Abstract
Regulation of programmed cell death by Bcl-xL is dependent on both its solution and integral membrane conformations. A conformational change from solution to membrane is also important in this regulation. This conformational change shows a pH-dependence similar to the translocation domain of diphtheria toxin, where an acid-induced molten globule conformation in the absence of lipid vesicles mediates the change from solution to membrane conformations. By contrast, Bcl-xLΔTM in the absence of lipid vesicles exhibits no gross conformational changes upon acidification as observed by near- and far-UV circular dichroism spectropolarimetry. Additionally, no significant local conformational changes upon acidification were observed by heteronuclear NMR spectroscopy of Bcl-xLΔTM. Under conditions that favor the solution conformation (pH 7.4), the free energy of folding for Bcl-xLΔTM (ΔG°) was determined to be 15.8 kcal·mol−1. Surprisingly, under conditions that favor a membrane conformation (pH 4.9), ΔG° was 14.6 kcal·mol−1. These results differ from those obtained with many other membrane-insertable proteins where acid-induced destabilization is important. Therefore, other contributions must be necessary to destabilize the solution conformation Bcl-xL and favor the membrane conformation at pH 4.9. Such contributions might include the presence of a negatively charged membrane or an electrostatic potential across the membrane. Thus, for proteins that adopt both solution and membrane conformations, an obligatory molten globule intermediate may not be necessary. The absence of a molten globule intermediate might have evolved to protect Bcl-xL from intracellular proteases as it undergoes this conformational change essential for its activity.
Keywords: Bcl-2 family, Bcl-xL, solution to membrane conformational change, diphtheria toxin, pH-dependence, pore-forming toxins, protein folding
The Bcl-2 proteins regulate programmed cell death by acting in the cytosol and organellar membranes (Adams and Cory 1998; Chao and Korsmeyer 1998; Green and Reed 1998; Ng and Shore 1998; Harris and Thompson 2000; Hengartner 2000). Some Bcl-2 proteins act by adopting at least two different structural conformations: a solution conformation and an integral membrane conformation. For example, pro-apoptotic Bax is a monomeric, helical bundle protein localized in the cytosol until an apoptotic signal causes translocation to the mitochondrial outer membrane (Suzuki et al. 2000). At the mitochondrial outer membrane, Bax inserts and folds into a large, multimeric integral membrane protein that is thought to regulate the release of cytochrome c (Hsu et al. 1997; Wolter et al. 1997; Shimizu et al. 1999; Antonsson et al. 2000, 2001; Saito et al. 2000).
Similar to Bax, the anti-apoptotic protein Bcl-xL is a soluble, primarily monomeric, helical bundle protein local-izedinparttothecytosol (Muchmore et al. 1996). However, in contrast to Bax, Bcl-xL inserts into the mitochondrial outer membrane and folds into a small, integral membrane protein (Hsu et al. 1997; Minn et al. 1997). For both proteins, the solution-to-membrane conformational change has been reconstituted in vitro with only recombinant proteins and vesicles from synthetic lipids, suggesting that this conformational change might not be receptor-mediated (Minn et al. 1997; Basanez et al. 2001).
The dual structural nature of the Bcl-2 proteins allows for dual mechanisms for their biological activity. In the case of Bcl-xL, the solution conformation acts by sequestering pro-apoptotic factors in the cytosol as a water-soluble helical bundle (Sedlak et al. 1995; Sattler et al. 1997). By contrast, the membrane conformation acts as a small, moderately selective cationic channel in the mitochondrial outer membrane (Minn et al. 1997; Vander Heiden et al. 2001). The exact mechanism of Bcl-xL activity in the membrane is still under debate, but mutants of Bcl-xL that possess altered ion channel properties also have altered apoptotic activities, confirming a biological role for the membrane conformation (Minn et al. 1997, 1999; Xie et al. 1998; Losonczi et al. 2000; Basanez et al. 2001; Vander Heiden et al. 2001). These properties of Bcl-xL were actually demonstrated with Bcl-xLΔTM1 that lacks the C-terminal hydrophobic anchor, which is not required for biological activity or ion channel activity (Muchmore et al. 1996; Minn et al. 1997).
The dual mechanisms for the anti-apoptotic activity of Bcl-xLΔTM in the cytosol and in the membrane raise the question of how a water-soluble protein undergoes a conformational change to become an integral membrane protein. This conformational change appears to have two requirements: acidic pH and presence of lipid vesicles (Basanez et al. 2001). In fact, Bcl-xLΔTM is only weakly associated with lipid vesicles at pH 7.0 but is fully associated at pH 4.5 as measured by a sedimentation assay (Basanez et al. 2001). The ion channel activity of Bcl-xLΔTM also shows a similar pH dependence with conductance occurring readily under acidic conditions but not readily at pH 7.0 (Minn et al. 1997). Because these experiments were performed in vitro with recombinant protein and vesicles derived from synthetic lipids, they suggest that the amino acid sequence alone can specify both solution and membrane conformations.
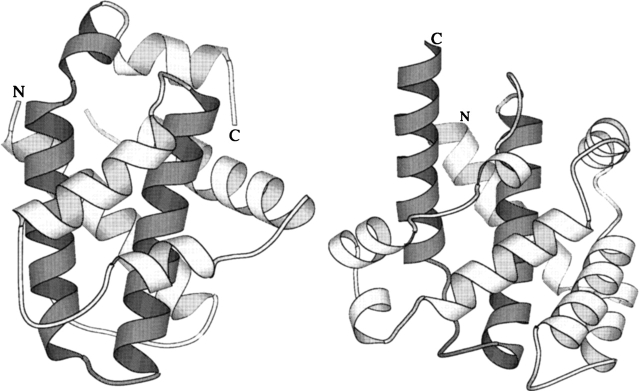
Many bacterial toxins also undergo a pH-dependent conformational change from solution to membrane conformations including the translocation domain from diphtheria toxin and the colicin family of pore-forming toxins (Parker et al. 1990; Lakey et al. 1992; London 1992; Lacy and Stevens 1998; Zakharov and Cramer 2002a). The solution structures of many of these proteins are known and they share a common helical bundle topology (Parker et al. 1989, 1992; Choe et al. 1992; Elkins et al. 1997). In fact, the motivation to explore ion channel properties of Bcl-xLΔTM arose, in part, from the structural similarity it shares with the translocation domain of diphtheria toxin (Fig. 1), which also binds to lipid vesicles in a pH-dependent manner (Sandvig and Olsnes 1980; Muchmore et al. 1996). Some of these helical bundles retain a helical conformation in the membrane (Oh et al. 1996; Lindeberg et al. 2000; Chenal et al. 2002; Zakharov and Cramer 2002b), but no high-resolution structure of a membrane conformation of these proteins has been determined. The topology of this helical membrane conformation must be quite different from the solution conformation, because the polar or charged residues on the surface of the solution conformation would need to be sequestered from the hydrophobic milieu of the membrane bilayer. Based on these considerations, the solution to membrane conformational change has been referred to folding inside-out (Lesieur et al. 1997).
Figure 1.
Structural similarity between Bcl-xL (left, from 1MAZ.pdb) and the translocation domain of diphtheria toxin (right, from 1DDT.pdb). The hydrophobic helical hairpin motifs, helices 5,6 of Bcl-xL and helices 8,9 of diphtheria toxin T-domain are represented by a darker shade of gray. These figures were generated using MOLSCRIPT (Kraulis 1991).
