Abstract
Objectives: To investigate the specificity of three anti-CD68 monoclonal antibodies (mAbs) for macrophages (Mφ) in immunohistochemistry (IHC) and flow cytometry (FACS).
Methods: IHC was performed on cryostat sections of rheumatoid arthritis (RA) and osteoarthritis (OA) synovial membranes using the anti-CD68 mAbs KP1, EBM11, and PGM1, and the fibroblast (FB) markers CD90 and prolyl 4-hydroxylase. Expression of CD68 was also analysed by FACS on the monocytic cell lines THP-1 and U937, as well as on synovial fibroblasts (SFB), skin FB, and gingival FB (both surface and intracellular staining).
Results: In IHC, there was an overlap between CD68 (mAbs KP1 and EBM11) and the FB markers CD90/prolyl 4-hydroxylase in the lining layer, diffuse infiltrates, and stroma of RA and OA synovial membranes. In FACS analysis of THP-1 and U937 cells, the percentage of cells positive for the anti-CD68 mAbs KP1 and EBM11 progressively increased from surface staining of unfixed cells, to surface staining of pre-fixed cells, to intracellular staining of the cells. Upon intracellular FACS of different FB, nearly all cells were positive for KP1 and EBM11, but only a small percentage for PGM1. In surface staining FACS, a small percentage of FB were positive for all three anti-CD68 mAbs.
Conclusion: An overlap between CD68 (mAbs KP1 or EBM11) and the FB markers CD90 or prolyl 4-hydroxylase may prevent unequivocal identification of Mφ in synovial tissue by IHC or in monocytic cells and FB upon intracellular FACS. This may be due to sharing of common markers by completely different cell lineages.
Full Text
The Full Text of this article is available as a PDF (1.7 MB).
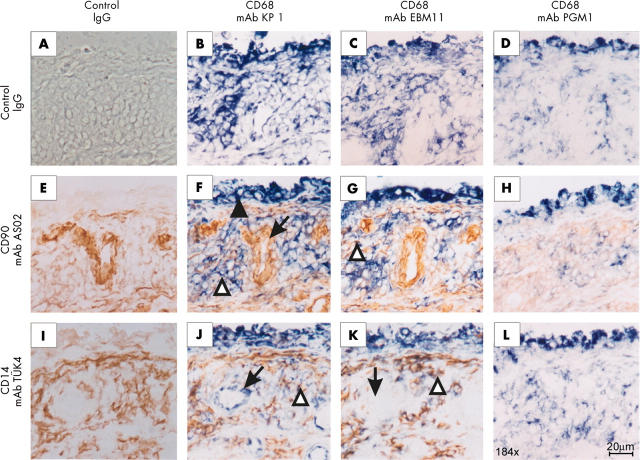
Figure 1.
Immunohistochemical analysis of synovial tissue from one representative patient with RA for CD68 (mAbs KP1, EBM11, and PGM1), the FB marker CD90 (mAb AS02), and the monocyte/ Mφ marker CD14 (mAb TÜK4). Control isotype matched mAbs showed no positive reaction (fig 1A). The anti-CD68 mAbs KP1 (B) and EBM11 (C) strongly stained cells in the lining layer (filled arrowhead in fig 1F), diffuse infiltrates, and stroma. The mAb KP1 also detected endothelial cells (B; arrow in fig 1J). The anti-CD90 mAb AS02 very faintly stained cells in the lining layer, whereas this mAb strongly stained endothelial cells (E; arrow in fig 1F). Cells in diffuse infiltrates and stroma were weakly stained by this mAb. The anti-CD14 mAb TÜK4 faintly stained cells in the lining layer (I). This mAb strongly stained cells in the diffuse infiltrates and the stroma. After double staining experiments with the anti-CD68 mAb KP1 or EBM11 (blue colour) and the anti-CD90 mAb AS02 (brown colour), double-positive cells in diffuse infiltrates were seen (F and G; open arrowheads figs 1F and G). After double staining experiments with the anti-CD68 KP1 or EBM11 (blue colour) and the anti-CD14 mAb TüK4 (brown colour), double-positive cells in diffuse infiltrates were seen (J and K; open arrowheads in figs 1J and K). The anti-CD68 mAb PGM1 strongly stained cells in the lining layer and diffuse infiltrates (D, H, and L). This strong staining was only seen after fixation of the tissue sections with PFA followed by heating in SSC.
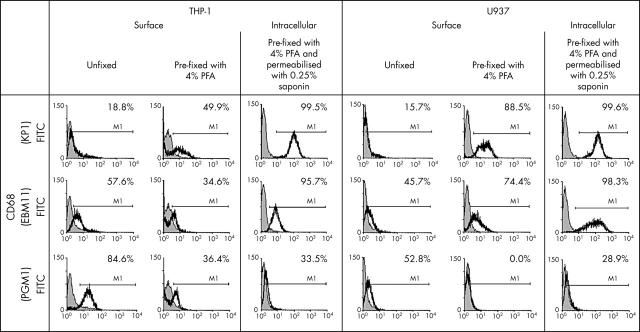
Figure 2.
