Abstract
The formation of disulfide bridges is a classical approach used to study the mobility, proximity and distances of residues in a variety of proteins, including ligand- and voltage-gated ion channels. We performed patch-clamp studies to investigate the interaction of a pair of cysteines introduced into the human skeletal muscle voltage-gated Na+ channel (hNav1.4) using the oxidation catalyst, Cu2+(1,10-phenanthroline)3 (CuPhen).
Our experiments resulted in a surprising finding, a reversible current inhibition of the mutant I1160C/L1482C containing two cysteines in the D3/and D4/S4–S5 loops, subjected to oxidative cross-linking in the presence of CuPhen.
We report here that CuPhen is an open channel blocker of both mutant and wild-type (WT) hNav1.4 channels, however, for WT channels a more than 10-fold higher concentration was needed to induce the same effect. Moreover, 1,10-phenanthroline was capable of blocking Na+ channels in the absence of Cu2+ ions. Our results indicate a use- and voltage-dependent binding and unbinding of CuPhen, reminiscent of the lidocaine quaternary derivative QX-314 and the neurotoxin batrachotoxin.
Care should be taken when using CuPhen as an oxidizing reagent in cross-linking experiments, since it may directly affect channel activity. Our results identify CuPhen (and phenantroline) as a novel use-dependent inhibitor of Na+ channels, a mechanism that is shared by drugs widely used in the treatment of epilepsy, neuropathic pain, cardiac arrhythmia and myotonia. We hypothesize that I1160C in D3/S4–S5 and the corresponding L1482C mutation in D4/S4–S5 could allosterically affect a binding site located in the inner pore region of the channel.
Keywords: Ion channel, use-dependent blocker, disulfide bridge, cross-linking, human skeletal muscle
Introduction
Transiently active, voltage-gated Na+ channels play a unique role in excitable cells, mediating the depolarizing and part of the repolarizing phase of the action potential. These channels are the targets of a variety of neurotoxins and drugs that modify their function by binding to different sites in the protein. A large pore-forming α-subunit encoding four homologous domains associates with modulatory β-subunits to form the channel (Catterall, 2000). Each domain contains six putative transmembrane segments S1–S6 arranged around the permeation pathway. Positive charges in the fourth transmembrane segments of the channel confer the sensitivity to changes of the membrane potential. Four linkers, called P-loops, connect the S5 and S6 segments of each domain and together form the selectivity filter of the channel, while the S6 helices provide the walls of the pore towards the intracellular side. The S4–S5 loops and the distal parts of S6 segments are involved in both fast channel inactivation and pharmacological block.
We have previously identified two amino acids, located in the S4–S5 loops in D3 and D4 that – when mutated to cysteines – disrupt fast inactivation of the channel in a cooperative manner (I1160C and L1482C; Popa et al., 2004). To investigate the physical distance between the two cysteines during channel gating, we used a classical approach, the disulfide bridging method. This strategy has been used to study protein mobility and proximity relationships between residues in both water-soluble and integral-membrane proteins (Careaga & Falke, 1992; Yu et al., 1995; Wu & Kaback, 1996). The formation of a disulfide bond, either spontaneously or upon application of oxidizing reagents, depends on the proximity of the two cysteine residues (Careaga & Falke, 1992) providing a rough assay of inter-residue distances. The most commonly used reagent to create an oxidative environment is copper2+(1,10-phenanthroline)3 (CuPhen), an enhancer of the rate of oxygen oxidation of sulfhydryl groups (Kobashi, 1968). It has been applied in disulfide cross-linking studies on G-protein-coupled receptors (Zeng et al., 1999), Na,K-ATPase (Ivanov et al., 2000), ligand-gated ion channels (Horenstein et al., 2001; Tousova et al., 2004) and voltage-gated ion channels (Bénitah et al., 1997; Aziz et al., 2002, Neale et al., 2003). Evidence has accumulated for the unexpected consequences of CuPhen use, apart from its role as a mild oxidizing reagent, such as binding of the 1,10 phenanthroline molecules between the two subunits of the bacterial aspartate receptor (Milburn et al., 1991; Scott et al., 1993) and more recently as a blocker of the vanilloid receptor TRPV1 (Tousova et al., 2004).
Our cross-linking experiments also resulted in a surprising finding, a reversible current inhibition consistent with use-dependent pore block for the mutant hNav1.4 containing the two cysteine residues in the S4–S5 loops of domains D3 and D4. Our results indicate that both CuPhen and 1,10 phenanthroline in the absence of Cu2+ ions are open-channel blockers of hNav1.4 channels.
