow component of the delayed rectifier potassium current (IKs) underlying the LQT1, whereas mutations in KCNH2 (or HERG) result in defects in the rapid component of the delayed rectifier current (IKr) responsible for the LQT2. Life-threatening arrhythmias including torsades de pointes tachyarrhythmia and sudden cardiac death tend to occur with physical or emotional stress in patients with LQT1 and LQT2 syndrome (Schwartz et al., 2001). Since adrenergic stimulation results in QT prolongation on the surface ECG, β-blockers have been considered to be effective in preventing cardiac events especially in patients with LQT1 (Dorostkar et al., 1999; Moss et al., 2000; Schwartz et al., 2001; Shimizu et al., 2002). Since the HERG channel function is defective in LQT2 patients and HERG channel dependency is increased in LQT1 patients, it is reasonable to consider that β-blockers without HERG channel blocking activity are more preferable for the treatment of these patients. In the present study, atenolol and metoprolol did not inhibit HERG currents significantly at least in clinically relevant concentrations. Thus, these drugs are suitable for treatment of LQT1 and LQT2 patients. High concentrations of propranolol also blocked HERG channels in the present study. Since overdose application of propranolol actually prolongs QT interval (Farhangi & Sansone, 2003), propranolol may not be the best choice for the treatment of patients with LQT1 and LTQ2. In contrast, carvedilol directly inhibited HERG channels at clinically relevant concentrations. Thus, carvedilol might not be recommended in the treatment of patients with LQT1 and LQT2.
Study limitations
The present study has certain limitations. We used HERG channels heterologously expressed in a human cell line. The possible effects of β-blockers on other ion channels as well as receptors in vivo should be considered. Although the direct inhibition of HERG channels by binding to a common binding site presented in this study is one of the mechanisms that regulate HERG channels, the existence of other pathways that regulate HERG channels has been reported. Recent studies revealed that increased intracellular levels of cAMP regulated HERG channels directly by binding to the cyclic nucleotide binding domain and indirectly through cAMP-dependent protein kinase (PKA)-mediated phosphorylation of the channel protein, resulting in net reduction of HERG current (Thomas et al., 1999; Cui et al., 2000; 2001). These effects were, at least in part, mediated by the activation of β1-adrenergic receptors (Karle et al., 2002). According to these results, β-blockers without HERG channel blocking activity competitively attenuate the β-receptor-mediated HERG current inhibition. On the other hand, β-blockers with HERG channel blocking activity have dual pathways to regulate cardiac HERG channels by both direct binding to the HERG channels and competitive antagonism with β-adrenergic agonists on their receptor site. Further studies are needed to identify the most potent regulator of HERG channel at various concentrations.
Secondly, β-blockers, especially carvedilol and propranolol, caused a negative shift in activation curves (Figure 3). One previous study reported that carvedilol did not shift the activation curve in an oocyte expression system (Karle et al., 2001). In our preliminary experiments, we confirmed using Western blot analysis that β1-receptors are expressed on the surface of HEK293 cells (data not shown). Since β-adrenergic activation causes a positive shift in the activation curve (Cui et al., 2000), it is possible that inhibition of baseline activity of endogenous β-receptors caused the negative shift of activation curve and offset the current reduction induced by direct HERG channels block. The negative shift of the activation curve may cause a net increase of current if other biophysical parameters remain unchanged. One of the reasons for the lack of current increase may be due to pronounced pharmacological blockade of HERG channels.
Finally, minK-related peptide 1 (MiRP1), encoded by KCNE2, coassembles with the pore-forming HERG subunit and probably reconstitutes native IKr (Abbott et al., 1999). Compared to channels formed by the HERG subunit alone, HERG/MiRP1 complexes show altered channel properties and increase the potency of channel block by E-4031. Moreover, the mutant forms of MiRP1 demonstrated diminished potassium currents or increased channel blockade by drugs (Sesti et al., 2000). Therefore, it is possible that drug sensitivity might be different between HERG and HERG/MiRP1 channels, although wild-type MiRP1 does not markedly influence the drug sensitivity of the channels (Numaguchi et al., 2000; Kamiya et al., 2001; Scherer et al., 2002; Friederich et al., 2004).
Acknowledgments
We thank Drs Henry J. Duff, Zhengfeng Zhou, and Qiuming Gong for the expert technical assistance and advice. This work was supported by Astrazeneca Research Grant (to T.N.), UOEH Research Grant for Promotion of Occupational Health (to T.N.), and by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to T.N. 17590194).
