Abstract
Human lung tryptase, a homotetrameric serine protease unique to mast cell secretory granules, has been implicated in the pathogenesis of asthma. A hypothesis that tethered symmetrical inhibitors might bridge two adjacent active sites was explored via a rationally designed series of bisbenzamidines. These compounds demonstrated a remarkable distanced-defined structure–activity relationship against human tryptase with one series possessing subnanomolar potencies. Additional evidence supporting the concept of active-site bridging is also presented.
Keywords: asthma, protease inhibitors, bisbenzamidines
Human asthma is a complex immunoinflammatory disease (1–3). After allergen exposure, inflammatory cascades produce early- and late-phase responses as well as a state of hyperresponsiveness characterized by the release and synthesis of a myriad of mediators including the leukotrienes, prostaglandins, cytokines, histamine, growth factors, chemokines, heparin, and proteases. Of the various proteases, mast cell tryptase has recently become appreciated as a potential amplification component and driving force behind the changes to the airway seen in asthma. Inhibitors of tryptase have been reported (4–6), including one that has advanced to clinical trials (7).
Tryptase is a homotetrameric serine protease found in abundant preformed levels in human mast cells (11–35 pg/cell in human lung mast cells) that has been characterized as a protease, peptidase, and cytokine (8, 9). It has been implicated in a variety of pathological conditions including asthma (10) and other inflammatory (11–15) and autoimmune (16, 17) diseases. A variety of in vitro studies have demonstrated that this tryptic enzyme has many interesting effects such as promoting mast cell degranulation (43), inducing eosinophil and neutrophil migration (18), inactivating fibrinogen (19, 20), processing high and low molecular weight kininogen (21), degrading neurogenic bronchodilatory feedback mechanisms (22), amplifying the effects of histamine on lung tissue (23), and stimulating the growth of fibroblasts, bronchial smooth muscle cells, and airway epithelial cells while inducing IL-8 and intercellular adhesion molecule-1 expression (24). Tryptase can also process prostromelysin to mature stromelysin (matrix metalloproteinase type 3), which can further activate collagenase I (25, 26). All of these various activities of tryptase could significantly contribute to the early- and late-phase bronchoconstriction as well as to the development of airway hyperresponsiveness displayed in human asthma. In chronic asthma and other long-term respiratory diseases, these activities could also drive the profound changes to the airway such as desquamation of the epithelial lining, fibrosis, and thickening of the underlying tissues (these changes are not treated by present therapeutics). It is the timing of tryptase release and its apparent autocrine effect in the allergen response that also make it such a compelling target.
Because tryptase is an enzyme consisting of four associated subunits, each capable of enzymatic proteolysis, the possibility exists for inhibition of more than one subunit with a single inhibitor molecule. This strategy of tethering two binding moieties together to produce an exponentially more potent inhibitor has been applied to relatively few medicinal chemistry problems; however, two recent examples are matrix metalloproteinase inhibitors, discovered via the “structure–activity relationship by NMR” technique (27–29), and acetylcholinesterase inhibitors (30). In each case, separate chemical moieties that displayed weak binding affinities were linked together to provide extremely potent enzyme inhibitors. The theoretical basis for the enhanced binding of these bifunctional molecules (A-B), originally proposed by Jencks (31), involves a summation of the observed intrinsic binding energy of moiety A, the observed intrinsic binding energy of moiety B, and a Gibbs energy of connection. This last term incorporates the change in the probability of binding that the connected molecule A-B displays over the individual fragments. In this study, weakly binding benzamidine moieties were bridged at various lengths and with various templates to provide subnanomolar inhibitors of human lung tryptase.
MATERIALS AND METHODS
Chemical Syntheses.
The compounds described herein were prepared by standard synthetic organic chemistry procedures. Reagents, starting materials, and solvents were purchased from Aldrich or Maybridge (Cornwall, U.K.) Chemical Company and used without further purification. Intermediates and final products were purified by flash silica gel chromatography (32) or RP-HPLC by using a Waters Prep LC 2000 with Rainin Microsorb C18 columns (Rainin Instruments). Intermediates and final products were characterized by 1H NMR (400 MHz, Bruker, Billerica, MA), 13C NMR (75 MHz, Bruker) and LRMS (Perkin–Elmer SCIEX electrospray). All compounds presented herein were determined to be ≥95% purity by 1H NMR analysis.
Determination of Ki.
