Abstract
An attractive alternative to the use of direct agonists at the cannabinoid receptor type 1 (CB1) in the control of neuropathic pain may be to potentiate the actions of endogenous cannabinoids. Thus, the effects of AM404, an inhibitor of anandamide uptake, were assessed in an experimental model of neuropathic pain in rats.
Daily treatment with AM404 prevented, time- and dose-dependently, the development of thermal hyperalgesia and mechanical allodynia in neuropathic rats. Antagonists at cannabinoid CB1 or CB2 receptors, or at the transient receptor potential vanilloid type 1 receptor, each partially reversed effects induced by AM404. A complete reversal was obtained when the three antagonists were given together, suggesting that all three receptors are involved.
AM404 treatment affected two pathways involved in the generation and maintenance of neuropathic pain, one mediated by nitric oxide (NO) and the other by cytokines. AM404 completely prevented the overproduction of NO and the overexpression of nNOS, inhibited the increase in tumour necrosis factor α (TNFα) and enhanced the production of interleukin-10. Both NO and TNFα are known to contribute to the apoptotic process, which plays an important role in the establishment of chronic pain states. AM404 treatment prevented the increase in the ratio between pro- and anti-apoptotic gene bax/bcl-2 expression observed in the spinal cord of neuropathic rats.
Taken together, these findings suggest that inhibition of endocannabinoid uptake, by blocking the putative anandamide carrier, results in the relief of neuropathic pain and may represent a novel strategy for treating chronic pain.
Keywords: Cannabinoid, endocannabinoid, neuropathic pain, AM404, VDM11, cytokines, nitric oxide, apoptosis, TNFα, IL-10
Introduction
There is increasing evidence that cannabinoids are effective in attenuating neuropathic pain. Antihyperalgesic and antiallodynic effects of cannabinoids have been demonstrated in several models of experimental neuropathy (Herzberg et al., 1997; Bridges et al., 2001; Fox et al., 2001; Costa et al., 2004a). Cannabinoid receptors type 1 (CB1) are strategically located along the nociceptive pathways and the activation of peripheral, spinal and supraspinal CB1 receptors has been shown to reduce nociception. Activation of cannabinoid receptors type 2 (CB2) inhibits nociception induced by a variety of acute and chronic stimuli, decreasing the activity of primary afferent neurones through the inhibition of the release of sensitizing substances (e.g. histamine, serotonin, prostaglandins) from neighbouring mast and immune cells (Malan et al., 2001). In spite of the potential antinociceptive activity of cannabinoids, the therapeutic utility of CB1 receptor agonists is limited by the risk of psychotropic side effects and that of CB2 receptor agonists is restricted by immunosuppressive effects. A valid alternative strategy for pain relief may be the modulation of the endocannabinoid system by potentiating its actions through the inhibition of endocannabinoid (particularly anandamide) inactivation. The use of so-called ‘indirect agonists' may make it possible to minimize the adverse side effects produced by the direct activation of cannabinoid receptors.
Therefore, the first aim of the present study was to verify whether pharmacological modulation of anandamide tone by treatment with AM404 (N-(4-hydroxyphenyl)-5Z,8Z,11Z,14Z-eicosatetraenamide), an inhibitor of endocannabinoid transport, known to enhance anandamide-induced antinociceptive effects in vivo (Beltramo et al., 1997), was able to inhibit the development and maintenance of a model of neuropathic pain, chronic constriction injury (CCI) of the sciatic nerve, in rats. As anandamide is a potent CB1 agonist and also activates CB2 and vanilloid transient receptor potential vanilloid type 1 (TRPV1) receptors, and AM404 behaves as a full agonist at TRPV1 receptors, selective CB1, CB2 and TRPV1 receptor antagonists were used in order to clarify the contribution of each receptor class to the observed effects of AM404.
Cytokines, such as tumour necrosis factor α (TNFα) or interleukin-10 (IL-10), appear to be important for the induction of pain behaviour after nerve injury, producing algogenic (Perkins et al., 1995) and antihyperalgesic (Wagner et al., 1998) effects, respectively. Nitric oxide (NO) is also involved in the transmission and modulation of nociceptive information at the periphery, spinal cord and supraspinal level. In fact, systemic administration of NO synthase (NOS) inhibitors may relieve chronic allodynia-like symptoms in rats with spinal cord injury (Hao & Xu, 1996).
Besides their pronociceptive properties, TNFα and NO are known to affect apoptotic pathways. High concentrations of NO or peroxynitrite induce apoptotic cell death in several cell types, including neurones (Heneka et al., 1998), through the activation of caspase signalling and ceramide generation. Furthermore, it is well established that TNFα is capable of inducing apoptosis, acting in parallel with ‘professional' death ligands such as FasL and Apo2L/TRAIL (for a review see Varfolomeev & Ashkenazi, 2004). Even though the precise biological role of this activity is unclear, TNFα's apoptotic capability contributes to its established pathological role in inflammatory and painful diseases. The initiation/maintenance of hyperalgesia in neuropathic pain is sustained by apoptotic death in the dorsal horn of the spinal cord (Whiteside & Munglani, 2001; de Novellis et al., 2004; Scholz et al., 2005), probably causing vacant synapses that may induce sprouting of neuronal processes leading to hyperalgesia. A further aim of the present work was thus to find out if daily treatment with AM404 was accompanied by a modulation of NO and cytokine pathways and with a reduction in the overexpression of proapoptotic genes in CCI animals.
Methods
Animals and surgical procedure
All experiments performed were in accordance with Italian State and European regulations governing the care and treatment of laboratory animals (permission no 101/2004B), and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain (Zimmermann, 1983). Painful neuropathy was induced in male Wistar rats weighing 200–220 g (Harlan, Italy). Animals were anaesthetized with sodium pentobarbital (60 mg kg−1 i.p.) and neuropathic pain induced as described by Bennett & Xie (1988). Briefly, the common sciatic nerve was exposed at the level of the mid thigh and, proximal to the trifurcation of the sciatic nerve, four ligatures were loosely tied around it at about 1 mm spacing so that the epineural circulation was preserved. Sham animals (sciatic exposure without ligation) were used as controls.
Drugs and treatment protocols
AM404 was dissolved in a drop of Tween80, diluted in dimethylsulphoxide (DMSO) (10% in saline) and used at doses of 3, 5 and 10 mg kg−1. VDM11 was dissolved in ethanol : cremophor : saline (1 : 1 : 18) and used at 10 mg kg−1. Neuropathic rats were given the compound or its vehicle by subcutaneous (s.c.) injection, once a day for 7 days, starting from the day after the surgical procedure. In one group of rats, treatment was extended to 14 days.
