Abstract
Background: Recently, it has been found that collagen type II arthritis susceptible mouse strains are hyperreactive to immune complexes (ICs), locally deposited into their knee joints.
Objective: To investigate whether this strain specific knee joint hyperreactivity is related to a disturbed regulation of activatory and inhibitory FcγR on their macrophages before and after stimulation with ICs.
Methods: Macrophages from collagen induced arthritis susceptible strains (DBA/1 and B10.RIII) and non-susceptible strains (C57BL/6 and BALB/c) were compared. FcγR levels on macrophages were detected at protein level by flow cytometric analysis and at mRNA level by reverse transcriptase-polymerase chain reaction. Macrophages were stimulated with ICs, and production of cytokines and enzymes was measured at different times.
Results: On synovial and peritoneal macrophages of DBA/1 mice a higher basal FcγRII and III expression was found, which was skewed towards the activating FcγRIII. In B10.RIII macrophages, however, FcγRIII levels were much lower. Regulation of FcγR mRNA levels in macrophages was tested after stimulation with ICs for one and three days. DBA/1 and B10.RIII macrophages showed a prolonged up regulation of activating FcγRI and III, whereas the inhibiting FcγRII was significantly down regulated compared with non-susceptible strains. In line with this, DBA/1 and B10.RIII macrophages showed a higher interleukin 1 (IL1) and matrix metalloproteinase (MMP) production after IC exposure, whereas IL6 production was significantly reduced.
Conclusions: This study indicates that macrophages derived from collagen type II arthritis susceptible mice show a disregulated FcγR expression before, and even more clearly, after activation by ICs involved in inflammation and cartilage degradation, resulting in prolonged expression of activatory FcγRI and III, down regulation of inhibitory FcγRII and increased release of IL1 and MMP.
Full Text
The Full Text of this article is available as a PDF (182.2 KB).
Figure 1.

Expression of FcγRs on macrophages of C57BL/6, BALB/c, DBA/1, and B10.RIII mice. The expression of FcγRII/III was significantly higher in DBA/1 and B10.RIII mice than in the other strains. However when FcγR expression was tested on peripheral blood mononuclear cells (PBMC) of these different strains, no difference in expression was found (A). When K9-361 was used to detect FcγRII, we found higher expression of this receptor in DBA/1 and B10.RIII mice (B). To detect FcγRIII, the binding of anti-FcγRII/III (2.4G2) to FcγRII was blocked using K9-361. DBA/1 mice express high levels of FcγRIII than the other strains (C).
Figure 2.
Regulation of expression of FcγRI (A), II (B), and III (C) by macrophages of C57BL/6, BALB/c, DBA/1, and B10.RIII after stimulation with HAGG. The number of PCR cycles needed to detect these receptors in unstimulated cells was subtracted from the number needed in stimulated cells, after correction for GAPDH content. Note that CIA sensitive mice show a prolonged up regulation of FcγRI and III and a prolonged down regulation of FcγRII, whereas BALB/c and C57BL/6 mice show an up regulation of FcγRII at a later time.
Figure 3.
