Abstract
To determine the function of germ cell nuclear factor (GCNF) in female reproduction, we generated an oocyte-specific GCNF knockout mouse model (GCNFfl/flZp3Cre+). These mice displayed hypofertility due to prolonged diestrus phase of the estrous cycle and aberrant steroidogenesis. These reproductive defects were secondary to a primary defect in the oocytes, in which expression of the paracrine transforming growth factor-β signaling molecules, bone morphogenetic protein 15 (BMP-15) and growth differentiation factor 9 (GDF-9), were up-regulated in GCNFfl/flZp3Cre+ females at diestrus. This was a direct effect of GCNF, as molecular studies showed that GCNF bound to DR0 elements within the BMP-15 and GDF-9 gene promoters and repressed their reporter activities. Consistent with these findings, abnormal double-oocyte follicles, indicative of aberrant BMP-15/GDF-9 expression, were observed in GCNFfl/flZp3Cre+ females. The Cre/loxP knockout of GCNF in the oocyte has uncovered a new regulatory pathway in ovarian function. Our results show that GCNF directly regulates paracrine communication between the oocyte and somatic cells by regulating the expression of BMP-15 and GDF-9, to affect female fertility.
Keywords: Cre/loxP/gene regulation/nuclear receptor/oocyte/ovary
Introduction
Germ cell nuclear factor (GCNF/RTR/NCNF, NR6A1) is an orphan member of the nuclear receptor superfamily (Chen et al., 1994; Nuclear Receptor Nomenclature Committee, 1999). GCNF functions as a transcription factor that binds to a direct repeat of the sequence AGGTCA with 0 base pair (bp) spacing between the half sites (DR0) element to repress gene transcription in vitro and in vivo (Chen et al., 1994; Yan et al., 1997; Cooney et al., 1998; Fuhrmann et al., 2001). It is expressed in early mouse embryos after the onset of gastrulation (Susens et al., 1997; Chung et al., 2001) and is essential for normal embryonic development and repression of Oct4 expression in somatic cells of early mouse embryos (Chung et al., 2001; Fuhrmann et al., 2001). The DNA binding domain (DBD) of GCNF is essential for the function of GCNF during embryonic development and for Oct4 repression as GCNFlox/lox embryos, in which the DBD of GCNF is deleted but the ligand binding domain (LBD) of GCNF is expressed in the mouse embryo, phenocopy GCNF null mutant (GCNF–/–) mouse embryos (Lan et al., 2002). In adult vertebrates, GCNF is predominantly expressed in the gonads of several species, including mouse, rat and human (Chen et al., 1994; Katz et al., 1997; Agoulnik et al., 1998; Zhang et al., 1998). In the murine testis, GCNF is expressed in postmeiotic round spermatids at both the mRNA and protein levels (Chen et al., 1994; Katz et al., 1997; Lan et al., 2003), while in human testis, it is expressed in pachytene spermatocytes (Agoulnik et al., 1998). In the ovary, GCNF is expressed in oocytes in mouse, Xenopus and zebrafish (Chen et al., 1994; Joos et al., 1996; Katz et al., 1997; Braat et al., 1999). In the mouse ovary, GCNF is exclusively expressed in the oocytes of primary, secondary and pre-ovulatory follicles, but not primordial follicles, at both the mRNA and protein levels (Chen et al., 1994; Katz et al., 1997; Lan et al., 2003). GCNF is also present in ovulated oocytes and pre-implantation embryos, indicating that GCNF may play a maternal role in zygotic development prior to implantation (Lan et al., 2003). The oocyte-specific expression pattern indicates that GCNF may be a transcription factor that plays a role in regulating some aspect of oocyte function.
To determine the role of GCNF during female reproduction and by-pass the embryonic lethality, we employed a Cre/loxP strategy (Figure 1A), similar to a previous report (Gu et al., 1994), to inactivate the GCNF gene by deleting the DBD encoding exon of the GCNF gene specifically in the oocytes of adult mice. By breeding the floxed GCNF mice with male zona pellucida protein 3 (Zp3) gene promoter-driven Cre transgenic mice (de Vries et al., 2000), we generated oocyte-specific GCNF knockout mice. The phenotype of these oocyte-specific GCNF knockout mice was determined. More importantly, the molecular mechanism of the phenotype was characterized. This study provides direct evidence that there exists a paracrine communication between oocytes and adjacent somatic steroidogenic cells during folliculogenesis that modulates the estrous cycle during female reproduction, and this function is mediated by GCNF-dependent regulation of bone morphogenetic protein 15 (BMP-15) and growth differentiation factor 9 (GDF-9) expression.