To study the involvement of cannabinoid and/or vanilloid receptors in the AM404-induced effect, the ability of specific CB1, CB2 and TRPV1 antagonists to reverse the antihyperalgesic and antiallodynic effect of AM404 was tested. On the last day of AM404 administration (7 days after the injury), the cannabinoid CB1 receptor specific antagonist SR141716 (0.5 mg kg−1 i.p.), the cannabinoid CB2 receptor selective antagonist SR144528 (1 mg kg−1 i.p.) or the vanilloid TRPV1 specific antagonist capsazepine (10 mg kg−1 i.p.) were given together with AM404 (10 mg kg−1 s.c.) or its vehicle. Another group of CCI rats received concomitantly the three antagonists and AM404. Sham animals received drug vehicles.
SR141716 and SR144528 were dissolved in a mixture of Tween80 : DMSO : distilled water (1 : 2 : 7). Capsazepine was dissolved in a 1 : 1 : 8 mixture of ethanol:Tween80:saline. The doses of SR141716 and SR144528 used here were based on previous work in rodents (Rinaldi-Carmona et al., 1998; Carta et al., 1999). The dose of capsazepine used was shown by Di Marzo et al. (2001) to antagonize the effects induced by the selective agonist of TRPV1 receptor, capsaicin, in rats and by ourselves (Costa et al., 2004b) to reverse the antihyperalgesic effect of cannabidiol in carrageenan-induced acute inflammation in rats.
Thermal hyperalgesia and mechanical allodynia
Responses to thermal and mechanical stimuli were measured before and 3, 7 and 14 days (90 min after the last treatment with AM404 or other compounds) after the surgical procedure. In the antagonism studies, nociception and other behaviour was tested on day 7 (90 and 180 min after the administration of compounds). Heat hypersensitivity was tested according to the Hargreaves procedure (Hargreaves et al., 1988) using the plantar test (Ugo Basile, Varese, Italy). Briefly, animals were placed in a clear plexiglass box and allowed to acclimatize. A constant intensity radiant heat source was aimed at the midplantar area of the hind paw. The time, in seconds, from initial heat source activation until paw withdrawal was recorded. Mechanical allodynia was assessed using the Dynamic Plantar Aesthesiometer (Ugo Basile, Varese, Italy). Animals were placed in a test cage with a wire mesh floor, and the tip of a von Frey-type filament was applied to the middle of the plantar surface of the hind paw. The filament exerted an increasing force starting below the threshold of detection and increasing until the animal removed its paw. Withdrawal threshold was expressed as threshold level in g.
Locomotor activity
Spontaneous motor activity was evaluated in a separate group of CCI and sham rats with an activity cage (Ugo Basile, Varese, Italy), placed in a sound-attenuated room. The cage was fitted with horizontal infrared beams, 2 cm from the floor, which were broken as the animal moved about the cage. Each break gave rise to a ‘horizontal count' and these were cumulatively recorded for 10 min. Activity was measured 90 min after AM404 administration.
Biochemical evaluations
The biochemical evaluations were performed on animals receiving the highest dose of AM404 (10 mg kg−1). At 7 days following surgery, 90 min after the last dose of AM404, nociceptive thresholds were recorded and animals were killed. Hind limbs were severed at the level of the calcaneous bone, weighed, crushed at 0–4°C and used for determination of nitrite/nitrate production. The ipsilateral L4, L5 and L6 dorsal root ganglia (DRG) were removed, frozen in liquid nitrogen and stored at –80°C until the determination of TNFα and IL-10 content. Sciatic nerves proximal to the ligature were removed (at least 1 cm). Part of each sample was immediately frozen in liquid nitrogen for later assay of the neuronal isoform of NOS (nNOS) and part was used to prepare nuclear extracts which were stored at −80°C until the transcription factor NF-κB was assayed. Lumbar spinal cord at L4–L5 was excised to measure pro- and antiapoptotic gene expression.
Cytokine assay
The ipsilateral L4, L5 and L6 DRG were homogenized in phosphate-buffered saline (PBS), pH 7.4, containing a mix of protease inhibitors, in a ratio of 20 μl of PBS mg−1 DRG, using an ultrasonic homogenizer (Branson Sonifier®W-250). The homogenates were centrifuged at 1500 × g, at 4°C for 10 min, and the supernatant was immediately used for the assay. The concentration of TNFα and of IL-10 was measured with commercially available sandwich enzyme-linked immunosorbent assay (ELISA), according to the procedures recommended by the manufacturer. Briefly, samples, including standards of known rat cytokine, were added into the wells of microtitre strips coated with an antibody specific for rat TNFα or IL-10, followed by the addition of a biotinylated second antibody. During the first incubation, the rat antigen binds simultaneously to the immobilized antibody on one site and to the solution phase biotinylated antibody on the second site. After removal of excess second antibody, streptavidin-peroxidase is added, which binds to the biotinylated antibody to complete the four-member sandwich. After a wash to remove the unbound enzyme, a substrate solution is added. The intensity of the coloured product (recorded at 450 nm with a spectrophotometer Multiskan® EX, ThermolabSystem) is directly proportional to the concentration of cytokine.
Assay for the transcription factor NF-κB
The transcription factor NF-κB was measured by an ELISA kit that detected NF-κB activation by a combination of NF-κB-specific oligonucleotide binding and subsequent detection of the p65 subunit of NF-κB with specific antibody. This method was used as an alternative to the electrophoretic mobility shift assay, as the ELISA has been reported to be more sensitive (Shen et al., 2002). Sciatic nerves were homogenized in 100 μl ice-cold hypotonic lysis buffer (supplied with the nuclear extract kit) mg−1 tissue. After centrifugation at 850 × g for 10 min, 500 μl of hypotonic buffer supplemented with 25 μl of Nonidet P-40 was added to the pellet and the mixture was centrifuged at 14,000 × g for 2 min at 4°C. Pellets were suspended in 50 μl of hypertonic lysis buffer and incubated with shaking for 30 min at 4°C. Samples were then centrifuged at 14,000 × g for 10 min at 4°C, and the supernatant containing nuclear extracts was stored at −80°C until use. Nuclear protein extracts (10 μg) were added onto the oligonucleotide-coated ELISA plate and then incubated for 1 h at room temperature. Primary antibody recognizing an epitope on p65, which is accessible only when NF-κB is activated and bound to its target DNA, was added to wells and incubated for 1 h. This is followed by the addition of a horseradish peroxidase (HRP)-conjugated secondary antibody and, after 1 h, the HRP substrate was added. The reaction was stopped after 5–10 min, and the absorbance was measured on a spectrophotometer (Multiskan® EX, ThermolabSystem) at 450 nm. Jurkat cell nuclear extracts were used as an activated NF-κB positive control. NF-κB wild-type and mutated consensus oligonucleotides were used in order to monitor the specificity of the assay: a wild-type oligonucleotide should compete with NF-κB for binding, while the mutated consensus oligonucleotide should have no effect on NF-κB binding.