Flow cytometry analysis of the human monocytic cell lines THP-1 and U937 for CD68 (mAbs KP1, EBM11, and PGM1) using unfixed cells, cells after pre-fixation with 4% PFA, or cells after pre-fixation and permeabilisation with 0.25% saponin (in each case, data from one representative experiment are shown). In both cell lines, the percentages of positive cells for the anti-CD68 mAbs KP1 and EBM11 increased from surface staining of unfixed cells over surface staining of pre-fixed cells to intracellular staining of pre-fixed and permeabilised cells. In contrast, the percentages of positive cells for the anti-CD68 mAb PGM1 was highest on unfixed cells and decreased after pre-fixation with PFA or after pre-fixation with PFA and permeabilisation with saponin (isotype control: shaded curve; specific antibodies: black line).
Figure 3.
Flow cytometry analysis of RA SFB, OA SFB, and JT SFB, skin FB, and gingival FB for intracellular expression of prolyl 4-hydroxylase or CD68 (mAbs KP1, EBM11, and PGM1) and surface expression of the fibroblast marker CD90 (in each case, data from one representative experiment are shown). The anti-CD68 mAbs KP1 and EBM11 showed a positive intracellular staining in a high percentage of SFB (RA, OA, and JT), skin FB, and gingival FB. In contrast, the anti-CD68 mAb PGM1 detected almost no cells in any of the FB populations. In all FB populations, nearly all cells stained positively for the FB marker prolyl 4-hydroxylase, whereas the percentage of positive cells for the FB marker CD90 varied depending on the FB population (isotype control: shaded curve; specific antibodies: black line).
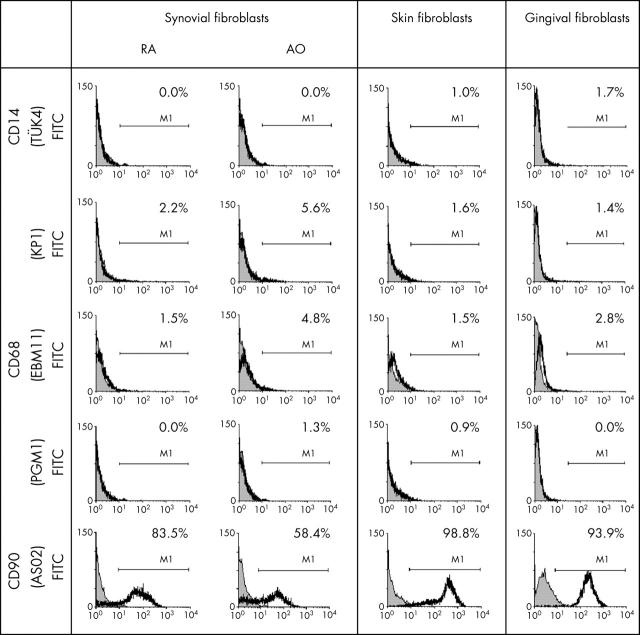
Figure 4.
Flow cytometry analysis of RA SFB, OA SFB, and JT SFB, skin FB, and gingival FB for surface expression of CD14, CD68 (mAbs KP1, EBM11, and PGM1) and the FB marker CD90 (in each case, data from one representative experiment are shown). In surface staining experiments with unfixed RA SFB, OA SFB, skin FB, and gingival FB, only a small percentage of the cells stained positively for the three anti-CD68 mAbs KP1, EBM11, and PGM1. Almost no cells stained positively for the macrophage marker CD14, whereas the percentage of positive cells for the FB marker CD90 varied depending on the FB population (isotype control: shaded curve; specific antibodies: black line).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barrera P., Blom A., van Lent P. L., van Bloois L., Beijnen J. H., van Rooijen N., de Waal Malefijt M. C., van de Putte L. B., Storm G., van den Berg W. B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000 Sep;43(9):1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cuevas E. C., Bateman A. C., Wilkins B. S., Johnson P. A., Williams J. H., Lee A. H., Jones D. B., Wright D. H. Microwave antigen retrieval in immunocytochemistry: a study of 80 antibodies. J Clin Pathol. 1994 May;47(5):448–452. doi: 10.1136/jcp.47.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Wilkinson L. S., Pitsillides A. A. Palisading cells of rheumatoid nodules: comparison with synovial intimal cells. Ann Rheum Dis. 1993 Nov;52(11):801–805. doi: 10.1136/ard.52.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Nielsen J. C., Torczynski E., Yu R., Franklin W. A. CD68 antigen expression by human retinal pigment epithelial cells. Exp Eye Res. 1992 Jul;55(1):21–28. doi: 10.1016/0014-4835(92)90087-9. [DOI] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Pileri S., Gambacorta M., Bigerna B., Durkop H., Eitelbach F., Thiele J., Pacini R., Cavaliere A. PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol. 1993 May;142(5):1359–1372. [PMC free article] [PubMed] [Google Scholar]
- Greaves David R., Gordon Siamon. Macrophage-specific gene expression: current paradigms and future challenges. Int J Hematol. 2002 Jul;76(1):6–15. doi: 10.1007/BF02982713. [DOI] [PubMed] [Google Scholar]
- Heinemann D. E., Lohmann C., Siggelkow H., Alves F., Engel I., Köster G. Human osteoblast-like cells phagocytose metal particles and express the macrophage marker CD68 in vitro. J Bone Joint Surg Br. 2000 Mar;82(2):283–289. [PubMed] [Google Scholar]