Heparin-stabilized human lung tryptase, purchased from Cortex Biochem (San Leandro, CA), was further purified on a Superdex 200 gel-filtration column (Amersham Pharmacia) to remove the excess unbound heparin. The active-site concentration of the enzyme was determined by spectrophotometric titration with 4-nitrophenyl 4′-guanidinobenzoate (33). Tryptase activity was measured according to the procedures of Schwartz (34) with minor modifications, by using Tosyl-Gly-Pro-Arg-p-nitroanilide (GPR-pNA) as a chromogenic substrate. The reaction was carried out in 50 mM Tris⋅HCl, pH 8.0, containing 150 mM NaCl and 0.02% Triton X-100 at 37°C in Costar ultralow cluster 96-well microtiter plate. The amount of pNA produced by tryptase was determined by measuring the change in absorbance at 405 nm on a SpectraMAX 340 plate reader (Molecular Devices). The Km for the substrate was determined by fitting the initial velocities of substrate hydrolysis to the Michaelis–Menten equation by using Sigma Plot (Jandel, San Rafael, CA) in an iterative fashion. Data were visualized by plotting according to the Lineweaver–Burk transformation. Inhibition assay was carried out in a total volume of 200 μl wherein tryptase (30 μl, final concentration 1 nM) was incubated with various concentrations of a sample (50 μl) to be tested in the above assay buffer for 5 min. The reaction was started by the addition of substrate GPR-pNA (40 μl, final concentration 320 μM), and the residual activity was measured after 15 min of incubation. The inhibition constant, Ki, was determined by fitting the inhibition data (measured IC50 values) to a two-site competitive binding equation by using the data analysis program graphpad prism (GraphPad, San Diego). For very potent inhibitors, this program extrapolated the low Ki values. Determination of Ki values against trypsin and plasmin were obtained similarly.
RESULTS AND DISCUSSION
The ability of benzamidine to inhibit serine proteases of the trypsin family is well documented (35–37). This moiety, an isostere for the guanidine side chain of arginine, binds readily to the P1 pocket via an amidine/carboxylate salt bridge with the aspartic acid at the base of this pocket. Indeed, benzamidine (1) was found to inhibit human lung tryptase (Ki = 22 μM), bovine trypsin (Ki = 33 μM), and human plasmin (22% inhibition at 100 μM). However, because tryptase is the only member of this tryptic enzyme family that associates in a polymeric manner, we speculated that bisbenzamidine inhibitors, tethered together at varying distances, might provide much greater potency and selectivity by binding at adjacent active sites.
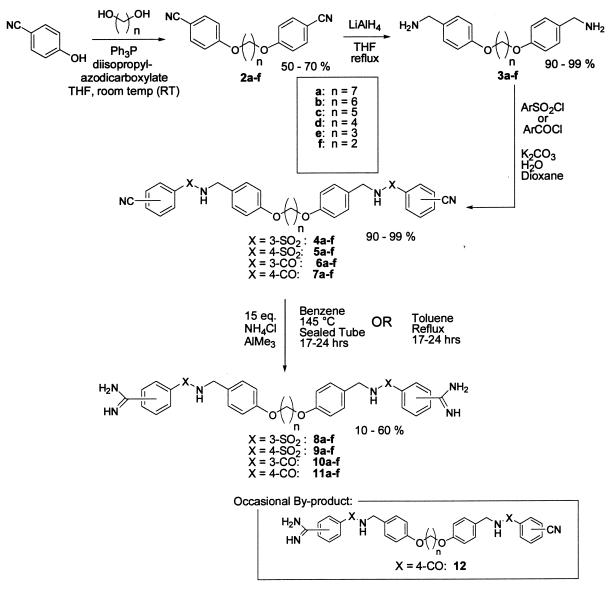
A concise synthetic route to a series of bisbenzamidines was designed and implemented (see Scheme S1). The particular structures targeted were chosen simply because the synthetic route to these compounds was highly precedented and likely to succeed. We felt that the linker region needed to possess only flexibility rather than any specific binding element and, as long as the benzamidine unit was presented at both ends, the compounds should effectively test the hypothesis of active site bridging. The aim of this study was not necessarily to identify a clinical candidate, but rather to produce pharmacological tools for probing the importance of tryptase in animal models of pulmonary disease. In the event, various bisethers 2a-f were readily prepared via Mitsunobu chemistry by using 4-cyanophenol and the required diol (38). Reduction of the nitrile and sulfonylation (or acylation) under Schotten–Baumann conditions with the appropriate cyanoarylsulfonyl chloride (or cyanoaryl acid chloride) provided the bisbenzonitriles 4a-f, 5a-f or 6a-f, 7a-f, respectively (39). The use of the Garigapati–Weinreb amidination (40, 41) in a sealed tube at high temperature or refluxing in toluene helps to overcome the insolubility of the monoamidine to return the required bisamidines 8–11 in moderate to low yields. Occasionally, the monoamidine intermediates 12 were isolated and tested. Although reverse-phase chromatography was needed for purifying several of the smaller bisamidines, this amidination procedure can yield clean products after a simple quench with silica gel and methanol followed by filtration and concentration. The excess ammonium chloride can be washed out of the resulting solids with a small quantity of water.