Methods
Mutagenesis
Site-directed mutagenesis to introduce the different alanine and cysteine mutations in S4–S5 loops of domains D3 and D4 of the α-subunit of the human skeletal muscle Na+ channel (hNav1.4, gene: SCN4A) was described previously (Alekov et al., 2001; Popa et al., 2004). Briefly, full-length wild-type (WT) and mutant constructs of single and double mutations were assembled in the expression vectors pRC/CMV for transfection into the mammalian cell line tsA201, or in pSP64 for in vitro translation into cRNA, which was injected into Xenopus oocytes. pSP64 vectors containing either WT or mutant cDNA were linearized with EcoRI. cRNA was prepared using the T7 mMessage mMachine SP6 kit (Ambion Inc., Austin, TX, U.S.A.).
Expression in tsA201 cells and oocytes
Mammalian tsA-201 cells were transfected using a standard calcium phosphate precipitation. Cells were cotransfected with constructs of either WT or mutant sodium channel α-subunits and a vector encoding the human CD8 cell surface protein. Magnetic polystyrene microspheres precoated with anti-CD8 antibody (Dynabeads M450, Dynal) were used to identify transfected cells (Lerche et al., 1997).
Oocyte preparation and injection was performed as described previously (Lerche et al., 1999). Adult Xenopus laevis frogs were anesthetized with 0.5% Tricaine (3-aminobenzoic acid ethyl ester methanesulfonate salt, Sigma-Aldrich, Munich, Germany) and pieces of ovary were surgically removed. The incisions were sutured and the animals allowed to recover. Frogs were humanely killed after the final collection. All experimental procedures using X. laevis were approved by the Regierungspraesidium Tuebingen, Germany. Oocytes were defolliculated using collagenase (2 mg ml−1 of type CLS III collagenase, Biochrom KG, Berlin, Germany) in OR-2 solution (in mM: 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.4) and stored at 18°C in frog Ringer solution (in mM: 115 NaCl, 2.5 KCl, 1.8 CaCl2 and 10 HEPES, pH 7.4) supplemented with 1% of fetal calf serum and 50 μg ml−1 gentamicin (Biochrom KG, Berlin, Germany). Diluted WT or mutant cRNA of Na+ channel α-subunits was injected, together with Na+ channel β1-subunit cRNA, in an estimated 1 : 10 molar ratio. Prior to macropatch recordings, the vitelline layer of each oocyte was removed with fine forceps.
Electrophysiology
Sodium currents from mammalian cells and Xenopus oocytes were recorded at room temperature 21–23°C using an EPC-7 patch clamp amplifier (List Electronics, Darmstadt, Germany), a Digidata 1200 digitizer and pClamp 6 data acquisition software (Axon Instruments, Union City, CA, U.S.A.). Leakage and capacitive currents were automatically subtracted using a prepulse protocol (-P/4). Currents were filtered at 3 and digitized at 20 kHz.
Most experiments were conducted in tsA201 cells using the whole-cell patch-clamp technique as described in Popa et al. (2004). Inside-out macropatches from Xenopus oocytes were used when it was necessary to apply several different solutions on the intracellular side of the membrane in a single experiment. Sodium currents of 0.3–7 nA were recorded from such inside-out patches using large, low-resistance (<0.8 MΩ) electrodes. These recordings were performed in a small chamber with a volume of 150 μl, which could be exchanged completely in less than 30 s.
The membrane was depolarized to various test potentials from a holding potential of −140 mV for whole-cell recordings and −120 mV for recordings from inside-out patches. The use-dependent inhibition was measured by applying trains of 25-ms depolarizations at a frequency of 0.1 Hz. Recordings were performed 10 min after establishing the whole-cell configuration to ensure stable conditions not only for channel block but also for voltage-dependent channel gating. To monitor use-dependent unbinding, the depolarizations were applied for 50 ms at 0.5 Hz. The voltage-dependent block and unblock could be repetitively induced for up to 1 h, without any changes in kinetics or steady-state levels.
Solutions
For whole-cell recordings, the pipette solution contained (in mM): 105 CsF, 35 NaCl, 10 EGTA, 10 HEPES, pH 7.4. The bathing solution contained (in mM): 150 NaCl, 2 KCl, 1.5 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4. For the measurements in reduced Na+ gradient across the membrane, we replaced half of the external NaCl by 75 mM KCl. Reversed solutions were used for inside-out patches from oocytes.
A CuPhen stock solution was prepared by dissolving Cu(II)SO4 and 1,10-phenanthroline in a 4 : 1 water/ethanol solution to concentrations of 150 and 500 mM, respectively (Careaga & Falke, 1992). For whole-cell recordings, this stock solution was diluted 1 : 1000 (or 1 : 100 for WT) to the pipette solution at the time of use. For recordings from inside-out macropatches, a final concentration of 7.5 μM CuPhen was used. The phenanthroline solution was prepared similarly by dissolving 1,10-phenanthroline in a 4 : 1 water/ethanol solution (500 mM).