Nitrite/nitrate assay
Nitric oxide production was assessed on the basis of nitrite/nitrate, which are the oxidation end-products of nitric oxide. Nitrite/nitrate concentrations were measured using the method of Misko et al. (1993) based on the acid-catalysed ring closure of 2,3-diaminonaphthalene with nitrite to form the highly fluorescent product 1-(H)-naphtotriazole. Paw tissue was homogenized with a T25, 18N Ultra-Turrax in a 1 : 4 (w v−1) Trizma HCl buffer (20 mM, pH 7.6) and centrifuged at 9000 × g for 10 min at 4°C. The supernatant was filtered through Microcon YM-10 (Millipore) (10,000 × g, 30 min, 4°C) to remove the proteins. The nitrite/nitrate content was then assessed fluorimetrically according the procedure of Misko et al. (1993), employing a FP-777 spectrofluorimeter (Jasco, Lecco, Italy), and calculated using a standard curve.
Determination of nNOS by Western blot analysis
Sciatic nerve was homogenized in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% sodium dodecylsulphate (SDS), 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.02% sodium azide, 1 mM phenylmethylsulphonyl fluoride, 10 μM leupeptin) and centrifuged at 100,000 × g at 4°C for 1 h. Cytosol was diluted in Laemli buffer (0.3 M Tris-HCl, pH 6.8, containing 10% SDS, 50% glycerol, 5% dithiothreitol and 0.05% bromophenol blue) to obtain 100 μg protein. The proteins were loaded onto a 7.5% SDS–polyacrylamide gel and then transferred onto a nitrocellulose membrane (Schleicher & Schuell, BAS 85) with the semidry method for 90 min at room temperature. The membrane was ‘blocked' with 5% non-fat dry milk in TBST (20 mM Tris base, pH 7.6, 137 mM NaCl and 0.1% Tween 20) at room temperature for 2 h and then incubated with primary polyclonal antibody directed against rat nNOS diluted 1 : 1000 in blocking solution, at 4°C overnight. After washing in TBST buffer, the blot was incubated with secondary antibody (anti-rabbit IgG, peroxidase linked F(ab')2 fragment, 1 : 1500 in 3% blocking solution) for 1 h at room temperature. After washing in TBST buffer, the blot was detected with an enhanced chemiluminescence detection kit. The blots were then incubated in a stripping buffer (67.5 mM Tris (pH 6.8), 2% SDS and 0.7% β-mercaptoethanol) overnight at 4°C and reprobed with a polyclonal anti-α-tubulin antibody (1 : 1000) as loading controls.
RNA extraction and RT–PCR
Total RNA was extracted from homogenized whole lumbar (L4–L5) spinal cord using an RNA Tri-Reagent according to the manufacturer's protocol. The extracted RNA was subjected to DNase I treatment at 37°C for 30 min. The total RNA concentration was determined by UV spectrophotometry. The mRNA levels of the genes under analysis were measured by RT–PCR amplification, as reported previously (de Novellis et al., 2004). Sequences for the rat mRNAs from GeneBank (DNASTAR INC., Madison, WI, U.S.A.) were used to design primer pairs for RT–PCRs (OLIGO 4.05 software, National Biosciences Inc., Plymouth, MN, U.S.A.). Each RT–PCR was repeated at least four times. A semiquantitative analysis of mRNA levels was carried out by the ‘GEL DOC 2000 UV SYSTEM'. The measured mRNA levels were normalized with respect to hypoxanthine-guanine phosphoribosyltransferase (HPRT), chosen as a housekeeping gene, and the gene expression values were expressed as arbitrary units (mean±s.e.m.). Amplification of genes of interest and HPRT were performed simultaneously.
Materials
AM404 and VDM11 were from Tocris Cookson, Bristol, U.K.; SR141716 and SR144528 were kindly supplied by Sanofi-Aventis, Montpellier, France. Capsazepine and the enhanced chemiluminescence detection kit were purchased from Sigma-Aldrich, Milano, Italy. Mixed protease inhibitors were supplied by Roche Diagnostics, Monza, Italy and the ELISA kit for IL-10 and TNFα by Biosource Int., Camarillo, CA, U.S.A. The nuclear extract kit and the ELISA for NF-κB were from Active Motif, Rixensart, Belgium. Antibody directed against rat nNOS was from Cayman Chemical, Ann Arbor, MI, U.S.A.; the anti-rabbit IgG, peroxidase linked F(ab')2 fragment from Amersham Pharmacia Biotech, Milano, Italy and the polyclonal anti-α-tubulin antibody from Sigma Aldrich, Milano, Italy. The RNA Tri-Reagent came from Molecular Research Center Inc., Cincinnati, OH, U.S.A. and the ‘GEL DOC 2000 UV SYSTEM' for mRNA assay from Bio-Rad, Hercules, CA, U.S.A.
Statistical analysis
The results were expressed as the mean±s.e.m. from groups of N animals as indicated in the text and legends. The data were analysed using one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test for multiple comparisons. Differences were considered significant at P<0.05. The correlation coefficient (r2) was calculated using GraphPAD Software (San Diego, CA, U.S.A.).
Results
Effect of AM404 on thermal hyperalgesia and mechanical allodynia
The effect on thermal hyperalgesia and mechanical allodynia of different doses of AM404 (3, 5 and 10 mg kg−1) administered s.c. once a day for 7 days to neuropathic rats is shown in Figure 1a and b, respectively. As expected, 7 days after the injury (CCI), rats developed a significant decrease in thermal withdrawal latency of the paw ipsilateral to the injury, as compared to sham-operated animals. After CCI, rats also developed mechanical allodynia to normally innocuous mechanical stimulation with a von Frey filament. Treatment of CCI rats with AM404 resulted in a dose-dependent relief of hypersensitivity (r2=0.8939, F=58.97, P<0.001 for thermal hyperalgesia and r2=0.7627, F=22.50, P<0.01 for mechanical allodynia), with a maximum effect elicited by 10 mg kg−1 of compound, a dose that completely prevented both thermal hyperalgesia and mechanical allodynia (Figure 1a and b).
Figure 1.
Effect of different doses of AM404 given daily to neuropathic (CCI) rats, for 1 week from the day after the surgery, on thermal hyperalgesia (a) and mechanical allodynia (b). Time course of the effect of AM404 at the highest dose (10 mg kg−1) on thermal hyperalgesia (c) and mechanical allodynia (d) in CCI rats. Withdrawal latency to heat and mechanical threshold of the injured paws are expressed as s and g, respectively, and data represent mean±s.e.m. of 8–10 rats. ***P<0.001, **P<0.01 vs sham/vehicle animals; °°°P<0.001, °°P<0.01, °P<0.05 vs CCI/vehicle animals.