Scheme 1.
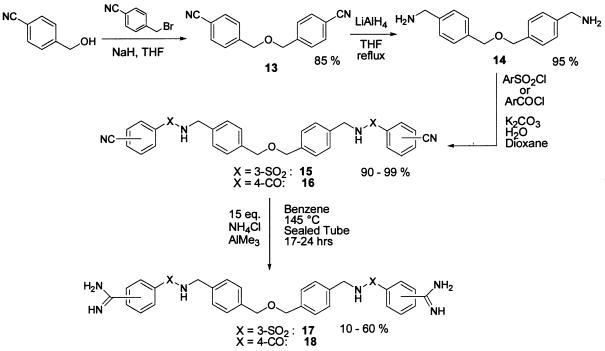
A contracted tether was designed around the mono-ether 13 (see Scheme S2). Hydride reduction to diamine 14 followed by sulfonylation or acylation and amidination as before provided the smaller bisamidines 17 and 18.
Scheme 2.
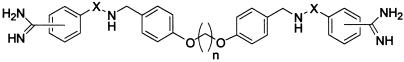
This series of four potential active site bridging inhibitors was then evaluated against human lung tryptase (see Table 1—selectivities are provided as magnitude increases over tryptase activity). At first glance, one notices that the para-amidine amide series (4-CO) demonstrates excellent subnanomolar potencies against the enzyme, while the corresponding para-amidine sulfonamide (4-SO2) series were generally much less active. One hypothesis for this observation is that the carbonyl group might favorably interact with the enzyme (presumably in and around the P1 pocket) via its planarity and particular benzamidine vector, whereas the tetrahedral SO2 cannot achieve the same geometry. Still another explanation could involve the electrophilic reactivity of the former and the nonreactivity of the latter. To test this hypothesis, the reversibility of these inhibitors was analyzed. When compounds 11c and 8c were separately incubated with the active enzyme and then dialyzed with 3,000× volumes of buffer, no activity could be recovered. On the other hand, denaturing the same enzyme-inhibitor complex with heat returned the intact inhibitors (data not shown). These results suggest that these compounds may have very slow off rates but that they are truly reversible, yet this does not rule out the possibility that the carbonyl moiety may be establishing a covalent interaction with the enzyme. An x-ray co-crystal would thus be required to probe the nature of carbonyl vs. sulfonyl activity against mast-cell tryptase.
Table 1.
Biochemical profile of active site bridging inhibitors of human tryptase
Another observation concerning the nature of these potent inhibitors was the nonconventional curve shape of their inhibitory response. Although a typical enzyme inhibitor produces a standard curve with a single slope, these compounds display a biphasic curve with a double slope that fits well into a two-site binding equation (data not shown). Two Ki values can be derived from these plots, one of which may represent the binding to a single active site, and the other may reflect the high-affinity effect of binding two active sites within the same complex. The data reported here are the latter Ki values.
The most striking structure–activity relationship evident from these data is the effect of tether length on tryptase inhibition. Aside from the para-amidine sulfonamide series (4-SO2), a distinct relationship between distance and inhibitory activity is manifested. A central chain of three to five carbon atoms produces very potent inhibition and, because tryptase is unique in its tetrameric structure, these inhibitors display excellent selectivity over other serine proteases (generally, trypsin and plasmin were the only other proteases that were affected).
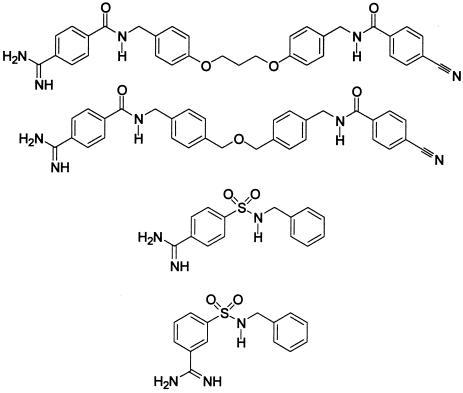
Intrigued by the potent activity of these C2 symmetric bisamidine inhibitors, monoamidine intermediates (12e, 19) were isolated, and several simple benzamidine derivatives were prepared (via chemistry depicted in Scheme S1) to further test the concept of active site bridging (see Table 2). None of these compounds were particularly active against tryptase relative to their bisamidine counterparts. For example, 12e, which contains all the potential binding elements of 11e except the second amidine, is 2,400 times less potent. This magnitude of potency enhancement is similar to that of the earlier examples cited (matrix metalloproteinase inhibitors and acetylcholinesterase inhibitors) and illustrates the important contribution of the Gibbs energy of connection.