Data analysis
All data were analyzed using a combination of pClamp, Excel (Microsoft, Redmond, MA, U.S.A.) and ORIGIN software (Microcal Software, Northampton, MA, U.S.A.). For statistic evaluation, Student's t-test was applied. All data are shown as means±s.e.m., unless otherwise indicated.
Results
Use-dependent block of I1160C/L1482C mutant hNav1.4 channels by CuPhen
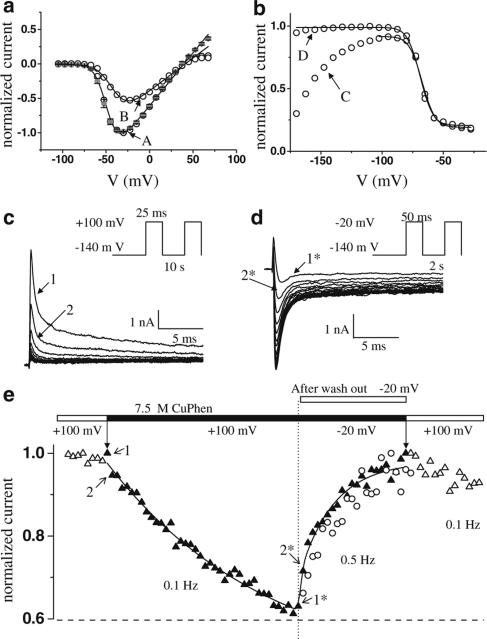
To assess the proximity of the cysteines introduced at positions 1160 and 1482 in the D3/and D4/S4–S5 loops, we applied 0.15 mM of CuPhen intracellularly in whole-cell recordings from tsA201 cells transfected with cDNA encoding the mutant channel. Current amplitudes recorded using a classical current–voltage (I–V) protocol saturated at highly positive voltages (Figure 1a, A). During a subsequent I–V protocol, the whole cell peak current amplitudes were much smaller for all depolarizing steps when compared with the amplitudes from previous protocol (Figure 1a, B), indicating that some of the channels were nonconducting in the later protocols. While this might have been an effect of a newly formed disulfide bridge between the introduced cysteines, we observed a very unusual increase of the peak current during the next voltage-clamp protocol used to record steady-state inactivation (Figure 1b, C): upon repetitive depolarizing pulses to −20 mV from different conditioning potentials, the channels recovered completely from the inhibition of the current amplitude (Figure 1b, D). We then tested the effect of repetitive depolarizations to +100 mV, which resulted in a continuous current decrease that could be completely reversed by consecutive depolarizations to −20 mV (Figure 1c and d). Since the reduction of a disulfide bridge requires the presence of excess thiols, which were not present in our solutions, the reversibility of current inhibition excludes the possibility of a disulfide bridge between I1160C and L1482C causing the inhibition of the channels, as was seen in previous work from other groups with cysteines in different ion channel regions (Bénitah et al., 1997; 1999; Neale et al., 2003; Xiong et al., 2003). In contrast, our results indicate a reversible channel block by CuPhen. There are two possible alternatives to the hypothesis that a disulfide bond is the unique cause of the current reduction: CuPhen could change the gating of the mutant channel by oxidation of the two cysteines (without disulfide bridging), or CuPhen could bind to a site on the channel to block its activity. If the inhibition due to CuPhen application was due to oxidation of cysteines changing the properties of the channels, then the block should persist after washing out the catalyst or be induced in the presence of another oxidizing reagent.
Figure 1.
Inhibition of the current and recovery from block in the presence of 0.15 mM CuPhen for the I1160C/L1482C mutant channel. (a) Two consecutive I–V's (current–voltage relationship) in the presence of CuPhen – open symbols, obtained by normalizing the peak currents during step depolarizations between –105 and 67.5 mV in steps of 7.5 mV from a holding potential of −140 mV to the maximum peak current recorded during the first I–V. The I–V in the absence of CuPhen is also represented for comparison (gray symbols, n=9). The inhibition was first noticed as a current saturation at voltages more positive than 40 mV (A), and then as a decrease of peak currents over the whole voltage range (B). (b) After recording the I–V's, two consecutive recordings of voltage-dependent channel availability (steady-state inactivation curve) were made. The increase in current during the first protocol (C) reflects recovery from inhibition. The currents were normalized to the maximum peak current during the second protocol (D). (c) and (d) show the development of and recovery from channel block, respectively, in response to repetitive depolarizations. (e) CuPhen block of the I1160C/L1482C mutant channel recorded in inside-out patches from X. laevis oocytes that had been injected with the cRNA of the mutant channel. The current did not decrease with consecutive depolarizations before application, nor after wash-out (open symbols), but only in the presence of CuPhen (filled symbols). The recovery from block developed similarly in the absence (open circles) or in the presence (filled triangles) of CuPhen. The values plotted are normalized peak currents recorded during consecutive depolarizations from a holding potential of −120 mV, with the frequency and at the voltages indicated in (e).