A change in the solution conformation in the absence of lipid vesicles is known for many proteins to lower the activation energy for the solution to membrane conformational change (Lesieur et al. 1997; Zakharov and Cramer 2002a). This change in the solution conformation can be large, such as a change in quaternary structure, or it can be small, such as a change in the tertiary structure commonly referred to as a molten globule conformation (Bychkova et al. 1996). A molten globule conformation is characterized by native-like secondary structure without the well-packed hydrophobic core found in native-like proteins (Ptitsyn et al. 1990; van der Goot et al. 1991). For example, an acid-induced conformational change in the pore-forming toxin Colicin A, even in the absence of lipid vesicles, results in formation of a molten globule conformation that more readily associates with lipid vesicles than the solution conformation that predominates at pH 7.4 (van der Goot et al. 1991). In fact, an acid-induced molten globule formation is the dominant mechanism for membrane insertion of many other proteins that undergo a solution to membrane conformational change including annexin 6, TRAIL, StAR, diphtheria toxin, and other toxins (Blewitt et al. 1985; van der Goot et al. 1991; Bychkova et al. 1996; Song et al. 2001; Chenal et al. 2002; Nam and Choi 2002).
For Bcl-xLΔTM, a mechanism for the solution to membrane conformational change is not known beyond the requirement for lipid vesicles and acidic conditions. Therefore, we first asked what changes are occurring to this protein under acidic conditions in the absence of lipid vesicles. Specifically we tested whether lowering the pH induces a change in the tertiary or quaternary structure by examining changes in the thermodynamic stability and structural properties of Bcl-xLΔTM.
Results
Thermodynamic stability of Bcl-xLΔTM upon acidification is slightly lowered
Bcl-xLΔTM is able to bind to lipid vesicles upon acidification (Minn et al. 1997; Basanez et al. 2001). This process could be facilitated by a change in structure, or dynamics, of the protein upon acidification even in the absence of lipid vesicles, which will be reflected in the thermodynamic stability of the protein. Therefore, the free energy of unfolding of Bcl-xLΔTM was determined at pH 7.4 and 4.9 by chemical denaturation monitoring the circular dichroism signal at 222 nm as a function of increasing GdnHCl concentration (Fig. 2). The data fit well to a two-state model for the unfolding reaction. At pH 7.4, the change in free energy of unfolding (ΔG°) for Bcl-xLΔTM was determined to be 15.8 ± 1.2 kcal · mol−1, while the corresponding value at pH 4.9 was 14.6 ± 1.0 kcal · mol−1. Chemical denaturation of Bcl-xLΔTM monitored by the change in intrinsic tryptophan fluorescence confirmed these results (data not shown).
Figure 2.
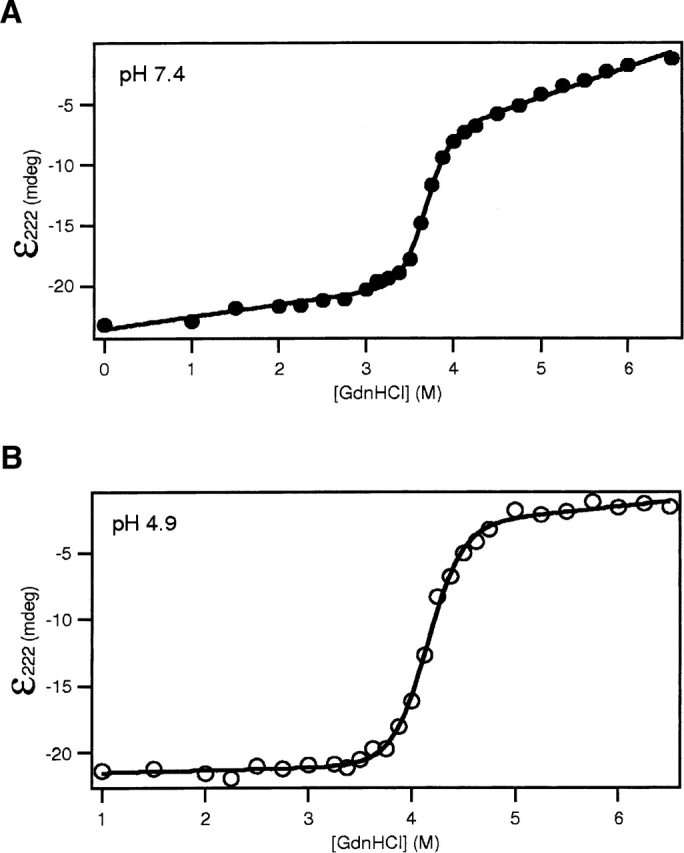
The thermodynamic stability of Bcl-xLΔTM is slightly reduced upon acidification. The circular dichroic signal at 222 nm of Bcl-xLΔTM was monitored as a function of GdnHCl and the resulting data fit as described in the text. The free energy of folding, ΔG° (H2O), under conditions that do not favor membrane insertion (pH 7.4 [•]) (A) is 15.8 ± 1.2 kcal · mol−1. Under conditions that favor membrane insertion (pH 4.9 [○]) (B), ΔG° (H2O) is 14.6 ± 1.0 kcal·mol−1. The mG value decreases from 4.3 ± 0.3 kcal·mol−1·M−1 at pH 7.4 to 3.5 ± 0.2 kcal · mol−1 · M−1 at pH 4.9.
While no pH-dependent change is observed in the thermodynamic stability for Bcl-xLΔTM, the relative enthalpic and entropic contributions to the free energy of folding might exhibit a pH-dependence that would inform on the mechanism of the solution to membrane conformational change. Presumably, the enthalpy of folding does have a pH-dependence because certain residues will become protonated from pH 7.4 to 5.0, and the enthalpy of protonation for these residues will contribute to the enthalpic contributions to the free energy of folding (Pfeil and Privalov 1976; Petrosian and Makhatadze 2000). To detect such enthalpy–entropy compensation to the overall free energy, we performed differential scanning calorimetry experiments at pH 7.4 and 4.9. The midpoint for the unfolding transition (Tm) is reduced upon acidification from 76°C to 71°C (data not shown). Unfortunately, the thermal unfolding transition was irreversible under these and other conditions; no further thermodynamic analysis was possible.
A decrease in pH does not induce a change in the quaternary structure
Given that the thermodynamic stability of Bcl-xLΔTM at pH 4.9 is still quite high (14.6 kcal · mol−1), we postulated that this protein might undergo a pH-dependent oligomerization as an intermediary step toward membrane insertion. We hypothesized that a decrease in pH might destabilize the monomer and favor oligomer formation providing the necessary free energy to achieve a membrane-insertion competent state and lead to the insertion of Bcl-xLΔTM into the membrane. Previously, Bcl-xLΔTM was reported to undergo dimerization although only in the presence of nonionic detergents (Xie et al. 1998). Additionally, an oligomeric insertion mechanism is common for many β-barrel toxins (Gouaux 1997; Heuck et al. 2001) and the annexin family of proteins (Beermann et al. 1998).
