Full Text
The Full Text of this article is available as a PDF (132.0 KB).
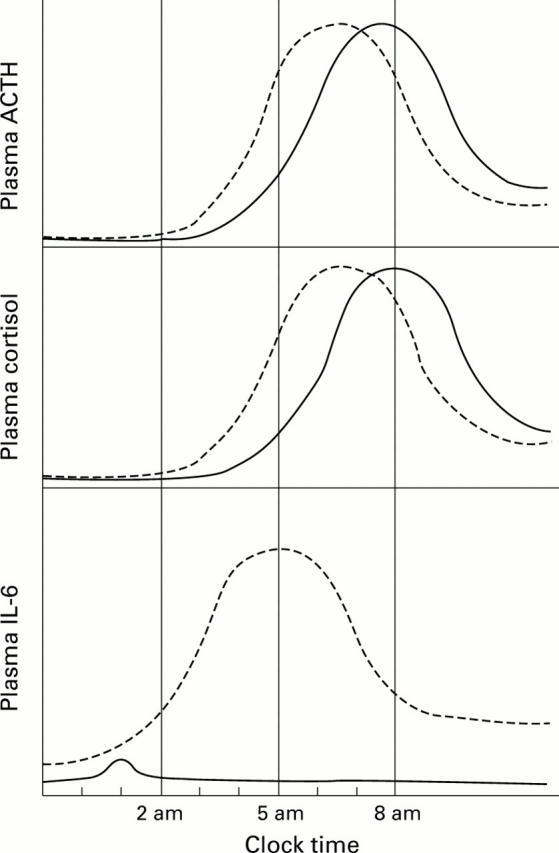
A heuristic scheme showing the circadian rhythmicity of plasma ACTH (upper panel), cortisol (middle panel), and interleukin-6 (IL-6) (lower panel) in normal controls (solid lines) and early, untreated patients with rheumatoid arthritis (interrupted lines). Note the early (phase advanced) circadian peaks of ACTH and cortisol in the patients, who have a later (phase delayed) marked peak elevation in their circadian plasma IL-6. The latter cytokine is a potent stimulator of the hypothalamic-pituitary-adrenal axis, which probably drives the phase advanced plasma ACTH and cortisol circadian peaks in the rheumatoid arthritis patients. Paradoxically, the mean plasma ACTH and cortisol concentrations over 24 h are not raised in the patients.15
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Taga T., Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Arvidson N. G., Gudbjörnsson B., Elfman L., Rydén A. C., Tötterman T. H., Hällgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis. 1994 Aug;53(8):521–524. doi: 10.1136/ard.53.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T., Jilka R. L., Boyce B. F., Girasole G., Broxmeyer H., Dalrymple S. A., Murray R., Manolagas S. C. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995 Jun;95(6):2886–2895. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Riley G. P., Hazleman B. L. Tendon lesions and soft tissue rheumatism--great outback or great opportunity? Ann Rheum Dis. 1996 Jan;55(1):1–3. doi: 10.1136/ard.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G. P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995 May 18;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Balleari E., Giusti M., Intra E., Accardo S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 1991 Jan;34(1):1–5. doi: 10.1002/art.1780340102. [DOI] [PubMed] [Google Scholar]
- De Benedetti F., Robbioni P., Massa M., Viola S., Albani S., Martini A. Serum interleukin-6 levels and joint involvement in polyarticular and pauciarticular juvenile chronic arthritis. Clin Exp Rheumatol. 1992 Sep-Oct;10(5):493–498. [PubMed] [Google Scholar]
- Elenkov I. J., Papanicolaou D. A., Wilder R. L., Chrousos G. P. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996 Sep;108(5):374–381. [PubMed] [Google Scholar]
- Farrell A. J., Blake D. R. Nitric oxide. Ann Rheum Dis. 1996 Jan;55(1):7–20. doi: 10.1136/ard.55.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjörnsson B., Skogseid B., Oberg K., Wide L., Hällgren R. Intact adrenocorticotropic hormone secretion but impaired cortisol response in patients with active rheumatoid arthritis. Effect of glucocorticoids. J Rheumatol. 1996 Apr;23(4):596–602. [PubMed] [Google Scholar]
- Harkness J. A., Richter M. B., Panayi G. S., Van de Pette K., Unger A., Pownall R., Geddawi M. Circadian variation in disease activity in rheumatoid arthritis. Br Med J (Clin Res Ed) 1982 Feb 20;284(6315):551–554. doi: 10.1136/bmj.284.6315.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan J. R. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 1995 Jul 20;333(3):142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Chrousos G. P. Hypothalamic-pituitary-adrenal-glucocorticoid axis function in rheumatoid arthritis. J Rheumatol. 1996 Apr;23(4):577–581. [PubMed] [Google Scholar]