To differentiate between a direct channel block by CuPhen and an indirect effect secondary to the oxidation, we expressed the I1160C/L1482C mutant channel in Xenopus oocytes and performed experiments with inside-out macropatches, which allowed the exchange of solutions on the intracellular side of the membrane. After wash-in of CuPhen, we observed a small tonic block in the resting state of the channel (not shown). As in the whole-cell experiments, a use-dependent block developed with repetitive depolarizing pulses to +100 mV; however, 20-fold lower concentrations were needed in inside-out patches to obtain effects similar to those seen in whole-cell recordings. Figure 1e shows for one macropatch the phasic recovery after current inhibition, both in the presence of and after washing out CuPhen. The block could not be established after wash-out (Figure 1e), or after applying another oxidizing agent, H2O2 (0.3%, not shown). In addition, the block could still be induced when macropatches were exposed for up to 40 min to both CuPhen and the sulfhydryl-reducing agent 1,4-dithiothreitol (DTT), applied in a ratio of 1 : 33 (not shown). Taken together, these results indicate that current inhibition is due to a direct action of CuPhen and is not a consequence of oxidation.
Voltage dependence of channel block by CuPhen
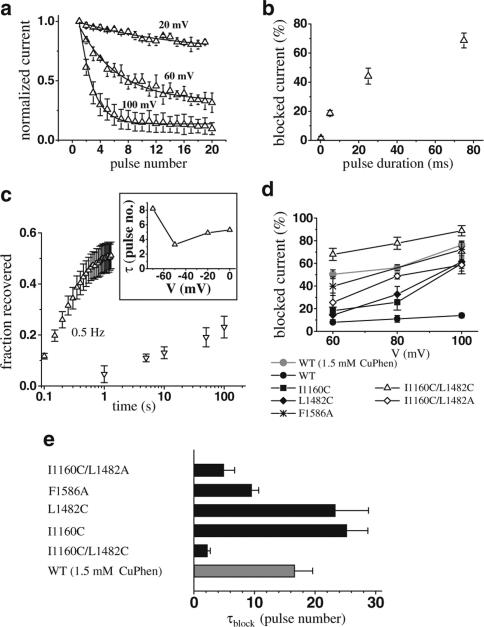
To identify the voltage dependence of the development of and recovery from inhibition, we applied trains of depolarizing pulses to different voltages from a holding potential of −140 mV. The block developed at voltages more positive than +20 mV in a use-dependent manner, and was strongly voltage-dependent (Figure 2a and d). Furthermore, the inhibition was positively correlated with the duration of the depolarizing pulse (Figure 2b); however, a train of short pulses, which depolarized the membrane for a time equal to the duration of one long pulse, was more effective (one 25-ms pulse: −38.7±5.3% inhibition, five 5-ms pulses −60.5±3.4% inhibition, P<0.02). The double mutant exhibits an impaired inactivation, with a persistent Na+ current of 24±2% of peak current remaining 70 ms after onset of the depolarization. This persistent current could explain the positive correlation of current inhibition with pulse duration via an open-channel block by CuPhen.
Figure 2.
Voltage dependence of the CuPhen block. (a) Peak current decrease during depolarizations applied at 0.1 Hz frequency, at indicated voltages. The lines represent fits of an exponential function to the data. Both rates and asymptotic levels are voltage-dependent. (b) The current block during a single depolarization to 100 mV increases with pulse duration. (c) Recovery from block developed rapidly with trains of depolarizations (protocol shown in Figure 1d) and in a voltage-dependent manner (inset, upward triangles, same protocol as in Figure 1d, but depolarizations to voltages as indicated in the inset), but very slowly when the membrane was kept at −20 mV (downward triangles). All mutations increased the steady-state levels of the block (d) and the association rate (e). For the WT channel, we used two different concentrations of CuPhen, 0.15 and 1.5 mM (gray symbols), whereas for all mutations we used 0.15 mM CuPhen. The values shown were obtained as described in (a), with one exception. Since there was only minimal block of WT channels with 0.15 mM, we were not able to fit consistently exponential functions to the peak current decay in this case. The data points for the WT at 0.15 mM therefore represent the relative reduction of peak current between the first and the 20th pulse to the voltage indicated on the abscissa, n=3–8.