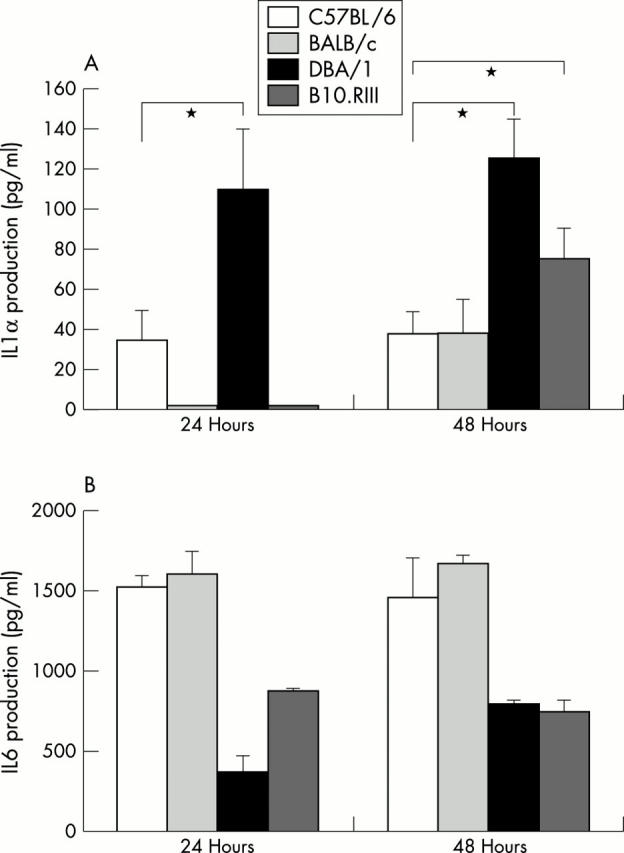
IL1α (A) and IL6 (B) production by macrophages of C57BL/6, BALB/c, DBA/1, and B10.RIII after stimulation by HAGG. IL1α production was measured by ELISA and was higher in DBA/1 mice at day 1 and in DBA/1 and B10.RIII at day 2, indicating higher cytokine production after stimulation with HAGG by macrophages of CIA sensitive strains. This is in contrast with IL6 levels produced by peritoneal macrophages after HAGG stimulation. DBA/1 and B10.RIII mice produced significantly less IL6 than the other strains.
Figure 4.
Collagenase/gelatinase production by macrophages of C57BL/6, BALB/c, DBA/1, and B10.RIII mice after stimulation with HAGG. Collagenase/gelatinase production was measured at day 2 after stimulation with HAGG, using a specific fluorescent substrate. The activity of the enzyme was measured by following fluorescence at different time points. Basal enzyme activity was subtracted from the levels of enzyme activity after stimulation with HAGG. Interestingly, enzyme activity of DBA/1 mice is largely increased at day 2 after stimulation with HAGG, compared with other strains.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams V. M., Cambridge G., Lydyard P. M., Edwards J. C. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000 Mar;43(3):608–616. doi: 10.1002/1529-0131(200003)43:3<608::AID-ANR18>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Arner E. C., Tortorella M. D. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995 Sep;38(9):1304–1314. doi: 10.1002/art.1780380919. [DOI] [PubMed] [Google Scholar]
- Baumann U., Köhl J., Tschernig T., Schwerter-Strumpf K., Verbeek J. S., Schmidt R. E., Gessner J. E. A codominant role of Fc gamma RI/III and C5aR in the reverse Arthus reaction. J Immunol. 2000 Jan 15;164(2):1065–1070. doi: 10.4049/jimmunol.164.2.1065. [DOI] [PubMed] [Google Scholar]
- Blom A. B., van Lent P. L., van Vuuren H., Holthuysen A. E., Jacobs C., van de Putte L. B., van de Winkel J. G., van den Berg W. B. Fc gamma R expression on macrophages is related to severity and chronicity of synovial inflammation and cartilage destruction during experimental immune-complex-mediated arthritis (ICA). Arthritis Res. 2000 Aug 31;2(6):489–503. doi: 10.1186/ar131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom A. B., van Lent P. L., Holthuysen A. E., van den Berg W. B. Immune complexes, but not streptococcal cell walls or zymosan, cause chronic arthritis in mouse strains susceptible for collagen type II auto-immune arthritis. Cytokine. 1999 Dec;11(12):1046–1056. doi: 10.1006/cyto.1999.0503. [DOI] [PubMed] [Google Scholar]
- Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999 Mar;26(3):717–719. [PubMed] [Google Scholar]
- Cambier J. C. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J Immunol. 1995 Oct 1;155(7):3281–3285. [PubMed] [Google Scholar]
- Chouchakova N., Skokowa J., Baumann U., Tschernig T., Philippens K. M., Nieswandt B., Schmidt R. E., Gessner J. E. Fc gamma RIII-mediated production of TNF-alpha induces immune complex alveolitis independently of CXC chemokine generation. J Immunol. 2001 Apr 15;166(8):5193–5200. doi: 10.4049/jimmunol.166.8.5193. [DOI] [PubMed] [Google Scholar]
- Clynes R., Dumitru C., Ravetch J. V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998 Feb 13;279(5353):1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- Clynes R., Ravetch J. V. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995 Jul;3(1):21–26. doi: 10.1016/1074-7613(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Clynes R., Takechi Y., Moroi Y., Houghton A., Ravetch J. V. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke T. D., Richer S., Hurd E., Jasin H. E. Localization of antigen-antibody complexes in intraarticular collagenous tissues. Ann N Y Acad Sci. 1975 Jun 13;256:10–24. doi: 10.1111/j.1749-6632.1975.tb36032.x. [DOI] [PubMed] [Google Scholar]