- Masi A. T., Da Silva J. A., Cutolo M. Perturbations of hypothalamic-pituitary-gonadal (HPG) axis and adrenal androgen (AA) functions in rheumatoid arthritis. Baillieres Clin Rheumatol. 1996 May;10(2):295–332. doi: 10.1016/s0950-3579(96)80019-7. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Feigenbaum S. L., Chatterton R. T., Cutolo M. Integrated hormonal-immunological-vascular (H-I-V triad) systems interactions in the rheumatic diseases. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):203–216. [PubMed] [Google Scholar]
- Masi A. T. Low dose glucocorticoid therapy in rheumatoid arthritis (RA): transitional or selected add-on therapy? J Rheumatol. 1983 Oct;10(5):675–678. [PubMed] [Google Scholar]
- Meikle A. W., Tyler F. H. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977 Aug;63(2):200–207. doi: 10.1016/0002-9343(77)90233-9. [DOI] [PubMed] [Google Scholar]
- Mihara M., Moriya Y., Kishimoto T., Ohsugi Y. Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor. Br J Rheumatol. 1995 Apr;34(4):321–325. doi: 10.1093/rheumatology/34.4.321. [DOI] [PubMed] [Google Scholar]
- Miura T., Inagaki N., Yoshida K., Nakajima T., Nagai H., Koda A. Mechanisms for glucocorticoid inhibition of immediate hypersensitivity reactions in rats. Jpn J Pharmacol. 1992 May;59(1):77–87. doi: 10.1254/jjp.59.77. [DOI] [PubMed] [Google Scholar]
- Morrison E., Capell H. Corticosteroids in the management of rheumatoid arthritis. Br J Rheumatol. 1996 Jan;35(1):2–4. doi: 10.1093/rheumatology/35.1.2. [DOI] [PubMed] [Google Scholar]
- Neeck G., Federlin K., Graef V., Rusch D., Schmidt K. L. Adrenal secretion of cortisol in patients with rheumatoid arthritis. J Rheumatol. 1990 Jan;17(1):24–29. [PubMed] [Google Scholar]
- O'Dell J. R., Haire C. E., Erikson N., Drymalski W., Palmer W., Eckhoff P. J., Garwood V., Maloley P., Klassen L. W., Wees S. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med. 1996 May 16;334(20):1287–1291. doi: 10.1056/NEJM199605163342002. [DOI] [PubMed] [Google Scholar]
- Perlstein R. S., Whitnall M. H., Abrams J. S., Mougey E. H., Neta R. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology. 1993 Mar;132(3):946–952. doi: 10.1210/endo.132.3.8382602. [DOI] [PubMed] [Google Scholar]
- Pickup M. E. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1979 Mar-Apr;4(2):111–128. doi: 10.2165/00003088-197904020-00004. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I. R., Wattie D. J., Evans M. C., Stapleton J. P. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med. 1996 Jun 10;156(11):1173–1177. [PubMed] [Google Scholar]
- Saag K. G., Koehnke R., Caldwell J. R., Brasington R., Burmeister L. F., Zimmerman B., Kohler J. A., Furst D. E. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994 Feb;96(2):115–123. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Slemenda C., Longcope C., Peacock M., Hui S., Johnston C. C. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996 Jan 1;97(1):14–21. doi: 10.1172/JCI118382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sothern R. B., Roitman-Johnson B., Kanabrocki E. L., Yager J. G., Roodell M. M., Weatherbee J. A., Young M. R., Nenchausky B. M., Scheving L. E. Circadian characteristics of circulating interleukin-6 in men. J Allergy Clin Immunol. 1995 May;95(5 Pt 1):1029–1035. doi: 10.1016/s0091-6749(95)70104-4. [DOI] [PubMed] [Google Scholar]
- Tilg H., Trehu E., Atkins M. B., Dinarello C. A., Mier J. W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994 Jan 1;83(1):113–118. [PubMed] [Google Scholar]
- Zanker B., Walz G., Wieder K. J., Strom T. B. Evidence that glucocorticosteroids block expression of the human interleukin-6 gene by accessory cells. Transplantation. 1990 Jan;49(1):183–185. doi: 10.1097/00007890-199001000-00040. [DOI] [PubMed] [Google Scholar]