To test for acid-induced formation of oligomers in solution, we used sedimentation equilibrium experiments. Six data sets collected using protein at two different concentrations and equilibrated at three different rotor speeds were globally fit to a modified Lamm-Svedberg equation (supplemental data). Analysis performed at pH 7.4 and 4.9 illustrated that there was no significant change in the oligomeric state of the protein as a function of pH (Fig. 3). The data at both pH values were well described by a fit to a single monomeric species. The molecular weight estimates were 25.4 ± 0.2 kDa at pH 7.4, and 27.3 ± 0.2 kDa at pH 4.9. The actual molecular weight of Bcl-xLΔTM is 23.8 kDa. The slight discrepancies in the molecular weights most likely are due to the uncertainty in the estimate for the partial specific volume (Kharakoz 1997), because fits of the data to a monomer–dimer equilibrium gave unreasonable values for KD (102 M). Therefore, Bcl-xLΔTM is monomeric at both conditions; however, we cannot exclude the possibility of oligomerization in the presence of the membrane.
Figure 3.
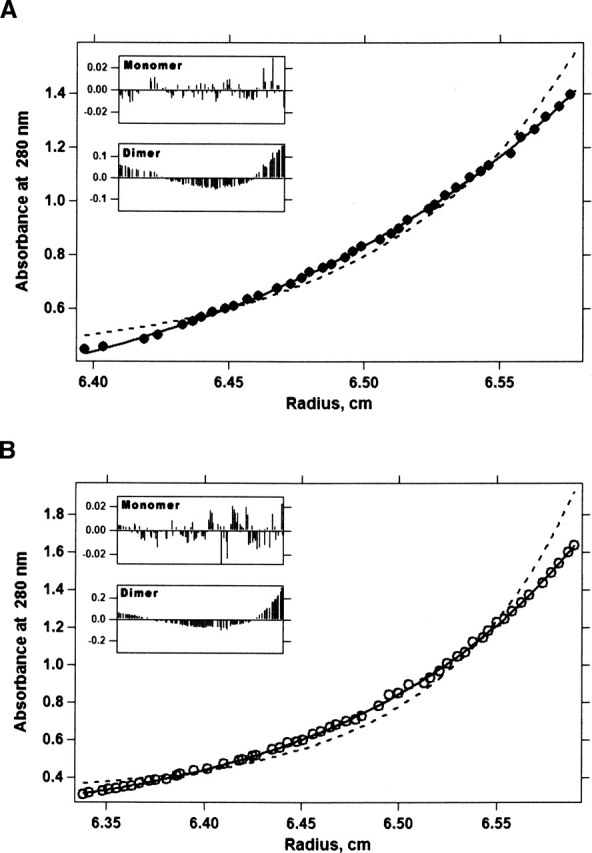
Bcl-xLΔTM is monomeric in solution upon acidification. Representative sedimentation equilibrium data are presented for 25 μM Bcl-xLΔTM at a XLI rotor speed of 19,000 rpm in solution at 25°C collected at pH 7.4 (A) and 4.9 (B). The data are well described by fitting to a single species (—) that is the molecular weight of monomeric Bcl-xLΔTM within experimental uncertainty. Every second data point is displayed (○) but all data were globally fit. The fit to the molecular weight of the dimer of Bcl-xLΔTM (- - -) and the residuals to the fits are also displayed. The global fit of the data from three concentrations and XLI rotor speeds of 17,000, 19,000, and 22,000 rpm was carried out using a modified Lamm-Svedberg equation as described in the text. The global fit to six data sets is presented as supplemental data.
Backbone structure of Bcl-xLΔTM upon acidification is retained
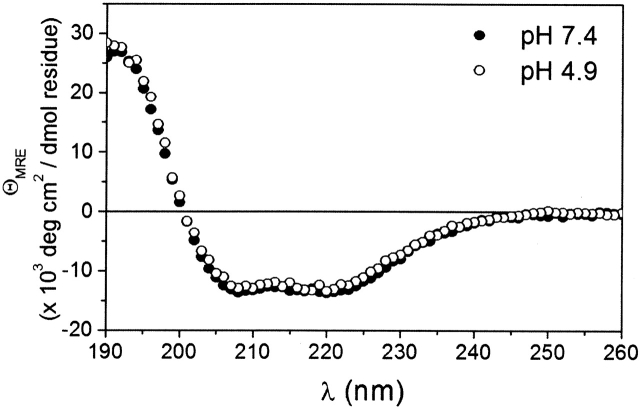
To test for any pH-dependent conformational change of the backbone of Bcl-xLΔTM, we recorded the far-UV circular dichroism spectrum as a function of pH (Fig. 4). The far-UV CD spectrum reports primarily on the secondary structure of a protein and for Bcl-xLΔTM at pH 7.4 is typical of a well-folded helical bundle protein with ~36% α-helicity. At pH 4.9, the spectrum was very similar to pH 7.4 (33% α-helicity), indicating no significant changes in the backbone structure occur upon acidification.
Figure 4.
Secondary structure of Bcl-xLΔTM is conserved upon acidification. The far-UV circular dichroism spectra were collected at pH 7.4 (•) and pH 4.9 (○).
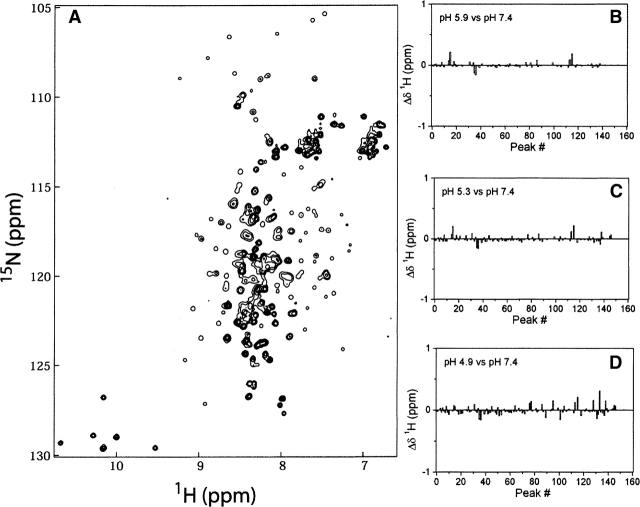
The circular dichroism spectrum measures mean residue ellipticity that is unable to report on site-specific changes in the backbone structure of Bcl-xLΔTM as a function of pH, whereas NMR spectroscopy can provide more detailed information. Therefore, to gain such information and to determine that the CD results did not arise from a concomitant loss and gain of helicity in the protein upon acidification, we examined the 1H-15N HSQC NMR spectrum as a function of pH (Fig. 5A). 1H-15N HSQC experiments provide residue-specific information primarily of the backbone structure of a protein. Upon lowering the pH, no significant change in the chemical shifts of the backbone amides of Bcl-xLΔTM were observed in either the 1H (Fig. 5B–D) or 15N data (not shown), which is consistent with the CD results. These results suggest that no significant structural changes, local or global, occur in the backbone structure of Bcl-xLΔTM from pH 7.4 to 4.9.
Figure 5.
The backbone structure of Bcl-xLΔTM does not undergo significant conformational change upon acidification. 1H-15N HSQC spectra of Bcl-xLΔTM were collected on a 0.6-mM sample of uniformly 15N labeled protein as a function of pH. (A) A representative 1H-15N HSQC spectrum for Bcl-xLΔTM at pH 7.4 is shown. The differences in amide proton chemical shift between pH 7.4 and pH 5.9 (B), pH 5.3 (C), and pH 4.9 (D) are displayed. Note that some minor differences are expected due to the salt dependence of the chemical shift (Schaller and Robertson 1995). Differences in amide nitrogen chemical shift also showed no significant changes as a function of pH (data not shown).