Recovery from channel block developed rapidly with repetitive depolarizing pulses to potentials between −60 and 0 mV in a voltage-dependent manner (Figure 2c inset). No recovery was observed when cells were held at −140 mV for more than 10 min (not shown), indicating that the blocker might be trapped by closure of the activation gate. Consistent with this hypothesis, recovery from channel block was also highly use-dependent. Holding the cells at −20 mV for 100 s led to a very slow recovery of only 50% of the blocked channels, whereas complete recovery occurred upon repetitive short pulses (to −20 mV) at a frequency of 0.5 Hz after approximately 1 s (Figure 2c).
CuPhen block of WT channels and role of S4–S5 and S6 mutations
WT channels were only minimally blocked by application of 0.15 mM of CuPhen, but at 1.5 mM CuPhen induced a similar voltage-dependent current inhibition to that seen in the mutant channels at the lower concentration (Figure 2d). For the single mutations I1160C and L1482C, we observed a much weaker block with 0.15 mM CuPhen than seen in the double mutant. Figure 2 shows the voltage dependence of channel block (d) and the time constants for development of block at +100 mV (e). Another double mutation, which contains an alanine in position 1482 (I1160C/L1482A), was also blocked by CuPhen, although less effectively than the double cysteine mutation. We also investigated channel block for a mutation within the pore region (D4/S6) which has previously been shown to affect channel block by local anesthetics (LAs) and batrachotoxin (BTX) (Ragsdale et al., 1996; Linford et al., 1998). Interestingly, in contrast to the WT channel, this mutant channel also showed a significant block upon application of 0.15 mM CuPhen, indicating that the S6 segment could be involved in binding of CuPhen. The block developed with intermediate time constants between the double and the single cysteine mutants in S4–S5 (Figure 2e), while recovery was faster (not shown).
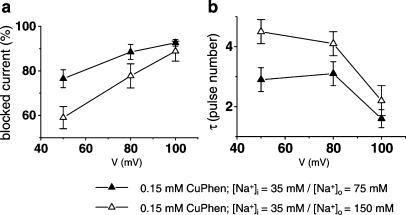
Dependence of CuPhen block on [Na+]o
The use dependence of both development of and recovery from channel block suggests that CuPhen is a pore blocker. To test this hypothesis further, we reduced [Na+]o from 150 to 75 mM and monitored the onset of current inhibition and asymptotic levels of the block for I1160C/L1482C channels. Since external Na+ ions should compete with a pore blocker for binding sites within the channel, we expected an increase in channel block by reducing [Na+]o, which could be clearly shown for both parameters investigated (Figure 3a and b). Upon strong depolarizations to +100 mV, these differences were no longer significant, probably because at this voltage external Na+ ions are driven out of the pore efficiently even at high [Na+]o, as has been also shown for tetraalkylammonium (TAA) block of Na+ channels (O'Leary et al., 1994).
Figure 3.
Dependence of channel block on [Na+]o. Increased asymptotic levels (a) and decreased time constants (b) of the block in conditions of reduced external Na+ concentration (from 150 to 75 mM). At 50 mV, the rate of channel block was 0.36±0.04 pulses−1 for [Na+]o=75 mM compared with 0.22±0.01 pulses−1 for [Na+]o=150 mM, P<0.05, and the asymptotic level of the block increased from 59±5 to 77±4% (P<0.05).
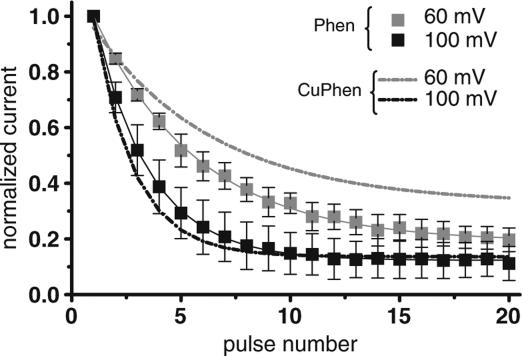
1,10 phenathroline-induced channel block in the absence of copper ions
Copper ions can accommodate three phenanthroline molecules to form Cu2+(Phen)3 complexes. We investigated the hypothesis that 1,10 phenanthroline is the relevant inhibitor of Na+ channels. Interestingly, we obtained very similar results when 0.5 mM of 1,10 phenanthroline was added to the pipette solution to those seen with 0.15 mM CuPhen for the mutant channel I1160C/L1482C (Figure 4). In addition, applying a CuPhen solution in which copper ions were added in excess resulted in a much weaker block, and CuSO4 did not induce channel inhibition (results not shown). These results indicate that 1,10 phenanthroline is the channel blocker.