- Daëron M., Latour S., Malbec O., Espinosa E., Pina P., Pasmans S., Fridman W. H. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995 Nov;3(5):635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- Deo Y. M., Graziano R. F., Repp R., van de Winkel J. G. Clinical significance of IgG Fc receptors and Fc gamma R-directed immunotherapies. Immunol Today. 1997 Mar;18(3):127–135. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- Ditzian-Kadanoff R. Testicular-associated immune deviation and prevention of adjuvant-induced arthritis by three tolerization methods. Scand J Immunol. 1999 Aug;50(2):150–158. doi: 10.1046/j.1365-3083.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Blades S., Cambridge G. Restricted expression of Fc gammaRIII (CD16) in synovium and dermis: implications for tissue targeting in rheumatoid arthritis (RA). Clin Exp Immunol. 1997 Jun;108(3):401–406. doi: 10.1046/j.1365-2249.1997.3941286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger M., Fischer G. F., Wolff K., Petzelbauer P. Immune complexes from vasculitis patients bind to endothelial Fc receptors independent of the allelic polymorphism of FcgammaRIIa. J Invest Dermatol. 1999 Jul;113(1):56–60. doi: 10.1046/j.1523-1747.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Isakov N. Immunoreceptor tyrosine-based activation motif (ITAM), a unique module linking antigen and Fc receptors to their signaling cascades. J Leukoc Biol. 1997 Jan;61(1):6–16. doi: 10.1002/jlb.61.1.6. [DOI] [PubMed] [Google Scholar]
- Johansson A. C., Sundler M., Kjellén P., Johannesson M., Cook A., Lindqvist A. K., Nakken B., Bolstad A. I., Jonsson R., Alarcón-Riquelme M. Genetic control of collagen-induced arthritis in a cross with NOD and C57BL/10 mice is dependent on gene regions encoding complement factor 5 and FcgammaRIIb and is not associated with loci controlling diabetes. Eur J Immunol. 2001 Jun;31(6):1847–1856. doi: 10.1002/1521-4141(200106)31:6<1847::aid-immu1847>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Johansson A. C., Vestberg M., Holmdahl R. Non-major histocompatibility complex dependent variations in lymphocyte activity between inbred mouse strains susceptible to various autoimmune diseases. Scand J Immunol. 2000 Jul;52(1):21–29. doi: 10.1046/j.1365-3083.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- Köhl J., Gessner J. E. On the role of complement and Fc gamma-receptors in the Arthus reaction. Mol Immunol. 1999 Sep-Oct;36(13-14):893–903. doi: 10.1016/s0161-5890(99)00111-x. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Munder M., Mallo M., Eichmann K., Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998 Jun 15;187(12):2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Y., Ueda S., Ohno H., Hamano Y., Tanaka M., Shiratori T., Yamazaki T., Arase H., Arase N., Karasawa A. Resistance of Fc receptor- deficient mice to fatal glomerulonephritis. J Clin Invest. 1998 Sep 15;102(6):1229–1238. doi: 10.1172/JCI3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Liao J. K., Galis Z. S., Tripathi S., Laufs U., Tripathi J., Chai N. N., Xu X. P., Jovinge S., Shah P. K. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999 Apr 23;274(17):11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V. Fc receptors. Curr Opin Immunol. 1997 Feb;9(1):121–125. doi: 10.1016/s0952-7915(97)80168-9. [DOI] [PubMed] [Google Scholar]
- Schiller C., Janssen-Graalfs I., Baumann U., Schwerter-Strumpf K., Izui S., Takai T., Schmidt R. E., Gessner J. E. Mouse FcgammaRII is a negative regulator of FcgammaRIII in IgG immune complex-triggered inflammation but not in autoantibody-induced hemolysis. Eur J Immunol. 2000 Feb;30(2):481–490. doi: 10.1002/1521-4141(200002)30:2<481::AID-IMMU481>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Silacci P., Dayer J. M., Desgeorges A., Peter R., Manueddu C., Guerne P. A. Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J Biol Chem. 1998 May 29;273(22):13625–13629. doi: 10.1074/jbc.273.22.13625. [DOI] [PubMed] [Google Scholar]