Bcl-xLΔTM upon acidification does not form a molten globule
In the case of many bacterial toxins, membrane insertion upon acidification is aided by the formation of a molten globule intermediate. In a molten globule, the secondary structure of the protein is similar to the native state and the general tertiary fold is retained (Ptitsyn et al. 1990; Creighton 1992). However, the structural integrity of the hydrophobic core of a molten globule is compromised with the apolar side chains adopting more rotameric positions than in the native state (Creighton 1992). Thus, the hydrophobic core of a molten globule is more fluid than that of a native protein.
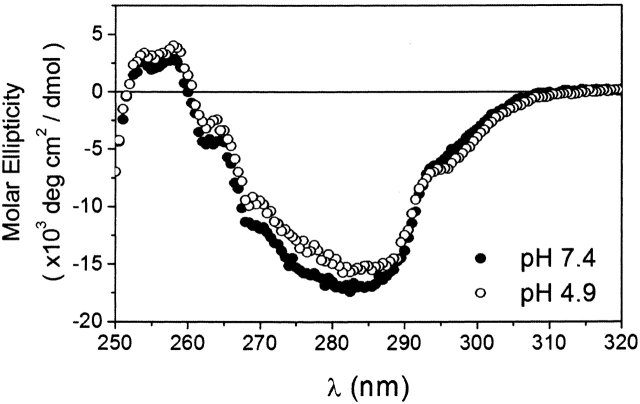
Therefore, we tested whether the ability of Bcl-xLΔTM to insert into membranes upon acidification arises from the formation of a molten globule that could be detected by observing changes in the hydrophobic core. The near-UV CD signal can report on the structural integrity of the aromatic residues in the hydrophobic core of the protein. In the case of a molten globule state, the aromatic residues are no longer well packed in an asymmetric environment, resulting in a loss of signal in this region of the spectrum. However, at pH 4.9, we observed no change in the near-UV CD signal from that observed at pH 7.4 (Fig. 6), suggesting the lack of an acid-induced molten globule state of Bcl-xLΔTM.
Figure 6.
The integrity of the hydrophobic core packing in Bcl-xLΔTM is conserved upon acidification. Near-UV spectra were collected at pH 7.4 (•) and pH 4.9 (○).
A hallmark of a molten globule state is the ability to bind hydrophobic dyes such as 8-anilino-1-naphthalene sulfonic acid (ANS). In fact, Bcl-xLΔTM has been reported in the past to bind ANS (Xie et al. 1998). However, the solution conformation of Bcl-xLΔTM reveals a hydrophobic cleft that would be expected to bind ANS without molten globule formation. For this reason, we measured the pH dependence of the methyl carbon chemical shifts, which are a more direct measure of the integrity of the hydrophobic core. These chemical shifts arise from methyl side chains of residues found primarily in the hydrophobic core of a protein. The spectrum collected at pH 7.4 showed well-dispersed resonances in the methyl region indicative of a well-packed hydrophobic core and typical of a native protein (Fig. 7A). Upon lowering the pH to 4.9, these methyl resonances were still well dispersed, indicating that the protein retained a well-packed hydrophobic core consistent with the absence of a molten globule state (Fig. 7B). The absence of any gross structural change in Bcl-xLΔTM between pH 7.4 and 4.9 argues for either subtle structural or dynamical changes in solution that we were unable to detect by these CD or NMR experiments, or the requirement of lipids for its pH-dependent solution-to-membrane conformational change.
Figure 7.
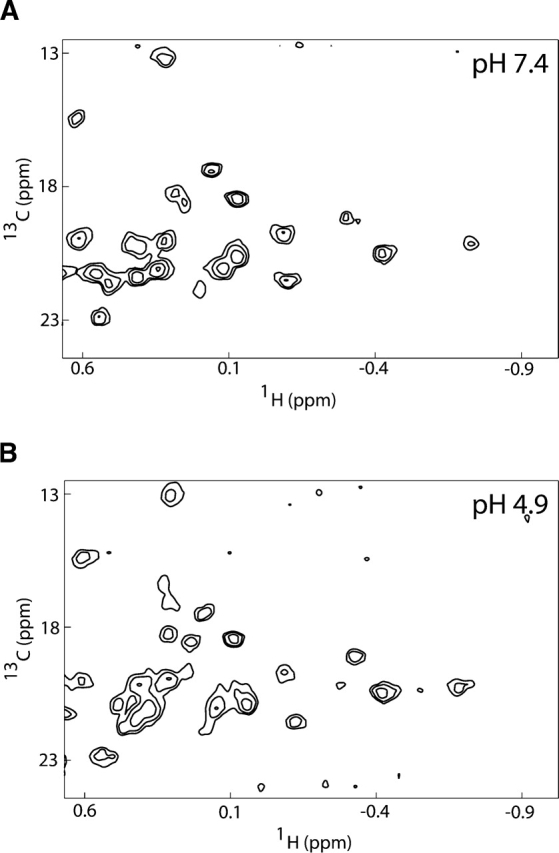
No indication of molten–globule formation upon acidification of Bcl-xLΔTM. 1H-13C HSQC spectra of Bcl-xLΔTM were collected on a 0.8-mM unlabeled protein sample (natural abundance 13C) as a function of pH. Data shown only for pH 7.4 (A) and pH 4.9 (B).
Discussion
Here, we present data that suggest the driving force for the solution to membrane conformational change of Bcl-xL is different than the translocation domain from diphtheria toxin by examining the thermodynamic and structural properties of Bcl-xLΔTM as a function of pH in the absence of lipid vesicles. In contrast to diphtheria toxin translocation domain, we find little change in the thermodynamic stability or structure of Bcl-xLΔTM from pH 7.4 to 4.9, while in the presence of lipid vesicles this pH change results in complete association of Bcl-xLΔTM with lipid vesicles (data not shown). The results presented here suggest that this protein does not insert through an obligatory molten globule intermediate that has been observed in the absence of membrane vesicles for other proteins such as cytochrome c, TRAIL, StAR, diphtheria toxin, and other toxins including colicin A, exotoxin A, and equinatoxin (Blewitt et al. 1985; van der Goot et al. 1991; Bychkova et al. 1996; Song et al. 2001; Chenal et al. 2002; Nam and Choi 2002).
The difference in the free energy of folding (ΔΔG°) of Bcl-xLΔTM between pH 7.4 and 4.9 in the absence of lipid vesicles is only 1.2 kcal · mol−1, which is within the uncertainty in the measurements and is small relative to its thermodynamic stability (14.6 kcal · mol−1 at pH 4.9). To confirm these observations, we repeated these measurements several times on different preparations of the protein. Therefore, even at a pH that favors membrane insertion, the solution conformation of Bcl-xLΔTM is still quite stable. We conclude that acid-induced destabilization of the solution conformation does not contribute to the energetics of the solution to membrane conformational change. Therefore, membrane insertion of Bcl-xLΔTM must derive from an increase in the number of acidic side chains that partition into the membrane upon protonation at lower pH.