Table 2.
Activity of monoamidines against human tryptase
On the basis of an analysis of the primary sequence of human lung tryptase and the x-ray crystal structures of other serine proteases, we had proposed that tryptase is not a C4 symmetrical tetramer but rather a dimer of dimers possibly associated through loop 5 interactions. In that proposed dimeric assembly, adjacent catalytic domains would then be in relatively close proximity, and a single inhibitory molecule, like compound 11c, could bridge these two active sites. In 1998, however, an x-ray crystal structure (3-Å resolution) of human tryptase was reported (42) that clarified the tetrameric geometry of the enzyme. In that study a square flat ringed arrangement was observed in which the four active sites are pointed toward the central core. Although this structure is slightly different than our proposal, the distances between adjacent and opposed active sites (≈20 Å and ≈40 Å) fit well with our results. For example, the distance between the two carbonyl groups in compound 11c, in the fully extended conformation, is 22.2 Å, whereas the distance between amide nitrogens is 18.8 Å. Thus compound 11c could occupy adjacent active sites in tryptase according to the dimensions reported.
In summary, potent selective nonpeptidic inhibitors of human lung tryptase have been designed, prepared, and tested to take advantage of the tetrameric nature of the enzyme. A remarkable distance-defined structure–activity relationship resulted that, along with other data, supports the concept of active site bridging. However, these tryptase inhibitors are symmetrical and possess high molecular weights that make them fairly unattractive as drug-discovery leads. On the other hand, some of the inhibitors are quite potent and selective for mast cell tryptase and are therefore potentially useful as pharmacological tools to delineate the role of tryptase in various animal models of inflammatory and pulmonary disorders. Studies of this aspect of our project will be reported in due course.
Acknowledgments
This paper is dedicated to Professor Edward M. Burgess on the occasion of his retirement from the Georgia Institute of Technology. We gratefully acknowledge Drs. Marv Caruthers, Jeff Yingling, Bhavana Shah, and Richard Nelson for helpful discussions.
References
- 1.Barnes P J. J Intern Med. 1992;231:453–461. doi: 10.1111/j.1365-2796.1992.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 2.Stinson S C. Chem Eng News. 1997;75:25–28. [Google Scholar]
- 3.Georgitis J W. Chest. 1999;115:210–217. doi: 10.1378/chest.115.1.210. [DOI] [PubMed] [Google Scholar]
- 4.Pohlig G, Fendrich G, Knecht R, Eder B, Piechottka G, Sommerhoff C P, Heim J. Eur J Biochem. 1996;241:619–626. doi: 10.1111/j.1432-1033.1996.00619.x. [DOI] [PubMed] [Google Scholar]
- 5.Caughey G H, Raymond W W, Bacci E, Lombardy R J, Tidwell R R. J Pharmacol Exp Ther. 1993;264:676–682. [PubMed] [Google Scholar]
- 6.Combrink K D, Gulgeze H B, Meanwell N A, Pearce B C, Zulan P, Bisacchi G S, Roberts D G M, Stanley P, Seiler S M. J Med Chem. 1998;41:4854–4860. doi: 10.1021/jm9804580. [DOI] [PubMed] [Google Scholar]
- 7.Clark J M, Moore W R, Tanaka R D. Drugs Future. 1996;21:811–816. [Google Scholar]
- 8.Walls A F. In: Asthma Rhinitis. Busse W W, Holgate S T, editors. Oxford: Blackwell Scientific; 1995. pp. 801–824. [Google Scholar]
- 9.He S, Walls A F. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel S E, Fowler A A, Schwartz L B. Am Rev Respir Dis. 1988;137:1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- 11.Rasp G, Hochstrasser K. Allergy (Copenhagen) 1993;48:72–74. doi: 10.1111/j.1398-9995.1993.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 12.Tetlow L C, Woolley D E. Ann Rheum Dis. 1995;54:549–555. doi: 10.1136/ard.54.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tetlow L C, Woolley D E. Ann Rheum Dis. 1995;54:896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavery J P, Lisse J R. Ann Allergy. 1994;72:425–427. [PubMed] [Google Scholar]