Changes in nociception and the effects of AM404 (10 mg kg−1) were time-dependent, as shown in Figure 1c and d. Both thermal and mechanical hypersensitivity were fully developed by 3 days, and this level was maintained for 14 days, after the injury. Treatment with AM404 partially relieved only thermal hyperalgesia in CCI rats after three doses, but abolished both thermal hyperalgesia and mechanical allodynia after 1 week of treatment. Because chronic treatment with AM404 might cause tolerance, some animals received this dose for a further week. As shown in Figure 1c and d, both the thermal withdrawal latency and mechanical withdrawal threshold of AM404-treated rats after 14 days were still at the same levels as after 7 days of treatment and completely overlapped those of sham animals. The same prolonged treatment did not affect the response to thermal and mechanical stimuli either of the paw contralateral to the injury or of sham animals (data not shown). The effects of a single administration of AM404, at 10 mg kg−1, were assessed in CCI rats, treated with vehicle for 7 days and then given AM404, 90 min before the nociceptive evaluation. The single dose of the compound did not alter either thermal nociceptive withdrawal latency (5.90±0.29 s for CCI vs 6.15±0.29 s for CCI+acute AM404) or withdrawal threshold to mechanical stimulus (18.62±0.89 g for CCI vs 18.44±1.29 g for CCI+acute AM404; n=5).
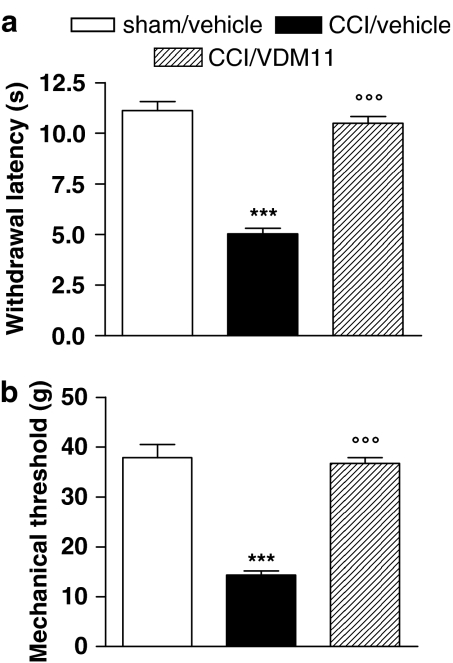
Effect of VDM11 on thermal hyperalgesia and mechanical allodynia
VDM11, a structural analogue of AM404, shows the same potency in inhibiting endocannabinoid cellular transport, but is devoid of effects on fatty acid amide hydrolase (FAAH), TRPV1 and cannabinoid receptors (De Petrocellis et al., 2000). Its effects on thermal hyperalgesia and mechanical allodynia in our model are shown in Figure 2. After seven daily doses of VDM11 (10 mg kg−1), both thermal hyperalgesia and mechanical allodynia were abolished (Figure 2a and b, respectively).
Figure 2.
Effect of VDM11 (10 mg kg−1) given daily to neuropathic (CCI) rats for 1 week from the day after the surgery on thermal hyperalgesia (a) and mechanical allodynia (b). Withdrawal latency to heat and mechanical threshold of the injured paws are expressed as s and g, respectively, and data represent mean±s.e.m. of five rats. ***P<0.001 vs sham/vehicle animals; °°°P<0.001 vs CCI/vehicle animals.
Effect of CB1, CB2 and TRPV1 receptor antagonists on AM404-induced effect
The ability of specific CB1, CB2 and TRPV1 antagonists to reverse the antihyperalgesic and antiallodynic effects of AM404 was tested on the last day of AM404 administration (day 7) and the results are shown in Figures 3 and 4, respectively. SR141716 (0.5 mg kg−1 i.p.) on its own did not modify the withdrawal threshold of the ipsilateral paw in CCI rats. When administered with AM404, this antagonist partially reversed the AM404-induced effects (Figures 3a and 4a). The cannabinoid CB2 receptor specific antagonist, SR144528, given alone, also did not affect the nociceptive hypersensitivity of CCI animals, whereas it was able to counteract partially the antihyperalgesic and antiallodynic effect of AM404 (Figures 3b and 4b). The TRPV1 receptor antagonist, capsazepine (10 mg kg−1 i.p.), when administered alone to CCI rats, did not affect their nociceptive response (Figures 3c and 4c). However, capsazepine given with AM404 (10 mg kg−1 s.c.) did partially reverse the antihyperalgesic and antiallodynic action of AM404 (Figures 3c and 4c). Figures 3d and 4d show that a combination of all three antagonists completely reversed the antihyperalgesic and antiallodynic properties of AM404, without altering the hypersensitivity of the model.
Figure 3.
Effect of SR141716 (0.5 mg kg−1 i.p.) (a), SR144528 (1 mg kg−1 i.p.) (b) and capsazepine (10 mg kg−1 i.p.) (c) given separately, and of all three antagonists given together (d) on AM404 (10 mg kg−1)-induced antihyperalgesia in neuropathic (CCI) rats, 180 min after the last AM404 administration (on day 7). Withdrawal latency to heat of the injured paws is expressed as s and data represent mean±s.e.m. of 8–10 rats. ***P<0.001, **P<0.01 vs sham/vehicle animals; °°°P<0.001, °°P<0.01, °P<0.05 vs CCI/vehicle animals.
Figure 4.
Effect of SR141716 (0.5 mg kg−1 i.p.) (a), SR144528 (1 mg kg−1 i.p.) (b) and capsazepine (10 mg kg−1 i.p.) (c) given separately, and of all three antagonists given together (d) on AM404 (10 mg kg−1)-induced antiallodynia in neuropathic (CCI) rats, 180 min after the last AM404 administration (on day 7). Withdrawal threshold to mechanical stimulus of the injured paws is expressed as g and data represent mean±s.e.m. of 6–7 rats. ***P<0.001, **P<0.01, *P<0.05 vs sham/vehicle animals; °°°P<0.001, °P<0.05 vs CCI/vehicle animals.
Effect of AM404 treatment on locomotor activity
Neither a single nor daily administration of AM404 (10 mg kg−1) modified locomotor activity in sham or CCI rats. Although the locomotor activity of CCI rats, 7 days after the injury, was significantly reduced (10 min activity counts, 1180±53.2) relative to the sham animals (1905±69.6 counts), neither response was affected by AM404 treatment (CCI+single AM404, 1029±98.0; CCI+7 days AM404, 1484±202.6 counts and sham+single AM404, 1675±97.50; sham+7 days AM404, 1920±117.2 counts; n=5–8 for all groups).
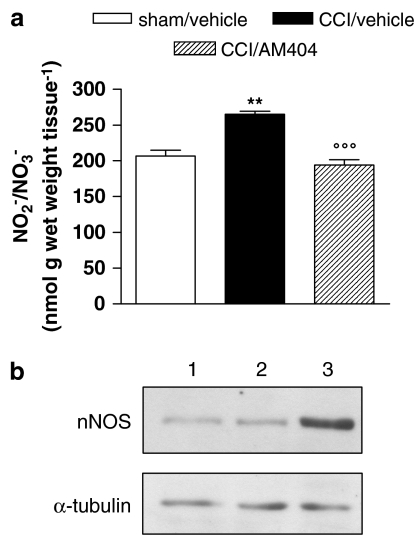
Effect of AM404 on the NO system
Seven days after the lesion, the nitrite/nitrate content in injured paws was increased, by about 30%, as compared to sham animals (Figure 5a). This increase was prevented by 7 days of treatment with AM404. The enhanced production of NO in CCI animals was associated with an increase in the concentration of nNOS protein in the cytosolic fraction of sciatic nerve homogenates, as shown by the representative immunoblot in Figure 5b. Treatment with AM404 also prevented this upregulation of nNOS expression.