Figure 4.
Phenanthroline blocks the I1160C/L1482C mutant channels in the absence of Cu2+ ions (squares). For comparison, the block in the presence of CuPhen is represented with dotted lines n=3–5.
Discussion
Our results revealed that CuPhen induces a use- and voltage-dependent inhibition of the hNav1.4 Na+ channel, which is enhanced by mutations in the D3/S4–S5, D4/S4–S5 and D4/S6 segments. Several aspects suggest that CuPhen is an open-channel pore blocker: (i) Repetitive pulses opening and closing the gates were necessary for both the development of and the recovery from channel block, suggesting in the latter case that CuPhen is trapped in the channel at hyperpolarized membrane potentials. (ii) Short pulses were more efficient in inhibiting the current of I1160C/L1482C mutant channels than a single pulse of equally long duration, and recovery occurred only very slowly during a prolonged depolarization, when the channels are mainly in the inactivated state. (iii) Our data revealed an enhancement of the CuPhen-induced block upon reduced [Na+]o, consistent with an antagonizing effect of Na+ ions in the pore (Wang, 1988). (iv) Current inhibition by CuPhen is reminiscent of the use-dependent block of voltage-gated Na+ channels by putative pore blockers such as some LAs, in particular by quaternary derivatives of lidocaine such as QX-314 (Strichartz, 1973). The neurotoxin BTX also exhibits both use-dependent binding and unbinding (Li et al., 2002). (v) It has been recently shown that CuPhen is an open-channel blocker of the nonselective cation channel, the vanilloid receptor TRPV1 (Tousova et al., 2004). This receptor exhibits a similar folding as voltage-gated ion channels, has a pore structure comparable to the Na+ channel and contains conserved amino acids in its S6 segments (Mohapatra et al., 2003; Ferrer-Montiel et al., 2004), including sites that are critical for LA and BTX binding (Linford et al., 1998; Wang et al., 2000). Finally, the S4–S5 loops harbor critical sites for the sensitivity of different Na+ channel isoforms to neurotoxins from the same class as BTX (Kimura et al., 2001). In our study, the block was also affected by the S4–S5 and S6 mutations investigated, including F1586A, which was predicted to be a receptor site for LAs and BTX (Ragsdale et al., 1996; Linford et al., 1998). The enhanced sensitivity of the mutant channels to CuPhen might result from the disruption of fast inactivation, the most prominent effect of the investigated mutants (Popa et al., 2004), as would be expected for an open-channel blocker. However, there was no clear correlation between the extent of CuPhen block and the degree of defective fast inactivation, as I1160C/L1482A has a much larger persistent current (Popa et al., 2004), but showed less intense channel inhibition compared to I1160C/L1482C. We therefore hypothesize that I1160C in D3/S4–S5 and the corresponding L1482C mutation in D4/S4–S5, especially when paired, and the F1586 mutation in D4/S6 allosterically affect the binding site of CuPhen (or the access to it), which could be located within the pore region and is guarded by the activation gate.
In addition, we found that 1,10 phenanthroline (Phen) induces a use- and voltage-dependent block in the absence of copper ions. Phen is a weak Lewis base. Its pK indicates that less than 0.5% will be positively charged at a pH of 7.4. One possibility could be that charged Phen is a potent inhibitor of the Na+ channel and electrostatic interactions are critical for binding. The guarded receptor model (Starmer et al., 1984) and binding within the pore at increasing electrical distances (Woodhull, 1973) could then elegantly explain the voltage dependence of the block. Alternatively, the neutral Phen molecules might induce the block. Due to their large permanent dipole moment of 4.11 D (Nishigaki et al., 1978), the Phen molecules align with the electric field across the membrane. The strength and occurrence of hydrogen bonds that could be formed between Phen and a putative receptor site within the pore region are critically determined by the orientation of the molecules involved, so that the local membrane electric field would directly act on binding of Phen. Such a scenario has been proposed for the neutral and small S-nitrosodithiothreitol, which has a similar mechanism of blocking Shaker K+ channels and competes with internal TEA (Brock et al., 2001).
Our study adds a new compound to the list of LAs, antiarrhythmic and anticonvulsant drugs known to inhibit voltage-gated Na+ channels in a use-dependent manner. Although its targets of action are key proteins in modulating electrical excitability or pain transduction, such as the voltage-gated Na+ channel (this study), or the vanilloid receptor 1 (Tousova et al., 2004), CuPhen and Phen most probably have no future in clinical applications, due to multiple other effects at the cellular level (Kobashi & Horecker, 1967; Watanabe et al., 2003). However, awareness of the blocking capability of CuPhen becomes critical when it is used as an oxidation catalyst to study Na+ channel biophysics.