Abbreviations
- COMET
Carvedilol Or Metoprolol European Trial
- DMSO
dimethyl sulphoxide
- HEK
human embryonic kidney
- HERG
human ether-a-go-go-related gene
- ICa
calcium current
- IKr
rapidly activating component of the delayed rectifier K+ current
- IKs
slowly activating component of the delayed rectifier K+ current
- Ito
transient outward current
- MiRP1
MinK-related peptide 1
References
- ABBOTT G.W., SESTI F., SPLAWSKI I., BUCK M.E., LEHMANN M.H., TIMOTHY K.W., KEATING M.T., GOLDSTEIN S.A.N. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Mechanisms and control of repolarization. Eur. Heart J. 1993;14 (Suppl H):3–13. doi: 10.1093/eurheartj/14.suppl_h.3. [DOI] [PubMed] [Google Scholar]
- CHENG J., NIWA R., KAMIYA K., TOYAMA J., KODAMA I. Carvedilol blocks the repolarizing K+ currents and the L-type Ca2+ current in rabbit ventricular myocytes. Eur. J. Pharmacol. 1999;376:189–201. doi: 10.1016/s0014-2999(99)00368-4. [DOI] [PubMed] [Google Scholar]
- CUI J., KAGAN A., QIN D., MATHEW J., MELMAN Y.F., MCDONALD T.V. Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J. Biol. Chem. 2001;276:17244–17251. doi: 10.1074/jbc.M010904200. [DOI] [PubMed] [Google Scholar]
- CUI J., MELMAN Y., PALMA E., FISHMAN G.I., MCDONALD T.V. Cyclic AMP regulates the HERG K+ channel by dual pathways. Curr. Biol. 2000;10:671–674. doi: 10.1016/s0960-9822(00)00516-9. [DOI] [PubMed] [Google Scholar]
- CURRAN M.E., SPLAWSKI I., TIMOTHY K.W., VINCENT G.M., GREEN E.D., KEATING M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- DOROSTKAR P.C., ELDAR M., BELHASSEN B., SCHEINMAN M.M. Long-term follow-up of patients with long-QT syndrome treated with β-blockers and continuous pacing. Circulation. 1999;100:2431–2436. doi: 10.1161/01.cir.100.24.2431. [DOI] [PubMed] [Google Scholar]
- DUPUIS D.S., KLAERKE D.A., OLESEN S.P. Effect of β-adrenoceptor blockers on human ether-a-go-go-related gene (HERG) potassium channels. Basic Clin. Pharmacol. Toxicol. 2005;96:123–130. doi: 10.1111/j.1742-7843.2005.pto960206.x. [DOI] [PubMed] [Google Scholar]
- FARHANGI V., SANSONE R.A. QTc prolongation due to propranolol overdose. Int. J. Psychiatry Med. 2003;33:201–202. doi: 10.2190/KLBE-UWHT-TARV-8E0M. [DOI] [PubMed] [Google Scholar]
- FRIEDERICH P., SOLTH A., SCHILLEMEIT S., ISBRANDT D. Local anaesthetic sensitivities of cloned HERG channels from human heart: comparison with HERG/MiRP1 and HERG/MiRP1 T8A. Br. J. Anaesth. 2004;92:93–101. doi: 10.1093/bja/aeh026. [DOI] [PubMed] [Google Scholar]
- KAMIYA K., MITCHESON J.S., YASUI K., KODAMA I., SANGUINETTI M.C. Open channel block of HERG K+ channels by vesnarinone. Mol. Pharmacol. 2001;60:244–253. doi: 10.1124/mol.60.2.244. [DOI] [PubMed] [Google Scholar]
- KARLE C.A., KREYE V.A., THOMAS D., ROCKL K., KATHOFER S., ZHANG W., KIEHN J. Antiarrhythmic drug carvedilol inhibits HERG potassium channels. Cardiovasc. Res. 2001;49:361–370. doi: 10.1016/s0008-6363(00)00265-0. [DOI] [PubMed] [Google Scholar]
- KARLE C.A., ZITRON E., ZHANG W., KATHOFER S., SCHOELS W., KIEHN J. Rapid component IKr of the guinea-pig cardiac delayed rectifier K+ current is inhibited by β1-adrenoreceptor activation, via cAMP/protein kinase A-dependent pathways. Cardiovasc. Res. 2002;53:355–362. doi: 10.1016/s0008-6363(01)00509-0. [DOI] [PubMed] [Google Scholar]