This result is notable because it differs from many proteins with solution conformations and acidic isoelectric points similar to Bcl-xLΔTM (calculated pI of 4.4), such as diphtheria toxin translocation domain, colicin A, and colicin B. In these cases, the primary driving force is the acid-induced destabilization of the solution conformation and not simply the acidification of side chains that favors the membrane conformation as we find for Bcl-xLΔTM (Ramsay et al. 1989; London 1992; Schendel and Cramer 1994; Lesieur et al. 1997; Sathish et al. 2002; Zakharov and Cramer 2002a)). For example, the rate-limiting step for the solution to membrane conformational change of colicin A is the acid-induced unfolding rate of the solution conformation in the absence of lipid vesicles, and not membrane binding (van der Goot et al. 1991). While the driving forces behind membrane insertion differ between Bcl-xLΔTM and many other toxins, our data do not exclude the possibility that the structural conformations involved for these proteins are similar. These toxins are thought to initially insert into membranes via a hydrophobic helical hairpin (Fig. 1) and then form an umbrella-like intermediate before becoming fully inserted. Our data do not address this issue and, given the structural similarity of the proteins, we expect that the Bcl-xLΔTM follows a similar pathway.
While the ΔΔG° is neglible, we did observe a pH-dependence to the denaturant dependence to the free energy of folding as reflected by the mG value. The mG value decreases from 4.3 ± 0.3 kcal · mol−1 · M−1 at pH 7.4 to 3.5 ± 0.2 kcal · mol−1 · M−1 at pH 4.9. Such a decrease in mG can be interpreted as the presence of an intermediate that is stabilized at acidic pH conditions, leading to a decrease in two-state character and a lower mG value (Whitten et al. 2001). This result might reflect an ensemble-based description of protein structure (Frauenfelder and Leeson 1998; Frauenfelder and McMahon 1998; Whitten et al. 2005). In this description, the macroscopic thermodynamic state of a protein is comprised of an ensemble of microstates that is quite heterogeneous. In this sense, protonation does not lead to changes in the macroscopic free energy of unfolding but can affect the distribution of microstates that could be reflected in a difference in the mG value that we observe.
Our results with Bcl-xLΔTM have implications for the full-length molecule. The requirement for acidic pH conditions in vitro for the solution to membrane conformational change of Bcl-xLΔTM in the presence of lipid vesicles is to potentially increase the likelihood that the protein lacking the C-terminal transmembrane anchor will associate with the membrane (Schendel et al. 1998). This acidic pH requirement might not be necessary for the full-length molecule. Or perhaps a lower decrease in pH is necessary for the full-length molecule to drive the equilibrium from solution to the membrane conformations in vivo. Interestingly, a slight decrease in the pH of the cytosol from ~7.4 to 6.8 during the initial phases of apoptosis has been reported (Matsuyama et al. 2000). Also, the anionic lipids on the surface of a membrane, like the mitochondrial outer membrane, cause a negative surface potential, increasing proton concentration near the surface and effectively lowering the local pH near the membrane surface (McLaughlin 1989; Menestrina et al. 1989; van der Goot et al. 1991; Murray et al. 1999).
Perhaps the reason for a difference between diphtheria toxin and Bcl-xL relates to the biological process that triggers these conformational changes. While the exact trigger for Bcl-xL is unknown, diphtheria toxin enters the cell via a clathrin-coated endosome that becomes acidic during maturation of the endosome (Draper and Simon 1980; Sandvig and Olsnes 1980). By contrast, Bcl-xL is exposed to a cytosolic environment that is more susceptible to intracellular proteases than the endosome. Therefore, the avoidance of a molten globule intermediate might have evolved to prevent unregulated degradation of Bcl-xL by intracellular proteases that would cleave a molten globule intermediate state more efficiently than the native state of Bcl-xL. Such proteases would not be present in the endosome and might have allowed the evolution of a diphtheria toxin that capitalizes on the acidic nature of the endosome. Based upon the results presented here, Bcl-xL evolved a different driving force for membrane insertion.
The significance of these results extends beyond the Bcl-2 and toxin field because many proteins link their protonation state to functional transitions such as hemoglobin (Ackers 1998). Typically, such changes in protonation state are accompanied by conformational changes; however, as demonstrated here, such conformational changes may not derive from protonation alone. Indeed, pH dependent cooperativity can be quantitatively described without invoking conformational changes for cases when strong electrostatic interactions exist between ligand binding sites and protonation sites (Spassov and Bashford 1998). It appears the Bcl-xL may be an example of this phenomenon.
In summary, to gain a clearer understanding of the pH-dependent solution to membrane conformational change of Bcl-xLΔTM we first analyzed the thermodynamic stability and solution conformation of Bcl-xLΔTM upon acidification in the absence of membranes. Contrary to many other membrane-insertable proteins, we find no evidence for acid-induced unfolding of the Bcl-xL solution conformation. Therefore, the main driving force for membrane insertion must derive from the free energy of binding to the membrane that only occurs upon protonation of acidic residues.
Materials and methods
Protein expression and purification
The gene encoding for human Bcl-xL(1–209), which lacks the C-terminal hydrophobic 24 amino acids, was subcloned into pHis-GB vector using EcoRI and SalI restriction sites by standard procedures (Sambrook and Russell 2001). This construct is an N-terminal fusion of the B1 domain from streptococcal protein G (GB1), and was selected to improve expression and solubility of Bcl-xL (Amezcua et al. 2002). The GB1 domain itself contains an N-terminal 6×His tagfor purificationbyNi2+ affinitychromatography. 6×His-GB1 is separated from Bcl-xLΔTM by a 15-residue linker that contains a recognition site for cleavage by TEV protease, which allows for the liberation of Bcl-xLΔTM from the fusion construct upon incubation with 6×His-TEV protease. Success of the subcloning procedure was confirmed by nucleotide sequencing, and the resulting plasmid transformed into Escherichia coli Rosetta cells (Novagen) for protein overexpression.
To increase the yield of protein in the soluble fraction, cells were grown at 37°C in 2 L of LB media to an OD600 of ~0.7, collected by centrifugation, and gently resuspended in 0.5 L of M9 minimal media and continued to grow at 37°C (Marley et al. 2001). After 1 h, protein expression was induced by the addition of 0.5 mM IPTG. The cells were harvested by centrifugation after 5–6 h at 37°C and resuspended in Buffer A (20 mM Tris, 0.5 M NaCl at pH 8.0), lysed by three passes through a French press. The protein present in the soluble fraction was then purified by affinity purification on a Ni2+ affinity column (His-Trap, GE Healthcare), followed by dialysis into TEV protease cleavage buffer (50 mM Tris, 50 mM NaCl, 5 mM β-mercaptoethanol at pH 8.0). The fusion protein was then treated overnight at 4°C with recombinant 6×His-TEV protease (1:50 w/w ratio) to release Bcl-xLΔTM from the GB1 domain. The reaction mixture was loaded onto the His-Trap column again and the flow-through fraction containing Bcl-xLΔTM was collected, concentrated, and quantitated using UV-absorbance (ɛ280 = 41,820 M−1cm−1 in 6 M GdnHCl). The yield of pure protein was ~10 mg/L. The purity was >95%, as judged by Coomassie-stained gel electrophoresis, and the identity of the protein was further confirmed by MALDI-TOF mass spectrometry. 15N-labeled samples were prepared in a similar manner in the presence of 15NH4Cl in the M9 minimal medium. All the proteins were stored at 4°C until used.