Figure 5.
Effect of AM404 (10 mg kg−1 s.c.) given daily to neuropathic (CCI) rats for 1 week from the day after the surgery on the NO system. (a) Nitrite/nitrate content measured in paw homogenate. Data represent mean±s.e.m. of 8–10 rats. **P<0.01 vs sham/vehicle animals; °°°P<0.001 vs CCI/vehicle animals. (b) Representative immunoreactive bands of nNOS protein in cytosolic fraction of sciatic nerve homogenate. Each lane was loaded with 100 μg of proteins. Lane 1: sham/vehicle; lane 2: CCI/AM404; lane 3: CCI/vehicle. α-tubulin is the protein loading control.
Effect of AM404 on cytokine levels
The content of TNFα in DRG was higher (84%, Figure 6a) on the injured side at 7 days after injury, when compared with sham animals. At the same time, there was a significant decrease in the IL-10 content of the DRG of CCI rats (37%, Figure 6b). Treatment with AM404 for the 7 days brought the TNFα content down to the level found in sham animals and stimulated production of IL-10 to almost three times that in sham rats.
Figure 6.
Effect of AM404 (10 mg kg−1 s.c.) given daily to neuropathic (CCI) rats for 1 week from the day after the surgery on (a) TNFα and (b) IL-10 content in DRG. Data represent mean±s.e.m. of 8–10 rats. ***P<0.001, **P<0.01 vs sham/vehicle animals; °°°P<0.001, °°P<0.01 vs CCI/vehicle animals.
Effect of AM404 on NF-κB
Assays for activated NF-κB showed that the DNA-binding activity of NF-κB subunit p65 was increased in the sciatic nerve of neuropathic rats, 7 days after the injury (Figure 7). AM404 treatment significantly inhibited this upregulation of NF-κB DNA-binding activity.
Figure 7.
Effect of AM404 (10 mg kg−1 s.c.) given daily to neuropathic (CCI) rats for 1 week from the day after the surgery, on NF-κB activation in the nuclear fraction of sciatic nerve expressed as arbitrary units (optical density (OD)) at 450 nm. Data represent mean±s.e.m. of 4–5 rats. *P<0.05 vs sham/vehicle animals; °P<0.05 vs CCI/vehicle animals.
Effect of AM404 on bax and bcl-2 gene expression
The semiquantitative analysis of mRNA levels measured by RT–PCR amplification showed no significant increase in the ratio between proapoptotic bax gene and antiapoptotic bcl-2 gene, 7 days after the injury, in extracts of the whole lumbar spinal cord of CCI animals (data not shown). However, at an earlier time, on day 3 after the injury, expression of the bax gene was increased (0.76±0.09 vs 0.19±0.03 units in CCI and sham rats, respectively), with no obvious change in the bcl-2 gene expression (0.89±0.09 vs 1.06±0.05 in CCI and sham rats, respectively). Consequently, the bax/bcl-2 ratio was increased by about 270% in CCI rats as compared to the sham rats (Figure 8a). Treatment with AM404 for the 3 days completely prevented this increase and the bax/bcl-2 ratio in the spinal cord of CCI animals treated with AM404 did not differ from that of sham animals (Figure 8a). Interestingly, AM404 treatment affected both genes, restricting the bax overexpression (CCI+AM404, 0.28±0.04 vs CCI alone, 0.76±0.09 units) and increasing the antiapoptotic bcl-2 gene expression (CCI+AM404, 1.30±0.08 vs CCI alone, 0.89±0.09 units). Further analysis of the proapoptotic bcl-xS and the antiapoptotic bcl-xL genes at 3 days showed a significant increase in the ratio between these genes in the spinal cord of CCI rats (Figure 8b). This increased ratio was suppressed by treatment with AM404 for the 3 days.
Figure 8.
Effect of AM404 (10 mg kg−1 s.c.) given daily to neuropathic (CCI) rats for 3 days on mRNA levels of the apoptotic genes (a) bax/bcl and (b) bcl-xS/bcl-xL in lumbar L4–L5 spinal cord. The measured mRNA levels were normalized with respect to hypoxanthine-guanine phosphoribosyltransferase (HPRT), chosen as housekeeping gene. The gene expression values are expressed as arbitrary units (% of control) and data represent mean±s.e. of 4–5 rats. *P<0.05 vs sham/vehicle animals; °P<0.05 vs CCI/vehicle animals.
Discussion
In this study, we report the antihyperalgesic and antiallodynic effects of the endocannabinoid transport inhibitor, AM404, in an experimental model of neuropathic pain, CCI of the sciatic nerve. Our results show, for the first time, that modulation of endocannabinoid tone by daily administration of AM404 for 1 week prevented the onset of thermal hyperalgesia and mechanical allodynia in CCI rats in a dose-dependent fashion. The same treatment did not affect the nociceptive thresholds in either sham-operated animals or the contralateral paw of neuropathic rats, indicating that the doses employed are not directly analgesic. In contrast to the effect of daily treatment, a single dose of the compound did not modify nociception in CCI animals, suggesting that a sustained modulation of the endocannabinoid tone is necessary to induce the relief of neuropathic pain. This was confirmed by the time dependence of the antihyperalgesic efficacy of the compound, which elicited only a partial relief of neuropathic pain after three doses, whereas 1 week of treatment was necessary to counteract allodynia.
It is important to note that this chronic treatment with AM404 did not induce tolerance and the antihyperalgesic and antiallodynic properties of the compound were still evident after a further week of treatment, that is, after a total of 14 days. Furthermore, in our hands, animals repeatedly given AM404 did not show any changes in overt behaviour, indicating that the doses used were well tolerated. As AM404 is known to depress motor function (Gonzalez et al., 1999; Giuffrida et al., 2000), it is possible that the antihyperalgesia and antiallodynia produced by the compound could be due to motor dysfunction. However, at the dose we used (10 mg kg−1), AM404 did not impair locomotor activity following acute or chronic administration in either CCI or sham rats. It is therefore unlikely that the relief of nociceptive responses induced by AM404 could be attributed to motor impairment. These findings confirm the idea that unwanted side effects would be unlikely after the potentiation of endocannabinoid tone, probably because this approach leads to the enhancement of endocannabinoid level mainly in those tissues in which there is an active synthesis of endocannabinoids.