- Sylvestre D., Clynes R., Ma M., Warren H., Carroll M. C., Ravetch J. V. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med. 1996 Dec 1;184(6):2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Beckerman K. P., Unanue E. R. Immune complexes inhibit antimicrobial responses through interleukin-10 production. Effects in severe combined immunodeficient mice during Listeria infection. J Clin Invest. 1995 Apr;95(4):1628–1634. doi: 10.1172/JCI117837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lent P. L., Holthuysen A. E., Van Rooijen N., Van De Putte L. B., Van Den Berg W. B. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998 Jul;57(7):408–413. doi: 10.1136/ard.57.7.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lent P. L., Van den Hoek A. E., Van den Bersselaar L. A., Spanjaards M. F., Van Rooijen N., Dijkstra C. D., Van de Putte L. B., Van den Berg W. B. In vivo role of phagocytic synovial lining cells in onset of experimental arthritis. Am J Pathol. 1993 Oct;143(4):1226–1237. [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Agnello V., Kunkel H. G. Gamma globulin complexes in synovial fluids of patients with rheumatoid arthritis. Partial characterization and relationship to lowered complement levels. Clin Exp Immunol. 1970 May;6(5):689–706. [PMC free article] [PubMed] [Google Scholar]
- Yang H. T., Jirholt J., Svensson L., Sundvall M., Jansson L., Pettersson U., Holmdahl R. Identification of genes controlling collagen-induced arthritis in mice: striking homology with susceptibility loci previously identified in the rat. J Immunol. 1999 Sep 1;163(5):2916–2921. [PubMed] [Google Scholar]
- Yanni G., Whelan A., Feighery C., Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994 Jan;53(1):39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T., Kubo S., Yoshino T., Ujike A., Matsumura K., Ono M., Ravetch J. V., Takai T. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999 Jan 4;189(1):187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent P. L., Holthuysen A. E., van Rooijen N., van de Loo F. A., van de Putte L. B., van den Berg W. B. Phagocytic synovial lining cells regulate acute and chronic joint inflammation after antigenic exacerbation of smouldering experimental murine arthritis. J Rheumatol. 1998 Jun;25(6):1135–1145. [PubMed] [Google Scholar]
- van Lent P. L., Holthuysen A. E., van den Bersselaar L. A., van Rooijen N., Joosten L. A., van de Loo F. A., van de Putte L. B., van den Berg W. B. Phagocytic lining cells determine local expression of inflammation in type II collagen-induced arthritis. Arthritis Rheum. 1996 Sep;39(9):1545–1555. doi: 10.1002/art.1780390915. [DOI] [PubMed] [Google Scholar]
- van Lent P. L., van den Bersselaar L. A., van den Hoek A. E., van de Loo A. A., van den Berg W. B. Cationic immune complex arthritis in mice--a new model. Synergistic effect of complement and interleukin-1. Am J Pathol. 1992 Jun;140(6):1451–1461. [PMC free article] [PubMed] [Google Scholar]
- van Meurs J. B., van Lent P. L., Singer I. I., Bayne E. K., van de Loo F. A., van den Berg W. B. Interleukin-1 receptor antagonist prevents expression of the metalloproteinase-generated neoepitope VDIPEN in antigen-induced arthritis. Arthritis Rheum. 1998 Apr;41(4):647–656. doi: 10.1002/1529-0131(199804)41:4<647::AID-ART11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- van de Loo F. A., Arntz O. J., Otterness I. G., van den Berg W. B. Protection against cartilage proteoglycan synthesis inhibition by antiinterleukin 1 antibodies in experimental arthritis. J Rheumatol. 1992 Mar;19(3):348–356. [PubMed] [Google Scholar]
- van den Berg W. B., Joosten L. A., Helsen M., van de Loo F. A. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994 Feb;95(2):237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