- 15.Gotis-Graham I, McNeil H P. Arthritis Rheum. 1997;40:479–489. doi: 10.1002/art.1780400314. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim M Z M, Reder A T, Lawand R, Takash W, Sallouh-Khatib S. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 17.Naukkarinen A, Harvima I T, Aalto M-L, Horsmanheimo M. Int J Dermatol. 1994;33:361–366. doi: 10.1111/j.1365-4362.1994.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 18.Walls A F, He S, Teran L M, Buckley M G, Jung K-S, Holgate S, Shute J K, Kairns J A. Int Arch Allergy Immunol. 1995;107:372–373. doi: 10.1159/000237039. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz L B, Bradford T R, Littman B H, Wintroub B U. J Immunol. 1985;135:2762–2767. [PubMed] [Google Scholar]
- 20.Harvima I T, Harvima R J, Penttilae I M, Eloranta T O, Horsmanheimo M, Fraeki J E. Int Arch Allergy Appl Immunol. 1989;90:104–108. doi: 10.1159/000235008. [DOI] [PubMed] [Google Scholar]
- 21.Imamura T, Dubin A, Moore W, Tanaka R, Travis J. Lab Invest. 1996;74:861–870. [PubMed] [Google Scholar]
- 22.Tam E K, Caughey G H. Am J Respir Cell Mol Biol. 1990;3:27–32. doi: 10.1165/ajrcmb/3.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Johnson P R A, Ammit A J, Carlin S M, Armour C L, Caughey G H, Black J L. Eur Respir J. 1997;10:38–43. doi: 10.1183/09031936.97.10010038. [DOI] [PubMed] [Google Scholar]
- 24.Cairns J A, Walls A F. J Immunol. 1996;156:275–283. [PubMed] [Google Scholar]
- 25.Lohi J, Harvima I, Keski-Oja J. J Cell Biochem. 1992;50:337–349. doi: 10.1002/jcb.240500402. [DOI] [PubMed] [Google Scholar]
- 26.Gruber B L, Marchese M J, Suzuki K, Schwartz L B, Okada Y, Nagase H, Ramamurthy N S. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuker S B, Hajduk P J, Meadows R P, Fesik S W. Science. 1996;274:1531–1534. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 28.Fesik, S. W., Shuker, S. B., Hajduk, P. J. & Meadows, R. P. (1997) Protein Eng.10, Suppl., 73.
- 29.Hajduk P J, Sheppard G, Nettesheim D G, Olejniczak E T, Shuker S B, Meadows R P, Steinman D H, Carrera G M, Marcotte P A, Severin J, et al. J Am Chem Soc. 1997;119:5818–5827. [Google Scholar]
- 30.Pang Y-P, Quiram P, Jelacic T, Hong F, Brimijoin S. J Biol Chem. 1996;271:23646–23649. doi: 10.1074/jbc.271.39.23646. [DOI] [PubMed] [Google Scholar]
- 31.Jencks W P. Proc Natl Acad Sci USA. 1981;78:4046–4050. doi: 10.1073/pnas.78.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Still W C, Kahn M, Mitra A. J Org Chem. 1978;43:2923. [Google Scholar]
- 33.Schwartz L B, Bradford T R, Lee D C, Chlebowski J F. J Immunol. 1990;144:2304–2311. [PubMed] [Google Scholar]
- 34.Schwartz L B, Bradford T R. J Biol Chem. 1986;261:7372–7379. [PubMed] [Google Scholar]
- 35.Michalski R, Nagel W, Robel K P. Naturwissenschaften. 1966;53:614. doi: 10.1007/BF00632281. [DOI] [PubMed] [Google Scholar]
- 36.Mares-Guia M, Shaw E, Cohen W. J Biol Chem. 1967;242:5777–5781. [PubMed] [Google Scholar]
- 37.Baker B R, Erickson E H. J Med Chem. 1967;10:1123–1128. doi: 10.1021/jm00318a030. [DOI] [PubMed] [Google Scholar]
- 38.Mitsunobu O. Synthesis. 1981. 1–28. [Google Scholar]
- 39.Sonntag M. Chem Rev. 1953;52:237–416. [Google Scholar]
- 40.Garigipati R S. Tetrahedron Lett. 1990. , 1969–1972. [Google Scholar]
- 41.Moss R A, Ma W, Merrer D C, Xue S. Tetrahedron Lett. 1995. , 8761–8764. [Google Scholar]
- 42.Pereira P J B, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, Sommerhoff C P, Bode W. Nature (London) 1998;392:306–311. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 43.He S, Gaca M D A, Walls A F. J Pharm Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]