Because a role for AM404 as a direct CB1 agonist was not supported either in vitro or in vivo, the antihyperalgesic and antiallodynic effects elicited by AM404 may be ascribed to its ability to enhance the extracellular level of anandamide. After release, anandamide is normally cleared by a rapid inactivation process consisting of uptake into cells (Beltramo et al., 1997), followed by catalytic hydrolysis via FAAH (Desarnaud et al., 1995). AM404 blocks anandamide transport in vitro and in vivo (Beltramo et al., 1997), but also shows little selectivity for the uptake process over FAAH (Jarrahian et al., 2000). We suggest that the ability of AM404 to interfere with FAAH is not crucial for AM404 effect on neuropathic pain; in fact, previous data reported the ineffectiveness of selective FAAH inhibitors in neuropathic pain (Jayamanne et al., 2006) and the development of nociceptive behaviour after nerve injury in FAAH−/− mice (Lichtman et al., 2004). Thus, AM404 can potentiate the antinociceptive properties of anandamide in our experimental condition. We would suggest that the endocannabinoid enhancement induced by AM404 was not able to evoke an analgesic response in normal, uninjured animals, whereas it was able to produce antihyperalgesia in neuropathic rats, probably because CB1 receptors are upregulated during pathological conditions (Siegling et al., 2001; Lim et al., 2003), making the cannabinoid system more sensitive to the action of agonists.
In addition, AM404 interacts with TRPV1 receptors (Zygmunt et al., 2000) at concentrations similar to those necessary to inhibit endocannabinoid transport, so that its effect on nociception in neuropathic rats may be due, at least in part, to its effects on the vanilloid system. TRPV1 receptors are mainly located in the primary sensory neurons and they are critical mediators of pain that occurs in the setting of tissue injury. Furthermore, the increased levels of TRPV1 observed in the DRG of neuropathic rats (Fukuoka et al., 2002) seem to contribute to thermal hyperalgesia. In the periphery, TRPV1 receptors are mainly expressed on unmyelinated C-fibres, known as polymodal nociceptors, responding to mechanical, chemical and thermal stimuli. Relatively few TRPV1 receptors were demonstrated on the thinly myelinated Aδ-fibres (Caterina et al., 1997) that can be activated by intense mechanical stimuli or by noxious heat. The Aβ-fibres mediating low threshold afferent mechanical inputs are insensitive to the TRPV1 agonist, capsaicin. However, induction of TRPV1 receptors on previously capsaicin-insensitive fibres by peripheral nerve injury (Rashid et al., 2003) could allow these targets to contribute to the antiallodynic effect of AM404. Anandamide has been shown to activate TRPV1 receptors directly (Zygmunt et al., 1999) as a partial agonist (Roberts et al., 2002). It is well known that the full agonist at this receptor, capsaicin, possesses powerful analgesic property through the long-lasting desensitization of TRPV1. We can speculate that repeated treatment with AM404, directly or through anandamide, can lead to receptor desensitization with subsequent antihyperalgesic and antiallodynic effects. Summarizing, AM404 could activate CB1 receptors indirectly through anandamide and TRPV1 receptors directly and/or indirectly via the endogenous fatty acid amide. However, an indirect action on TRPV1 seems unlikely, as the block of anandamide uptake prevents anandamide from accessing its intracellular binding site at TRPV1 receptor.
We tested the hypothesis that both CB1 and TRPV1 receptors were involved in the actions of AM404 by evaluating the ability of selective receptor antagonists to reverse AM404-induced effects. Our findings showed that both SR141716 and capsazepine partially reversed the AM404-induced effect, confirming our hypothesis that both receptor types mediate the relief of neuropathic pain elicited by the compound. Surprisingly, in our hands, the cannabinoid CB2 receptor antagonist, SR144528, was also able to partially reverse the AM404-induced response. In the light of the weak agonist activities of anandamide at the CB2 receptor, the mechanism underlying the involvement of this receptor remains to be clarified. It may be possible that, in the presence of AM404, the extracellular concentrations of the fatty acid amide reached levels able to activate also CB2 receptors at the site of injury. Alternatively, the role of CB2 receptors may be linked to other endogenous cannabinoids able to activate these receptors, such as 2-arachidonoyl-glycerol and virhodamine, whose levels might also increase after AM404, as suggested by indirect evidence (Fezza et al., 2002; Huang et al., 2002; Wilson & Nicoll, 2002). Activation of CB2 receptors could contribute to relief of neuropathic pain indirectly, by decreasing the release of sensitizing substances from neighbouring mast and immune cells or, more directly, by modulating the activation of microglial cells expressing CB2 receptors (Zhang et al., 2003) located in the spinal cord. In summary, the findings herein reported strongly suggest that inhibition of endocannabinoid removal by blocking the putative anandamide carrier results in the relief of neuropathic pain and may represent a novel strategy for treating chronic pain. A further support to this approach arises from our preliminary findings obtained employing VDM11, a more selective inhibitor of endocannabinoid transport. Repeated administration of this compound also abolished thermal hyperalgesia and mechanical allodynia, but, unlike AM404, the VDM11-induced effect was insensitive to capsazepine (data not shown). This finding strengthens our hypothesis that anandamide, retained in the extracellular space through the blockade of endocannabinoid trasporter, would not directly activate TRPV1 receptors and suggests that the involvement of TRPV1 in the effects of AM404 is due to the binding of this compound to vanilloid receptors.
Despite a wealth of data detailing the physiological and neuroanatomical changes associated with the development of neuropathic pain, the precise molecular mechanism that underlies its propagation and maintenance still remains poorly understood. One possible mechanism relates the increase in proinflammatory cytokines to the neuropathy-associated hyperalgesia (Sommer et al., 1998b); furthermore, the use of endogenously or synthetically produced anti-inflammatory cytokines (Wagner et al., 1998) or antibodies against TNFα or its receptors, as well as the use of TNFα antagonists or synthesis inhibitors such as thalidomide, attenuates the hyperalgesia following CCI (Sommer et al., 1998a, 1998b). An additional potential mediator in the evolution of neuropathic pain is NO, a diffusible, multifunctional, transcellular messenger that may contribute to the development of hyperalgesia directly by sensitizing peripheral nerve or indirectly by influencing the local inflammatory process. Peripheral nerve injuries have been shown to induce an increase in NOS expression in DRG (Luo et al., 1999) and in the sciatic nerve (Costa et al., 2004a). In addition to their pronociceptive action on sensory neurons, both TNFα and NO act as proapoptotic messengers (for reviews, see Varfolomeev & Ashkenazi, 2004 and Li & Wogan, 2005). The production of TNFα and NO is under the control of the transcription factor NF-κB, which also regulates the expression of other genes, including those encoding different cytokines and chemokines. Cytokines that are stimulated by NF-κB, such as TNFα, can also directly activate the NF-κB pathway, thus establishing a positive autoregulatory loop that can amplify the inflammatory responses. NF-κB also stimulates the expression of enzymes, such as inducible and neuronal isoform of NOS. In addition to activating the expression of genes involved in the control of immune and inflammatory response, NF-κB is also a key mediator of genes involved in the control of the cellular proliferation and apoptosis (Barkett & Gilmore, 1999). Although under physiological conditions the activation of NF-κB induces resistance to apoptotic stimuli, in response to other stimuli, NF-κB activation may lead to induction of apoptosis (Kuhnel et al., 2000). Thus, another critical point in the present study was to determine whether AM404 treatment can result in modulation of NO and cytokine pathways and in a reduction of the apoptosis occurring in CCI animals. Our findings confirm the pivotal role of cytokines in neuropathic pain as there was, in CCI animals, an enhanced production of TNFα and a significant decrease in IL-10 level, as well as an increased expression of neuronal isoform of NOS and increased production of NO. The overproduction of all these mediators is sustained by the activation of NF-κB signalling. Chronic treatment of CCI rats with AM404 restored the level of TNFα to normal and enhanced IL-10 content. In addition, AM404 brought NO output down to that of control animals, concomitant with inhibition of nNOS overexpression. The sequelae of AM404 treatment remain to be elucidated, but we can postulate that this effect may be related to the capability of AM404 to repress the NF-κB-mediated transactivation of the genes encoding for proinflammatory cytokines and NOS. A recent in vitro study showing that anandamide inhibits NF-κB activation in TNFα-stimulated cells (Sancho et al., 2003), supports our hypothesis. The strong increase in IL-10 content, elicited by AM404, would contribute to the effects on pain hypersensitivity, as exogenous IL-10 has been shown to prevent the progression of injury-induced pain behaviour following excitotoxic spinal cord injury (Plunkett et al., 2001).