Acknowledgments
We thank Dr Stephanie Schorge for her helpful comments and language editing. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG, Le1030/5-2, Le1030/9-1). HL is a Heisenberg fellow of the DFG.
Abbreviations
- BTX
batrachotoxin
- CuPhen
Cu2+(phenanthroline)3
- hNav1.4
human skeletal muscle Na+ channel
- LAs
local anesthetics
References
- ALEKOV A.K., PETER W., MITROVIC N., LEHMANN-HORN F., LERCHE H. Two mutations in the IV/S4–S5 segment of the human skeletal muscle Na+ channel disrupt fast and enhance slow inactivation. Neurosci. Lett. 2001;306:173–176. doi: 10.1016/s0304-3940(01)01895-x. [DOI] [PubMed] [Google Scholar]
- AZIZ Q.H., PARTRIDGE C.J., MUNSEY T.S., SIVAPRASADARAO A. Depolarization induces intersubunit cross-linking in a S4 cysteine mutant of the Shaker potassium channel. J. Biol. Chem. 2002;277:42719–42725. doi: 10.1074/jbc.M207258200. [DOI] [PubMed] [Google Scholar]
- BÉNITAH J.P., CHEN Z., BALSER J.R., TOMASELLI G.F., MARBAN E. Molecular dynamics of the sodium channel pore vary with gating: interactions between P-segment motions and inactivation. J. Neurosci. 1999;19:1577–1585. doi: 10.1523/JNEUROSCI.19-05-01577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÉNITAH J.P., RANJAN R., YAMAGISHI T., JANECKI M., TOMASELLI G.F., MARBAN E. Molecular motions within the pore of voltage-dependent sodium channels. Biophys. J. 1997;73:603–613. doi: 10.1016/S0006-3495(97)78096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK M.W., MATHES C., GILLY W.F. Selective open-channel block of Shaker (Kv1) potassium channels by S-nitrosodithiothreitol (SNDTT) J. Gen. Physiol. 2001;118:113–133. doi: 10.1085/jgp.118.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREAGA C.L., FALKE J.J. Thermal motions of surface alpha-helices in the D-galactose chemosensory receptor. Detection by disulfide trapping. J. Mol. Biol. 1992;226:1219–1235. doi: 10.1016/0022-2836(92)91063-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATTERALL W.A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- FERRER-MONTIEL A., GARCIA-MARTINEZ C., MORENILLA-PALAO C., GARCIA-SANZ N., FERNANDEZ-CARVAJAL A., FERNANDEZ-BALLESTER G., PLANNELS-CASES R. Molecular architecture of the vanilloid receptor. Insights for drug design. Eur. J. Biochem. 2004;271:1820–1826. doi: 10.1111/j.1432-1033.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- HORENSTEIN J., WAGNER D.A., CZAJKOWSKI C., AKABAS M.H. Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nat. Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- IVANOV A., ZHAO H., MODYANOV N.N. Packing of the transmembrane helices of Na,K-ATPase: direct contact between β subunit and H8 segment of α subunit revealed by oxidative cross-linking. Biochemistry. 2000;39:9778–9785. doi: 10.1021/bi001004j. [DOI] [PubMed] [Google Scholar]
- KIMURA T., YAMAOKA K., KINOSHITA E., MAEJIMA H., YUKI T., YAKEHIRO M., SEYAMA I. Novel site on sodium channel α-subunit responsible for the differential sensitivity of grayanotoxin in skeletal and cardiac muscle. Mol. Pharmacol. 2001;60:865–872. [PubMed] [Google Scholar]
- KOBASHI K. Catalytic oxidation of sulfhydryl groups by o-phenanthroline copper complex. Biochim. Biophys. Acta. 1968;158:239–245. doi: 10.1016/0304-4165(68)90136-0. [DOI] [PubMed] [Google Scholar]
- KOBASHI K., HORECKER B.L. Reversible inactivation of rabbit muscle aldolase by o-phenanthroline. Arch. Biochem. Biophys. 1967;121:178–186. doi: 10.1016/0003-9861(67)90022-7. [DOI] [PubMed] [Google Scholar]
- LERCHE H., PETER W., FLEISCHHAUER R., PIKA-HARTLAUB U., MALINA T., MITROVIC N., LEHMANN-HORN F. Role in fast inactivation of the IV/S4–S5 loop of the human muscle Na+ channel probed by cysteine mutagenesis. J. Physiol. 1997;505:345–352. doi: 10.1111/j.1469-7793.1997.345bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI H.-L., HADID D., RAGSDALE D.S. The batrachotoxin receptor on the voltage-gated sodium channel is guarded by the channel activation gate. Mol. Pharmacol. 2002;61:905–912. doi: 10.1124/mol.61.4.905. [DOI] [PubMed] [Google Scholar]