Chemical denaturation studies
The free energy of unfolding of Bcl-xLΔTM was determined by chemical denaturation titration experiments using GdnHCl. The unfolding reaction was monitored by observing the changes in the ellipticity at 222 nm using a Jasco J-810 spectropolarimeter. The temperature was maintained at 25°C and the scan speed was 10 nm/min. Each data point represents the average of five accumulations with a response time of 2 sec. The data of ɛ222 versus [GdnHCl] were fit to a two-state model using a nonlinear least-squares fit to the following equation using a script written in Igor Pro 4.04:
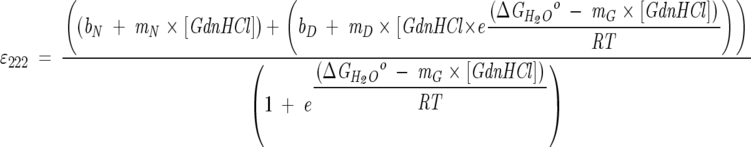 |
where bN, mN, and bD, mD represent the ordinate and slope of the native and the denatured baselines, respectively (Santoro and Bolen 1988). Values for the native and the denatured state baselines were estimated by multiple fits to the raw data to minimize the χ2 of the fit. The global folding stability extrapolated to standard conditions is given by ΔG°H2O, and mG is the slope of the linear extrapolation curve that represents the denaturant dependence of the Gibbs free energy.
Analytical ultracentrifugation
Sedimentation equilibrium experiments were performed on Bcl-xLΔTM at 25°C in a Beckman XL-I analytical ultracentrifuge; 10 μM, 25 μM, and 50 μM Bcl-xLΔTM samples in 20 mM sodium phosphate buffer at pH 7.4 and in 20 mM sodium acetate buffer at pH 4.9 were subjected to centrifugation at the following speeds: 17,000, 19,000 and 22,000 rpm. Equilibrium was achieved after 12 h, and the resulting data sets were globally fit to a modified Lamm-Svedberg equation as described (Hill et al. 2000). Excellent fits were obtained by fitting to a single species using the Marquardt-Levenberg nonlinear least-square algorithm (Levenberg 1944; Marquardt 1963). The partial specific volume was estimated to be 0.7217 from the amino acid composition using the method of Cohn and Edsall (1943). The density of the buffers were estimated to be 0.99971 (phosphate) and 0.999723 (acetate) using the program Sednterp (www.jphilo.mailway.com).
Circular dichroism spectroscopy
The far-UV circular dichroism spectrum was collected on a 7-μM sample of Bcl-xLΔTM in 20 mM sodium phosphate at pH 7.4 or 20 mM sodium acetate at pH 4.9 using a JASCO Model 710 CD Spectropolarimeter. The far-UV spectra were collected from 260 nm to 190 nm at a scan rate of 10 nm/min. The near-UV spectra from 320 nm to 250 nm were collected at a scan rate of 10 nm/min, with a protein concentration of 40 μM. All the spectra were collected at 25°C, corrected for buffer ellipticity, and represented the signal average of five accumulations. The percent α-helicity was estimated by the method of Luo and Baldwin (1997).
NMR spectroscopy
Unlabeled or 15N-labeled NMR samples of Bcl-xLΔTM (0.5 mM) were prepared in 20 mM sodium phosphate buffer plus 10% D2O at pH 7.4 (meter reading) and titrated down to pH 4.9 by the addition of small amounts of 0.1 N hydrochloric acid. The NMR experiments were performed at 25°C either on a Varian INOVA 500 MHz or a Bruker AVANCE 600 MHz spectrometer equipped with a triple resonance probe with triple axis gradients. The 1H-15N heteronuclear single quantum coherence (HSQC) spectra were collected with 1024 × 128 complex points (acquisition times of 64 msec and 32 msec in the direct and the indirect detected dimension, respectively) with the 15N carrier offset at 117.5 ppm. The 1H-13C HSQC was collected at natural abundance (2048 transients) with 1024 × 40 complex points with acquisition times of 64 and 7.4 msec in 1H and 13C, respectively, with 13C carrier offset placed at 17 ppm. The data were processed with nmrPipe (Delaglio et al. 1995) and displayed using NMRView (Johnson and Blevins 1994; Johnson 2004).
Electronic supplemental material
Global fits of six sets of equilibrium sedimentation data from Bcl-xLΔTM (two concentrations at three speeds each) to a single species of monomer molecular weight.
Acknowledgments
We thank Bertrand Garcia-Moreno and Kevin R. MacKenzie for helpful comments, and Frederick J. Tan and Corina E. Rogge for reading the manuscript. We also thank Kevin H. Gardner for generously providing the pHis-GB plasmid. This work was supported in part by the NIH GM067180 and American Cancer Society Award IRG-58-005-41, and funds generously provided by JHU.
Abbreviations
TM, transmembrane anchor
NMR, nuclear magnetic resonance
CD, circular dichroism
GdnHCl, guanidine hydrochloride
HSQC, heteronuclear single quantum coherence
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051807706.
Supplemental material: see www.proteinscience.org
References
- Ackers, G.K. 1998. Deciphering the molecular code of hemoglobin allostery. Adv. Protein Chem. 51: 185–253. [DOI] [PubMed] [Google Scholar]
- Adams, J.M. and Cory, S. 1998. The Bcl-2 protein family: Arbiters of cell survival. Science 281: 1322–1326. [DOI] [PubMed] [Google Scholar]
- Amezcua, C.A., Harper, S.M., Rutter, J., and Gardner, K.H. 2002. Structure and interactions of PAS kinase N-terminal PAS domain: Model for intramolecular kinase regulation. Structure (Camb.) 10: 1349–1361. [DOI] [PubMed] [Google Scholar]
- Antonsson, B., Montessuit, S., Lauper, S., Eskes, R., and Martinou, J.C. 2000. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 345 (Pt. 2): 271–278. [PMC free article] [PubMed] [Google Scholar]
- Antonsson, B., Montessuit, S., Sanchez, B., and Martinou, J.C. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276: 11615–11623. [DOI] [PubMed] [Google Scholar]
- Basanez, G., Zhang, J., Chau, B.N., Maksaev, G.I., Frolov, V.A., Brandt, T.A., Burch, J., Hardwick, J.M., and Zimmerberg, J. 2001. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J. Biol. Chem. 276: 31083–31091. [DOI] [PubMed] [Google Scholar]
- Beermann, B.B., Hinz, H.J., Hofmann, A., and Huber, R. 1998. Acid induced equilibrium unfolding of annexin V wild type shows two intermediate states. FEBS Lett. 423: 265–269. [DOI] [PubMed] [Google Scholar]
- Blewitt, M.G., Chung, L.A., and London, E. 1985. Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry 24: 5458–5464. [DOI] [PubMed] [Google Scholar]
- Bychkova, V.E., Dujsekina, A.E., Klenin, S.I., Tiktopulo, E.I., Uversky, V.N., and Ptitsyn, O.B. 1996. Molten globule-like state of cytochrome c under conditions simulating those near the membrane surface. Biochemistry 35: 6058–6063. [DOI] [PubMed] [Google Scholar]
- Chao, D.T. and Korsmeyer, S.J. 1998. BCL-2 family: Regulators of cell death. Annu. Rev. Immunol. 16: 395–419. [DOI] [PubMed] [Google Scholar]
- Chenal, A., Savarin, P., Nizard, P., Guillain, F., Gillet, D., and Forge, V. 2002. Membrane protein insertion regulated by bringing electrostatic and hydrophobic interactions into play. A case study with the translocation domain of diphtheria toxin. J. Biol. Chem. 277: 43425–43432. [DOI] [PubMed] [Google Scholar]
- Choe, S., Bennett, M.J., Fujii, G., Curmi, P.M., Kantardjieff, K.A., Collier, R.J., and Eisenberg, D. 1992. The crystal structure of diphtheria toxin. Nature 357: 216–222. [DOI] [PubMed] [Google Scholar]
- Cohn, E.J. and Edsall, J.T. 1943. Protein, amino acids, and peptides as ions and dipolar ions. Reinhold, New York.