- KIKUCHI K., NAGATOMO T., ABE H., KAWAKAMI K., DUFF H.J., MAKIELSKI J.C., JANUARY C.T., NAKASHIMA Y. Blockade of HERG cardiac K+ current by antifungal drug miconazole. Br. J. Pharmacol. 2005;144:840–848. doi: 10.1038/sj.bjp.0706095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINKER N.J., COLONNA P., KEKWICK C.A., TILL J., CAMM A.J., WARD D.E. Assessment of QT dispersion in symptomatic patients with congenital long QT syndromes. Am. J. Cardiol. 1992;69:634–638. doi: 10.1016/0002-9149(92)90155-r. [DOI] [PubMed] [Google Scholar]
- MADEJA M., MUSSHOFF U., SPECKMANN E.J. Follicular tissues reduce drug effects on ion channels in oocytes of Xenopus laevis. Eur. J. Neurosci. 1997;9:599–604. doi: 10.1111/j.1460-9568.1997.tb01636.x. [DOI] [PubMed] [Google Scholar]
- MCPHILLIPS J.J., SCHWEMER G.T., SCOTT D.I., ZINNY M., PATTERSON D. Effects of carvedilol on blood pressure in patients with mild to moderate hypertension. A dose response study. Drugs. 1988;36 (Suppl 6):82–91. doi: 10.2165/00003495-198800366-00015. [DOI] [PubMed] [Google Scholar]
- MILNES J.T., CROCIANI O., ARCANGELI A., HANCOX J.C., WITCHEL H.J. Blockade of HERG potassium currents by fluvoxamine: incomplete attenuation by S6 mutations at F656 or Y652. Br. J. Pharmacol. 2003;139:887–898. doi: 10.1038/sj.bjp.0705335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHESON J.S., CHEN J., LIN M., CULBERSON C., SANGUINETTI M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS A.J., ZAREBA W., HALL W.J., SCHWARTZ P.J., CRAMPTON R.S., BENHORIN J., VINCENT G.M., LOCATI E.H., PRIORI S.G., NAPOLITANO C., MEDINA A., ZHANG L., ROBINSON J.L., TIMOTHY K., TOWBIN J.A., ANDREWS M.L. Effectiveness and limitations of β-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- NUMAGUCHI H., MULLINS F.M., JOHNSON J.P., Jr, JOHNS D.C., PO S.S., YANG I.C., TOMASELLI G.F., BALSER J.R. Probing the interaction between inactivation gating and Dd-sotalol block of HERG. Circ. Res. 2000;87:1012–1018. doi: 10.1161/01.res.87.11.1012. [DOI] [PubMed] [Google Scholar]
- PERRY M., DE GROOT M.J., HELLIWELL R., LEISHMAN D., TRISTANI-FIROUZI M., SANGUINETTI M.C., MITCHESON J.S. Structural determinants of HERG channel block by clofilium and ibutilide. Mol. Pharmacol. 2004;66:240–249. doi: 10.1124/mol.104.000117. [DOI] [PubMed] [Google Scholar]
- POOLE-WILSON P.A., SWEDBERG K., CLELAND J.G., DI LENARDA A., HANRATH P., KOMAJDA M., LUBSEN J., LUTIGER B., METRA M., REMME W.J., TORP-PEDERSEN C., SCHERHAG A., SKENE A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- REDFERN W.S., CARLSSON L., DAVIS A.S., LYNCH W.G., MACKENZIE I., PALETHORPE S., SIEGL P.K., STRANG I., SULLIVAN A.T., WALLIS R., CAMM A.J., HAMMOND T.G. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- REMME W.J., CLELAND J.G., DI LENARDA A., HANRATH P., LUTIGER B., KOMAJDA M., METRA M., SCHERHAG A., CHARLESWORTH A., TORP-PEDERSEN C. Carvedilol better protects against vascular events than metoprolol in heart failure: results from COMET. J. Am. Coll. Cardiol. 2004;43:A205–A206. doi: 10.1016/j.jacc.2006.10.059. [DOI] [PubMed] [Google Scholar]
- RIDLEY J.M, MILNES J.T., WITCHEL H.J., HANCOX J.C. High affinity HERG K+ channel blockade by the antiarrhythmic agent dronedarone: resistance to mutation of the S6 residues Y652 and F656. Biochem. Biophys. Res. Commun. 2004;325:883–891. doi: 10.1016/j.bbrc.2004.10.127. [DOI] [PubMed] [Google Scholar]