- LINFORD N.J., CANTRELL A.R., QU Y., SCHEUER T., CATTERALL W.A. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13947–13952. doi: 10.1073/pnas.95.23.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILBURN M.V., PRIVE G.G., MILLIGAN D.L., SCOTT W.G., YEH J., JANCARIK J., KOSHLAND D.E., JR, KIM S.H. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991;254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- MOHAPATRA D.P., WANG S.Y., WANG G.K., NAU C. A tyrosine residue in TM6 of the vanilloid receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol. Cell. Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- NEALE E.J., ELLIOTT D.J., HUNTER M., SIVAPRASADARAO A. Evidence for intersubunit interactions between S4 and S5 transmembrane segments of the Shaker potassium channel. J. Biol. Chem. 2003;278:29079–29085. doi: 10.1074/jbc.M301991200/6493. [DOI] [PubMed] [Google Scholar]
- NISHIGAKI S., YOSHIOKA H., NAKATSU K. The crystal and molecular structure of o-phenanthroline. Acta Crystallogr. 1978;34:875–879. [Google Scholar]
- O'LEARY M.E., KALLEN R.G., HORN R. Evidence for a direct interaction between internal tetra-alkylammonium cations and the inactivation gate of cardiac sodium channels. J. Gen. Physiol. 1994;104:523–539. doi: 10.1085/jgp.104.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPA M.O., ALEKOV A.K., BAIL S., LEHMANN-HORN F., LERCHE H. Cooperative effect of S4–S5 loops in domains D3 and D4 on fast inactivation of the Na+ channel. J. Physiol. 2004;561:39–51. doi: 10.1113/jphysiol.2004.065912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGSDALE D.S., MCPHEE J.C., SCHEUER T., CATTERALL W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT W.G., MILLIGAN D.L., MILBURN M.V., PRIVE G.G., YEH J., KOSHLAND D.E., JR, KIM S.H. Refined structures of the ligand-binding domain of the aspartate receptor from Salmonella typhimurium. J. Mol. Biol. 1993;232:555–573. doi: 10.1006/jmbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- STARMER C.F., GRANT A.O., STRAUSS H.C. Mechanism of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys. J. 1984;46:15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRICHARTZ G.R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J. Gen. Physiol. 1973;62:37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOUSOVA K., SUSANKOVA K., TEISINGER J., VYKLICKY L., VLACHOVA V. Oxidizing reagent copper-o-phenanthroline is an open channel blocker of the vanilloid receptor TRPV1. Neuropharmacology. 2004;47:273–285. doi: 10.1016/j.neuropharm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- WANG G.K. Cocaine-induced closures of single batrachotoxin-activated Na+ channels in planar lipid bilayers. J. Gen. Physiol. 1988;92:747–765. doi: 10.1085/jgp.92.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S.-Y., NAU C., WANG G.K. Residues in Na+ channel D3–S6 segment modulate both batrachotoxin and local anesthetics affinities. Biophys. J. 2000;79:1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE K., TOKUMOTO T., ISHIKAWA K. 1,10-Phenanthroline phosphorylates (activates) MAP kinase in Xenopus oocytes. Cell Signal. 2003;15:1139–1147. doi: 10.1016/s0898-6568(03)00116-5. [DOI] [PubMed] [Google Scholar]
- WOODHULL A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J., KABACK H.R. A general method for determining helix packing in membrane proteins in situ: helices I and II are close to helix VII in the lactose permease of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14498–14502. doi: 10.1073/pnas.93.25.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG W., LI R.A., TIAN Y., TOMASELLI G.F. Molecular motions of the outer ring of charge of the sodium channel: do they couple to slow inactivation. J. Gen. Physiol. 2003;122:323–332. doi: 10.1085/jgp.200308881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU H., KONO M., MCFEE T.D., OPRIAN D.D. A general method for mapping tertiary contacts between aminoacids residues in membrane-embedded proteins. Biochemistry. 1995;34:14963–14969. doi: 10.1021/bi00046a002. [DOI] [PubMed] [Google Scholar]
- ZENG F.-Y., HOPP A., SOLDNER A., WESS J. Use of disulfide cross-linking strategy to study muscarinic receptor structure and mechanism of activation. J. Biol. Chem. 1999;274:16629–16640. doi: 10.1074/jbc.274.23.16629. [DOI] [PubMed] [Google Scholar]