Apoptotic cell death following CCI has been previously demonstrated with the TUNEL technique in the spinal cord (Kawamura et al., 1997; Azkue et al., 1998; Scholz et al., 2005), but not in the DRG (Whiteside & Munglani, 2001). In agreement with others (Maione et al., 2002), our data showed that CCI increased apoptosis in the spinal cord 3 days after the injury, as revealed by changes in the ratio between proapoptotic bax and bcl-xS genes and antiapoptotic bcl-2 and bcl-xL genes. Our data also suggest that the apoptosis is limited to the first few days following nerve injury, contributing to the aetiology of neuropathic pain. Some studies suggest that the death of GABAergic neurones in the dorsal horn after nerve injury in rats contributes to the development and maintenance of chronic pain (Ibuki et al., 1997; Eaton et al., 1998; Moore et al., 2002; Scholz et al., 2005). Apoptosis in the spinal cord may lead to structural changes that could result in the generation of vacant synapses that may induce sprouting of neuronal processes from deeper laminae into the dorsal horn. These sprouts would increase the sensitivity of the nociceptive system. Treatment with AM404 completely prevented the bcl family-dependent apoptotic pathway causing, in turn, an antihyperalgesic and an antiallodynic effect.
In summary, our data provide evidence that AM404 relieves neuropathic pain acting via CB1, CB2 and TRPV1 receptors without producing any relevant cannabimimetic effects and that this action is accompanied by a modulation of nitric oxide and cytokine pathways and by a reduction in the extent of apoptosis occurring in neuropathic animals. Taken together, these findings suggest that the elevation of the extracellular endocannabinoid level might have therapeutic benefit in alleviating neuropathic pain, although the concomitant activation of TRPV1 receptors may be also required. Although less potent or specific as an inhibitor of endocannabinoid uptake than other compounds (VDM11, UCM707), AM404 has the additional advantages of being able to directly activate vanilloid receptors, of exhibiting anti-oxidant properties due to the presence of a phenolic group in its chemical structure and of inhibiting, at least in vitro, purified COX-1 and COX-2 (Hogestatt et al., 2005), activities that could be useful to counteract neurogenic inflammation occurring during neuropathy.
Acknowledgments
We are grateful to Sanofi-Aventis for kindly supplying SR141716 and SR144528. This work has been supported by grant from the Italian Ministry for Education, University and Research (MIUR).
Abbreviations
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CCI
chronic constriction injury
- DMSO
dimethyl sulphoxide
- DRG
dorsal root ganglia
- FAAH
fatty acid amide hydrolase
- IL-10
interleukin-10
- SDS
sodium dodecylsulphate
- TNFα
tumour necrosis factor α
- TRPV1
transient receptor potential vanilloid type 1
References
- AZKUE J.J., ZIMMERMANN M., HSIEH T.F., HERDEGEN T. Peripheral nerve injury induces NMDA receptor-mediated, delayed degeneration in spinal neurons. Eur. J. Neurosci. 1998;10:2204–2206. doi: 10.1046/j.1460-9568.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- BARKETT M., GILMORE T.D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- BELTRAMO M., STELLA N., MALIGNANO A., LIN S.Y., MAKRIYANNIS A., PIOMELLI D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;2777:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- BENNETT G.J., XIE Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- BRIDGES D., AHMAND K., RICE A.S. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTA G., GESSA G.L., NAVA F. Dopamine D2 receptor antagonists prevent Ä9-tetrahydrocannabinol-induced antinociception in rats. Eur. J. Pharmacol. 1999;384:153–156. doi: 10.1016/s0014-2999(99)00696-2. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- COSTA B., COLLEONI M., CONTI S., TROVATO A.E., BIANCHI M., SOTGIU M.L., GIAGNONI G. Repeated treatment with the synthetic cannabinoid WIN55,212-2 reduces both hyperalgesia and production of pronociceptive mediators in a rat model of neuropathic pain. Br. J. Pharmacol. 2004a;141:4–8. doi: 10.1038/sj.bjp.0705587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTA B., GIAGNONI G., FRANKE C., TROVATO A.E., COLLEONI M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br. J. Pharmacol. 2004b;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE NOVELLIS V., SINISCALCO D., GALDERISI U., FUCCIO C., NOLANO M., SANTORO L., CASCINO A., ROTH K.A., ROSSI F., MAIONE S. Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology. 2004;46:468–479. doi: 10.1016/j.neuropharm.2003.10.014. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., DAVIS J.B., PERTWEE R.G., DIMARZO V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- DESARNAUD F., CADAS H., PIOMELLI D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J. Biol. Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., LASTRES-BECKER I., BISOGNO T., DE PETROCELLIS L., MILONE A., DAVIS J.B., FERNANDEZ-RUIZ J.J. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur. J. Pharmacol. 2001;420:123–131. doi: 10.1016/s0014-2999(01)01012-3. [DOI] [PubMed] [Google Scholar]
- EATON M.J., PLUNKETT J.A., KARMALLY S., MARTINEZ M.A., MONTANEZ K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J. Chem. Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- FEZZA F., BISOGNO T., MINASSI A., APPENDINO G., MECHOULAM R., DI MARZO V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513:294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- FOX A., KESINGLAND A., GENTRY C., MCNAIR K., PATEL S., URBAN L., JAMES I. The role of central and peripheral cannabinoid 1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- FUKUOKA T., TOKUNAGA A., TACHIBANA T., DAI Y., YAMANAKA H., NOGUCHI K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- GIUFFRIDA A., RODRIGUEZ DE FONSECA F., NAVA F., LOUBET-LESCOULIE P., PIOMELLI D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur. J. Pharmacol. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- GONZALEZ S., ROMERO J., DE MIGUEL R., LASTRES-BECKER I., VILLANUA M.A., MAKRIYANNIS A., RAMOS J.A., FERNANDEZ-RUIZ J.J. Extrapyramidal and neuroendocrine effects of AM404, an inhibitor of the carrier-mediated transport of anandamide. Life Sci. 1999;65:327–336. doi: 10.1016/s0024-3205(99)00251-9. [DOI] [PubMed] [Google Scholar]