- Hiraoka T., Izumi Y., Sueda T. Immunochemical detection of CD14 on human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1998 Aug;13(4):246–252. doi: 10.1111/j.1399-302x.1998.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Hogg N., Palmer D. G., Revell P. A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology. 1985 Dec;56(4):673–681. [PMC free article] [PubMed] [Google Scholar]
- Kinne R. W., Bräuer R., Stuhlmüller B., Palombo-Kinne E., Burmester G. R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000 Apr 12;2(3):189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R. W., Palombo-Kinne E., Emmrich F. Activation of synovial fibroblasts in rheumatoid arthritis. Ann Rheum Dis. 1995 Jun;54(6):501–504. doi: 10.1136/ard.54.6.501-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllyharju J. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 1998 Feb;16(7):357–368. doi: 10.1016/s0945-053x(98)90009-9. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Haringman J. J., Post W. J., Versendaal J., Breedveld F. C., Tak P. P. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology (Oxford) 1999 Nov;38(11):1074–1080. doi: 10.1093/rheumatology/38.11.1074. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Reece R. J., Barg E. C., Smeets T. J., Farnell J., Rosenburg R., Veale D. J., Breedveld F. C., Emery P., Tak P. P. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 2000 Aug;43(8):1820–1830. doi: 10.1002/1529-0131(200008)43:8<1820::AID-ANR18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Micklem K., Rigney E., Cordell J., Simmons D., Stross P., Turley H., Seed B., Mason D. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989 Sep;73(1):6–11. doi: 10.1111/j.1365-2141.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. S., Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991 Oct 1;174(4):827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasad M. P., Terpstra V., Kondratenko N., Quehenberger O., Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach A., Kraft R., Herrmann K., Haustein U. F., Anderegg U. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res. 1998 Jul;290(7):360–366. doi: 10.1007/s004030050318. [DOI] [PubMed] [Google Scholar]
- Sack U., Stiehl P., Geiler G. Distribution of macrophages in rheumatoid synovial membrane and its association with basic activity. Rheumatol Int. 1994;13(5):181–186. doi: 10.1007/BF00390265. [DOI] [PubMed] [Google Scholar]
- Smith S. C., Folefac V. A., Osei D. K., Revell P. A. An immunocytochemical study of the distribution of proline-4-hydroxylase in normal, osteoarthritic and rheumatoid arthritic synovium at both the light and electron microscopic level. Br J Rheumatol. 1998 Mar;37(3):287–291. doi: 10.1093/rheumatology/37.3.287. [DOI] [PubMed] [Google Scholar]
- Strobl H., Scheinecker C., Csmarits B., Majdic O., Knapp W. Flow cytometric analysis of intracellular CD68 molecule expression in normal and malignant haemopoiesis. Br J Haematol. 1995 Aug;90(4):774–782. doi: 10.1111/j.1365-2141.1995.tb05195.x. [DOI] [PubMed] [Google Scholar]
- Umino T., Sköld C. M., Pirruccello S. J., Spurzem J. R., Rennard S. I. Two-colour flow-cytometric analysis of pulmonary alveolar macrophages from smokers. Eur Respir J. 1999 Apr;13(4):894–899. doi: 10.1034/j.1399-3003.1999.13d33.x. [DOI] [PubMed] [Google Scholar]
- Wilkins B. S., Jones D. B. Heterogeneity of expression of CD68 and other macrophage-associated antigens in human long-term bone marrow culture. Biologicals. 1996 Dec;24(4):333–337. doi: 10.1006/biol.1996.0047. [DOI] [PubMed] [Google Scholar]
- Wong P., Cuello C., Bertouch J. V., Roberts-Thomson P. J., Ahern M. J., Smith M. D., Youssef P. P. The effects of pulse methylprednisolone on matrix metalloproteinase and tissue inhibitor of metalloproteinase-1 expression in rheumatoid arthritis. Rheumatology (Oxford) 2000 Oct;39(10):1067–1073. doi: 10.1093/rheumatology/39.10.1067. [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Kunisch E., Pfeiffer R., Hirth A., Stahl H. D., Sack U., Laube A., Liesaus E., Roth A., Palombo-Kinne E. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture--primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2000 Nov 21;3(1):72–76. doi: 10.1186/ar142. [DOI] [PMC free article] [PubMed] [Google Scholar]