- RODEN D.M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-CHAPULA J.A., FERRER T., NAVARRO-POLANCO R.A., SANGUINETTI M.C. Voltage-dependent profile of human ether-a-go-go-related gene channel block is influenced by a single residue in the S6 transmembrane domain. Mol. Pharmacol. 2003;63:1051–1058. doi: 10.1124/mol.63.5.1051. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JIANG C., CURRAN M.E., KEATING M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- SCHERER C.R., LERCHE C., DECHER N., DENNIS A.T., MAIER P., FICKER E., BUSCH A.E., WOLLNIK B., STEINMEYER K. The antihistamine fexofenadine does not affect I(Kr) currents in a case report of drug-induced cardiac arrhythmia. Br. J. Pharmacol. 2002;137:892–900. doi: 10.1038/sj.bjp.0704873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOLZ E.P., ZITRON E., KIESECKER C., LUECK S., KATHOFER S., THOMAS D., WERETKA S., PETH S., KREYE V.A., SCHOELS W., KATUS H.A., KIEHN J., KARLE C.A. Drug binding to aromatic residues in the HERG channel pore cavity as possible explanation for acquired Long QT syndrome by antiparkinsonian drug budipine. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003;368:404–414. doi: 10.1007/s00210-003-0805-5. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ P.J., PRIORI S.G., SPAZZOLINI C., MOSS A.J., VINCENT G.M., NAPOLITANO C., DENJOY I., GUICHENEY P., BREITHARDT G., KEATING M.T., TOWBIN J.A., BEGGS A.H., BRINK P., WILDE A.A., TOIVONEN L., ZAREBA W., ROBINSON J.L., TIMOTHY K.W., CORFIELD V., WATTANASIRICHAIGOON D., CORBETT C., HAVERKAMP W., SCHULZE-BAHR E., LEHMANN M.H., SCHWARTZ K., COUMEL P., BLOISE R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- SESTI F., ABBOTT G.W., WEI J., MURRAY K.T., SAKSENA S., SCHWARTZ P.J., PRIORI S.G., RODEN D.M., GEORGE A.L., Jr, GOLDSTEIN S.A. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10613–10618. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU W., ANTZELEVITCH C. Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- SHIMIZU W., TANABE Y., AIBA T., INAGAKI M., KURITA T., SUYAMA K., NAGAYA N., TAGUCHI A., AIHARA N., SUNAGAWA K., NAKAMURA K., OHE T., TOWBIN J.A., PRIORI S.G., KAMAKURA S. Differential effects of beta-blockade on dispersion of repolarization in the absence and presence of sympathetic stimulation between the LQT1 and LQT2 forms of congenital long QT syndrome. J. Am. Coll. Cardiol. 2002;39:1984–1991. doi: 10.1016/s0735-1097(02)01894-6. [DOI] [PubMed] [Google Scholar]
- SMITH C., TEITLER M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc. Drugs Ther. 1999;13:123–126. doi: 10.1023/a:1007784109255. [DOI] [PubMed] [Google Scholar]
- TAMARGO J., CABALLERO R., GOMEZ R., VALENZUELA C., DELPON E. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- THOMAS D., ZHANG W., KARLE C.A., KATHOFER S., SCHOLS W., KUBLER W., KIEHN J. Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J. Biol. Chem. 1999;274:27457–27462. doi: 10.1074/jbc.274.39.27457. [DOI] [PubMed] [Google Scholar]
- TRUDEAU M.C., WARMKE J.W., GANETZKY B., ROBERTSON G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- VISKIN S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- YAO X., MCINTYRE M.S., LANG D.G., SONG I.H., BECHERER J.D., HASHIM M.A. Propranolol inhibits the human ether-a-go-go-related gene potassium channels. Eur. J. Pharmacol. 2005;519:208–211. doi: 10.1016/j.ejphar.2005.05.010. [DOI] [PubMed] [Google Scholar]
- ZHOU Z., GONG Q., YE B., FAN Z., MAKIELSKI J.C., ROBERTSON G.A., JANUARY C.T. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys. J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]