- HAO J.X., XU X.J. Treatment of chronic allodynia-like response in spinally injured rats: effects of systemically administered nitric oxide synthase inhibitors. Pain. 1996;66:313–319. doi: 10.1016/0304-3959(96)03039-4. [DOI] [PubMed] [Google Scholar]
- HARGREAVES K.M., DUBNER R., BROWN F., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HENEKA M.T., LOSCHMANN P.A., GLEICHMANN M., WELLER M., SCHULZ J.B., WULLNER U., KLOCKGETHER T. Induction of nitric oxide synthase and nitric oxide-mediated apoptosis in neuronal PC12 cells after stimulation with tumor necrosis factor-alpha/lipopolysaccharide. J. Neurochem. 1998;71:88–94. doi: 10.1046/j.1471-4159.1998.71010088.x. [DOI] [PubMed] [Google Scholar]
- HERZBERG U., ELIAV E., BENNETT G.J., KOPIN I.J. The analgesic effect of R(+)-WIN55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci. Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- HOGESTATT E.D., JONSSON B.A.G., ERMUND A., ANDERSSON D.A., BJORK H., ALEXANDER J.P., CRAVATT B.F., BASBAUM A.I., ZYGMUNT P.M. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBUKI T., HAMA A.T., WANG X.T., PAPPAS G.D., SAGEN E.J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- JARRAHIAN A., MANNA S., EDGEMONT W.S., CAMPBELL W.B., HILLARD C. Structure–activity relationship among N-arachidonoylethanolamine (anandamide) head group analogues for the anandamide transporter. J. Neurochem. 2000;74:2597–2606. doi: 10.1046/j.1471-4159.2000.0742597.x. [DOI] [PubMed] [Google Scholar]
- JAYAMANNE A., GREENWOOD R., MITCHELL V.A., ASLAN S., PIOMELLI D., VAUGHAN C.V. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br. J. Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMURA T., AKIRA T., WANTANABE M., KAGITANI Y. Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology. 1997;36:1023–1030. doi: 10.1016/s0028-3908(97)00096-8. [DOI] [PubMed] [Google Scholar]
- KUHNEL F., ZENDER L., PAUL Y., TIETZE M.K., TRAUTWEIN C., MANNS M., KUBICKA S. NF-κB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J. Biol. Chem. 2000;275:6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- LI C.Q., WOGAN G.N. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2005;226:1–15. doi: 10.1016/j.canlet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., SHELTON C.C., ADVANI T., CRAVATT B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- LIM G., SUNG B., JI R.R., MAO J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of WIN 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–283. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- LUO Z.D., CHAPLAN S.R., SCOTT B.P., CIZKOVA D., CALCUTT N.A., YAKSH T.L. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: lack of a role in allodynia development. J. Neurosci. 1999;19:9201–9208. doi: 10.1523/JNEUROSCI.19-21-09201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIONE S., SINISCALCO D., GALDERISI U., DE NOVELLIS V., ULIANO R., DI BERNARDO G., BERRINO L., CASCINO A., ROSSI F. Apoptotic genes expression in the lumbar dorsal horn in a model of neuropathic pain in rat. Neuroreport. 2002;13:101–106. doi: 10.1097/00001756-200201210-00024. [DOI] [PubMed] [Google Scholar]
- MALAN T.P., IBRAHIM M.M., DENG H., LIU Q., MATA H.P., VANDERAH T., PORRECA F., MAKRIYANNIS A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., SCHILLING R.J., SALVEMINI D., MOORE W.M., CURRIE M.G. A fluorimetric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- MOORE K.A., KOHNO T., KARCHEWSKI L.A., SCHOLZ J., BABA H., WOOLF C.J. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS M.N., KELLY D., DAVIS A.J. Bradykinin B1 and B2 receptor mechanisms and cytokine-induced hyperalgesia in the rat. Can. J. Physiol. Pharmacol. 1995;73:832–836. doi: 10.1139/y95-113. [DOI] [PubMed] [Google Scholar]
- PLUNKETT J.A., YU C.G., EASTON J.M., BETHEA J.R., YEZIERSKI R.P. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp. Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- RASHID M.H., INOUE M., KONDO S., KAWASHIMA T., BAKOSHI S., UEDA H. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J. Pharmacol. Exp. Ther. 2003;304:940–948. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.-M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.-C., LE FUR G. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- ROBERTS L.A., CHRISTIE M.J., CONNOR M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br. J. Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHO R., CALZADO M.A., DI MARZO V., APPENDINO G., MUNOZ E. Anandamide inhibits nuclear factor-κB activation through a cannabinoid receptor-independent pathway. Mol. Pharmacol. 2003;63:429–438. doi: 10.1124/mol.63.2.429. [DOI] [PubMed] [Google Scholar]
- SCHOLZ J., BROOM D.C., YOUN D.H., MILLS C.D., KOHNO T., SUTER M.R., MOORE K.A., DECOSTERD I., COGGESHALL R.E., WOOLF C.J. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J. Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Z., PEEDIKAYIL J., OLSON G.K., SIEBERT P.D., FANG Y. Multiple transcription factor profiling by enzyme-linked immunoassay. Biotechniques. 2002;32:1168–1170. doi: 10.2144/02325dd07. [DOI] [PubMed] [Google Scholar]
- SIEGLING A., HOFMANN H.A., DENZER D., MAULER F., DE VRY J. Cannabinoid CB1 receptor upregulation in a rat model of chronic neuropathic pain. Eur. J. Pharmacol. 2001;415:R5–R7. doi: 10.1016/s0014-2999(01)00798-1. [DOI] [PubMed] [Google Scholar]
- SOMMER C., MARZINIAK M., MYERS R.R. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain. 1998a;74:83–91. doi: 10.1016/S0304-3959(97)00154-1. [DOI] [PubMed] [Google Scholar]
- SOMMER C., SCHMIDT C., GEORGE A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp. Neurol. 1998b;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- VARFOLOMEEV E.E., ASHKENAZI A. Tumor necrosis factor: an apoptosis junkie. Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- WAGNER R., JANJIGIAN M., MYERS R.R. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- WHITESIDE G.T., MUNGLANI R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J. Neurosci. Res. 2001;64:168–173. doi: 10.1002/jnr.1062. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endocannabinoid signalling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- ZHANG J., HOFFERT C., VU H.K., GROBLEWSKI T., AHMAD S., O'DONNELL D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., CHUANG H., MOVAHED P., JULIUS D., HOGESTATT E.D. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]