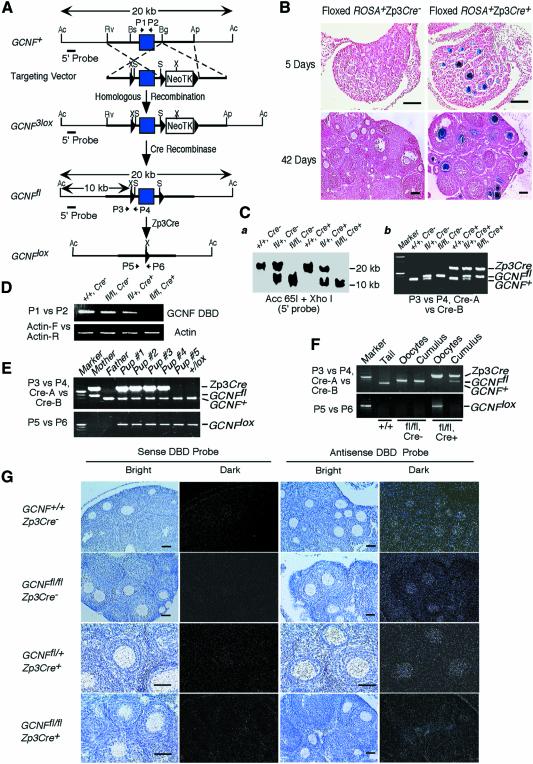
Fig. 1. Generation of an oocyte-specific GCNF knockout mouse model. (A) Structures of the GCNF3lox, GCNFfl and GCNFlox alleles and the targeting vector. LoxP sites, filled triangles; the DBD-encoding exon, filled box. Restriction enzyme sites of Acc65I (Ac), ApaI (Ap), BglI (Bg), BsiEI (Bs), EcoRV (Rv), XhoI (X) and SalI (S) in each allele are shown. Positions of PCR primers (P1 to P6), and the 5′ probe for Southern blot analysis are indicated. (B) Cre recombinase activity on the floxed ROSA transgene in the ovaries determined by β-galactosidase staining. Bar scale, 200 µm. (C) Genotype analysis of tail biopsies. (a) Southern blot analysis showing a 20 kb band for the GCNF+ allele and a 10 kb band for the GCNFfl allele. (b) PCR analysis using primers (P3 and P4) to distinguish the GCNFfl and GCNF+ alleles, and primers (Cre-A and Cre-B) to determine the presence of Zp3Cre transgene. (D) RT–PCR analysis showing the loss of the DBD encoding region of the GCNF mRNA in the GCNFfl/flZp3Cre+ ovary. (E) Genotyping of tail DNA showing the complete deletion of the GCNF DBD encoding exon in the progenies from the cross between GCNFfl/flZp3Cre+ females and wild-type males. (F) PCR analysis showing complete deletion of the GCNF DBD encoding exon in ovulated oocytes, but not in cumulus cells. (G) In situ hybridization showing the loss of the DBD region of the GCNF mRNA in the oocytes of GCNFfl/flZp3Cre+ mice. Bar scale, 50 µm.
Results
Generation of GCNFfl/fl mice
To generate the floxed GCNF mice, we constructed a targeting vector that introduced a gene selection cassette, consisting of the neomycin (Neo) and herpes simplex virus thymidine kinase (TK) genes flanked by loxP sites, downstream of exon 4 of the GCNF gene and a single loxP site 500 bp upstream of exon 4 in the GCNF gene (Lan et al., 2002). Subsequent electroporation of embryonic stem (ES) cells (129 AB1.2 cells) with the targeting vector and selection in Geneticin® (G418) led to ES cell clones containing a GCNF3lox allele (Figure 1A). Two correctly targeted ES cell clones carrying the GCNF3lox allele were amplified and then transiently transfected with a Cre expression plasmid (pOG231) (O’Gorman et al., 1997) to delete the NeoTK selection cassette and leave loxP sites upstream and downstream of exon 4. Southern blot and PCR analyses demonstrated the absence of the NeoTK cassette, and the presence of two loxP sites flanking the DBD encoding exon in eight of 80 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil (FIAU)-resistant ES cell clones (our unpublished data). Five GCNFfl/+ ES clones were injected into C57BL/6 blastocysts to generate chimeric mice. Chimeric mice from each clone transmitted the GCNFfl allele to their offspring. Intercrossing of heterozygous floxed GCNF mice (GCNFfl/+) from each line produced GCNF+/+, GCNFfl/+ and GCNFfl/fl progenies at the expected 1:2:1 Mendelian ratio, for both males and females. GCNFfl/fl mice were healthy and fertile, and had normal GCNF expression in the testis (our unpublished data) and ovary (Figure 1G). These results indicate that insertion of loxP sites upstream and downstream of the DBD encoding exon of the GCNF gene does not cause any hypomorphic effects due to disrupted expression.