- Creighton, T.E. 1992. The molten globule state. In Protein folding, pp. 243–300. W.H. Freeman and Co., New York.
- Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. 1995. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6: 277–293. [DOI] [PubMed] [Google Scholar]
- Draper, R.K. and Simon, M.I. 1980. The entry of diphtheria toxin into the mammalian cell cytoplasm: Evidence for lysosomal involvement. J. Cell Biol. 87: 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins, P., Bunker, A., Cramer, W.A., and Stauffacher, C.V. 1997. A mechanism for toxin insertion into membranes is suggested by the crystal structure of the channel-forming domain of colicin E1. Structure 5: 443–458. [DOI] [PubMed] [Google Scholar]
- Frauenfelder, H. and Leeson, D.T. 1998. The energy landscape in non-biological and biological molecules. Nat. Struct. Biol. 5: 757–759. [DOI] [PubMed] [Google Scholar]
- Frauenfelder, H. and McMahon, B. 1998. Dynamics and function of proteins: The search for general concepts. Proc. Natl. Acad. Sci. 95: 4795–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux, E. 1997. Channel-forming toxins: Tales of transformation. Curr. Opin. Struct. Biol. 7: 566–573. [DOI] [PubMed] [Google Scholar]
- Green, D.R. and Reed, J.C. 1998. Mitochondria and apoptosis. Science 281: 1309–1312. [DOI] [PubMed] [Google Scholar]
- Harris, M.H. and Thompson, C.B. 2000. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 7: 1182–1191. [DOI] [PubMed] [Google Scholar]
- Hengartner, M.O. 2000. The biochemistry of apoptosis. Nature 407: 770–776. [DOI] [PubMed] [Google Scholar]
- Heuck, A.P., Tweten, R.K., and Johnson, A.E. 2001. β-barrel pore-forming toxins: Intriguing dimorphic proteins. Biochemistry 40: 9065–9073. [DOI] [PubMed] [Google Scholar]
- Hill, R.B., Hong, J.K., and DeGrado, W.F. 2000. Hydrogen bonded cluster can specify the native state of a protein. J. Am. Chem. Soc. 122: 746–747. [Google Scholar]
- Hsu, Y.T., Wolter, K.G., and Youle, R.J. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. 94: 3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B.A. 2004. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol. Biol. 278: 313–352. [DOI] [PubMed] [Google Scholar]
- Johnson, B.A. and Blevins, R.A. 1994. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4: 603–615. [DOI] [PubMed] [Google Scholar]
- Kharakoz, D.P. 1997. Partial volumes and compressibilities of extended polypeptide chains in aqueous solution: Additivity scheme and implication of protein unfolding at normal and high pressure. Biochemistry 36: 10276–10285. [DOI] [PubMed] [Google Scholar]
- Kraulis, P.J. 1991. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24: 946–950. [Google Scholar]
- Lacy, D.B. and Stevens, R.C. 1998. Unraveling the structures and modes of action of bacterial toxins. Curr. Opin. Struct. Biol. 8: 778–784. [DOI] [PubMed] [Google Scholar]
- Lakey, J.H., Gonzalez-Manas, J.M., van der Goot, F.G., and Pattus, F. 1992. The membrane insertion of colicins. FEBS Lett. 307: 26–29. [DOI] [PubMed] [Google Scholar]
- Lesieur, C., Vecsey-Semjen, B., Abrami, L., Fivaz, M., and Gisou van der Goot F., 1997. Membrane insertion: The strategies of toxins (review). Mol. Membr. Biol. 14: 45–64. [DOI] [PubMed] [Google Scholar]
- Levenberg, K. 1944. A method for the solution of certain problems in least squares. Quart. Appl. Math. 2: 164–168. [Google Scholar]
- Lindeberg, M., Zakharov, S.D., and Cramer, W.A. 2000. Unfolding pathway of the colicin E1 channel protein on a membrane surface. J. Mol. Biol. 295: 679–692. [DOI] [PubMed] [Google Scholar]
- London, E. 1992. Diphtheria toxin: Membrane interaction and membrane translocation. Biochim. Biophys. Acta 1113: 25–51. [DOI] [PubMed] [Google Scholar]
- Losonczi, J.A., Olejniczak, E.T., Betz, S.F., Harlan, J.E., Mack, J., and Fesik, S.W. 2000. NMR studies of the anti-apoptotic protein Bcl-xL in micelles. Biochemistry 39: 11024–11033. [DOI] [PubMed] [Google Scholar]
- Luo, P. and Baldwin, R.L. 1997. Mechanism of helix induction by trifluoro-ethanol: A framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 36: 8413–8421. [DOI] [PubMed] [Google Scholar]
- Marley, J., Lu, M., and Bracken, C. 2001. A method for efficient isotopic labeling of recombinant proteins. J. Biomol. NMR 20: 71–75. [DOI] [PubMed] [Google Scholar]
- Marquardt, D. 1963. An algorithm for least-squares estimation of nonlinear parameters. SIAM J. Appl. Math. 11: 431–441. [Google Scholar]
- Matsuyama, S., Llopis, J., Deveraux, Q.L., Tsien, R.Y., and Reed, J.C. 2000. Changes in intramitochondrial and cytosolic pH: Early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2: 318–325. [DOI] [PubMed] [Google Scholar]
- McLaughlin, S. 1989. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem. 18: 113–136. [DOI] [PubMed] [Google Scholar]
- Menestrina, G., Forti, S., and Gambale, F. 1989. Interaction of tetanus toxin with lipid vesicles. Effects of pH, surface charge, and transmembrane potential on the kinetics of channel formation. Biophys. J. 55: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn, A.J., Velez, P., Schendel, S.L., Liang, H., Muchmore, S.W., Fesik, S.W., Fill, M., and Thompson, C.B. 1997. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature 385: 353–357. [DOI] [PubMed] [Google Scholar]
- Minn, A.J., Kettlun, C.S., Liang, H., Kelekar, A., Vander Heiden, M.G., Chang, B.S., Fesik, S.W., Fill, M., and Thompson, C.B. 1999. Bcl-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. EMBO J. 18: 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore, S.W., Sattler, M., Liang, H., Meadows, R.P., Harlan, J.E., Yoon, H.S., Nettesheim, D., Chang, B.S., Thompson, C.B., Wong, S.L., et al. 1996. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381: 335–341. [DOI] [PubMed] [Google Scholar]
- Murray, D., Arbuzova, A., Hangyas-Mihalyne, G., Gambhir, A., Ben-Tal, N., Honig, B., and McLaughlin, S. 1999. Electrostatic properties of membranes containing acidic lipids and adsorbed basic peptides: Theory and experiment. Biophys. J. 77: 3176–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, G.H. and Choi, K.Y. 2002. Association of human tumor necrosis factor-related apoptosis inducing ligand with membrane upon acidification. Eur. J. Biochem. 269: 5280–5287. [DOI] [PubMed] [Google Scholar]
- Ng, F.W. and Shore, G.C. 1998. Bcl-XL cooperatively associates with the Bap31 complex in the endoplasmic reticulum, dependent on procaspase-8 and Ced-4 adaptor. J. Biol. Chem. 273: 3140–3143. [DOI] [PubMed] [Google Scholar]
- Oh, K.J., Zhan, H., Cui, C., Hideg, K., Collier, R.J., and Hubbell, W.L. 1996. Organization of diphtheria toxin T domain in bilayers: A site-directed spin labeling study. Science 273: 810–812. [DOI] [PubMed] [Google Scholar]
- Parker, M.W., Pattus, F., Tucker, A.D., and Tsernoglou, D. 1989. Structure of the membrane-pore-forming fragment of colicin A. Nature 337: 93–96. [DOI] [PubMed] [Google Scholar]
- Parker, M.W., Tucker, A.D., Tsernoglou, D., and Pattus, F. 1990. Insights into membrane insertion based on studies of colicins. Trends Biochem. Sci. 15: 126–129. [DOI] [PubMed] [Google Scholar]
- Parker, M.W., Postma, J.P., Pattus, F., Tucker, A.D., and Tsernoglou, D. 1992. Refined structure of the pore-forming domain of colicin A at 2.4 Å resolution. J. Mol. Biol. 224: 639–657. [DOI] [PubMed] [Google Scholar]
- Petrosian, S.A. and Makhatadze, G.I. 2000. Contribution of proton linkage to the thermodynamic stability of the major cold-shock protein of Escherichia coli CspA. Protein Sci. 9: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeil, W. and Privalov, P.L. 1976. Thermodynamic investigations of proteins. III. Thermodynamic description of lysozyme. Biophys. Chem. 4: 41–50. [DOI] [PubMed] [Google Scholar]
- Ptitsyn, O.B., Pain, R.H., Semisotnov, G.V., Zerovnik, E., and Razgulyaev, O.I. 1990. Evidence for a molten globule state as a general intermediate in protein folding. FEBS Lett. 262: 20–24. [DOI] [PubMed] [Google Scholar]
- Ramsay, G., Montgomery, D., Berger, D., and Freire, E. 1989. Energetics of diphtheria toxin membrane insertion and translocation: Calorimetric characterization of the acid pH induced transition. Biochemistry 28: 529–533. [DOI] [PubMed] [Google Scholar]
- Saito, M., Korsmeyer, S.J., and Schlesinger, P.H. 2000. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2: 553–555. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. and Russell, D.W. 2001. Molecular cloning: A laboratory manual, 3d ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sandvig, K. and Olsnes, S. 1980. Diphtheria toxin entry into cells is facilitated by low pH. J. Cell Biol. 87: 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro, M.M. and Bolen, D.W. 1988. Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl α-chymotrypsin using different denaturants. Biochemistry 27: 8063–8068. [DOI] [PubMed] [Google Scholar]
- Sathish, H.A., Cusan, M., Aisenbrey, C., and Bechinger, B. 2002. Guanidine hydrochloride induced equilibrium unfolding studies of colicin B and its channel-forming fragment. Biochemistry 41: 5340–5347. [DOI] [PubMed] [Google Scholar]
- Sattler, M., Liang, H., Nettesheim, D., Meadows, R.P., Harlan, J.E., Eberstadt, M., Yoon, H.S., Shuker, S.B., Chang, B.S., Minn, A.J., et al. 1997. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science 275: 983–986. [DOI] [PubMed] [Google Scholar]
- Schaller, W. and Robertson, A.D. 1995. pH, ionic strength, and temperature dependences of ionization equilibria for the carboxyl groups in turkey ovomucoid third domain. Biochemistry 34: 4714–4723. [DOI] [PubMed] [Google Scholar]
- Schendel, S.L. and Cramer, W.A. 1994. On the nature of the unfolded intermediate in the in vitro transition of the colicin E1 channel domain from the aqueous to the membrane phase. Protein Sci. 3: 2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel, S.L., Montal, M., and Reed, J.C. 1998. Bcl-2 family proteins as ion-channels. Cell Death Differ. 5: 372–380. [DOI] [PubMed] [Google Scholar]
- Sedlak, T.W., Oltvai, Z.N., Yang, E., Wang, K., Boise, L.H., Thompson, C.B., and Korsmeyer, S.J. 1995. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. 92: 7834–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, S., Narita, M., and Tsujimoto, Y. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487. [DOI] [PubMed] [Google Scholar]
- Song, M., Shao, H., Mujeeb, A., James, T.L., and Miller, W.L. 2001. Molten–globule structure and membrane binding of the N-terminal protease-resistant domain (63–193) of the steroidogenic acute regulatory protein (StAR). Biochem. J. 356: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassov, V. and Bashford, D. 1998. Electrostatic coupling to pH-titrating sites as a source of cooperativity in protein-ligand binding. Protein Sci. 7: 2012–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, M., Youle, R.J., and Tjandra, N. 2000. Structure of Bax: Co-regulation of dimer formation and intracellular localization. Cell 103: 645–654. [DOI] [PubMed] [Google Scholar]
- van der Goot, F.G., Gonzalez-Manas, J.M., Lakey, J.H., and Pattus, F. 1991. A “molten-globule” membrane-insertion intermediate of the pore-forming domain of colicin A. Nature 354: 408–410. [DOI] [PubMed] [Google Scholar]
- Vander Heiden, M.G., Li, X.X., Gottleib, E., Hill, R.B., Thompson, C.B., and Colombini, M. 2001. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 276: 19414–19419. [DOI] [PubMed] [Google Scholar]
- Whitten, S.T., Wooll, J.O., Razeghifard, R., Garcia-Moreno, E.B., and Hilser, V.J. 2001. The origin of pH-dependent changes in m-values for the denaturant-induced unfolding of proteins. J. Mol. Biol. 309: 1165–1175. [DOI] [PubMed] [Google Scholar]
- Whitten, S.T., Garcia-Moreno, E.B., and Hilser, V.J. 2005. Local conformational fluctuations can modulate the coupling between proton binding and global structural transitions in proteins. Proc. Natl. Acad. Sci. 102: 4282–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter, K.G., Hsu, Y.T., Smith, C.L., Nechushtan, A., Xi, X.G., and Youle, R.J. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., Schendel, S., Matsuyama, S., and Reed, J.C. 1998. Acidic pH promotes dimerization of Bcl-2 family proteins. Biochemistry 37: 6410–6418. [DOI] [PubMed] [Google Scholar]
- Zakharov, S.D. and Cramer, W.A. 2002a. Colicin crystal structures: Pathways and mechanisms for colicin insertion into membranes. Biochim. Biophys. Acta 1565: 333–346. [DOI] [PubMed] [Google Scholar]
- ———. 2002b. Insertion intermediates of pore-forming colicins in membrane two-dimensional space. Biochimie 84: 465–475. [DOI] [PubMed] [Google Scholar]