Oocyte-specific Cre recombinase activity
Previously generated Zp3Cre transgenic mice have been shown to express Cre recombinase specifically in the ovary (de Vries et al., 2000). To confirm the cell specificity of Cre recombinase in Zp3Cre transgenic mice, we used the floxed ROSA reporter mice (Soriano, 1999). X-gal staining of tissues from progeny of matings between male Zp3Cre transgenic mice with female floxed ROSA mice at different ages was performed. Specific β-galactosidase activity was observed only in the ovary (Figure 1B), and not in the oviduct, uterus, heart, kidney, liver, pituitary and brain in the adult floxed ROSA+Zp3Cre+ females (our unpublished data). Within the ovary, the Cre recombinase activity was observed only in oocytes from the primary to pre-ovulatory stages; no expression was noted in oocytes of primordial follicles or in somatic cells (Figure 1B). Therefore, the Cre recombinase in the Zp3Cre transgenic mice is specifically expressed in the oocytes of primary, secondary and pre-ovulatory follicles, which is similar to the GCNF ovarian expression pattern (Katz et al., 1997; Lan et al., 2003), and is efficiently able to delete the floxed DNA fragment in the mouse genome, as 100% of those oocytes at the primary and later follicular stages stained positively for β-galactosidase activity.
Creation of oocyte-specific GCNF knockout mice
Breeding of male Zp3Cre transgenic mice with female GCNFfl/fl mice produced bigenic heterozygous animals (GCNFfl/+Zp3Cre+). Male GCNFfl/+Zp3Cre+ mice were then bred with GCNFfl/+ females to generate GCNFfl/flZp3Cre+ offspring with an expected Mendelian rate of 12.5%. The genotypes of these animals were determined by Southern blot and PCR analyses (Figure 1C). About half of GCNFfl/flZp3Cre+ mice (310/625) were females, that were oocyte-specific GCNF knockout mice. These oocyte-specific GCNF knockout mice grew normally and were indistinguishable from their GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+ littermates (our unpublished data).
To determine whether the DBD encoding region of GCNF mRNA was completely deleted by the Cre recombinase in the GCNFfl/flZp3Cre+ ovaries, total ovarian RNA was isolated from 1-month-old littermates of GCNFfl/flZp3Cre+ mice and three control groups including GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+ mice, and then subjected to RT–PCR with specific primers for exon 4 of the GCNF gene encoding the DBD. As shown in Figure 1D, the DBD region of GCNF mRNA was absent in the GCNFfl/flZp3Cre+ ovary, but was present in the ovaries of all three control littermates. In situ hybridization also showed that the DBD-encoding region of the GCNF transcript was completely deleted in the GCNFfl/flZp3Cre+ ovaries (Figure 1G). To confirm the above results, female GCNFfl/flZp3Cre+ mice were bred with GCNF+/+ males, and the genotype of the offspring was determined by PCR analysis. As shown in Figure 1E, all the progeny lacked a GCNFfl allele; rather they had a recombined GCNFlox allele generated by the Cre recombinase in the oocytes. PCR analysis of genomic DNA isolated from ovulated oocytes and cumulus granulosa cells showed the absence of GCNFfl allele and presence of GCNFlox allele in ovulated oocytes but not cumulus granulosa cells, confirming complete deletion of GCNFfl allele by Zp3Cre recombinase specifically in oocytes and not the adjacent granulosa cells in GCNFfl/flZp3Cre+ females (Figure 1F). No recombined GCNFlox allele was present in genomic DNA isolated from pituitary and brain of adult GCNFfl/flZp3Cre+ females (our unpublished data). Taken together, these results show that the GCNF DBD-encoding exon was completely deleted by the Zp3Cre recombinase only in oocytes, not in granulosa cells, nor in other organs such as the uterus, pituitary or brain.
Reduced fertility and prolonged estrous cycle in the GCNFfl/flZp3Cre+ females
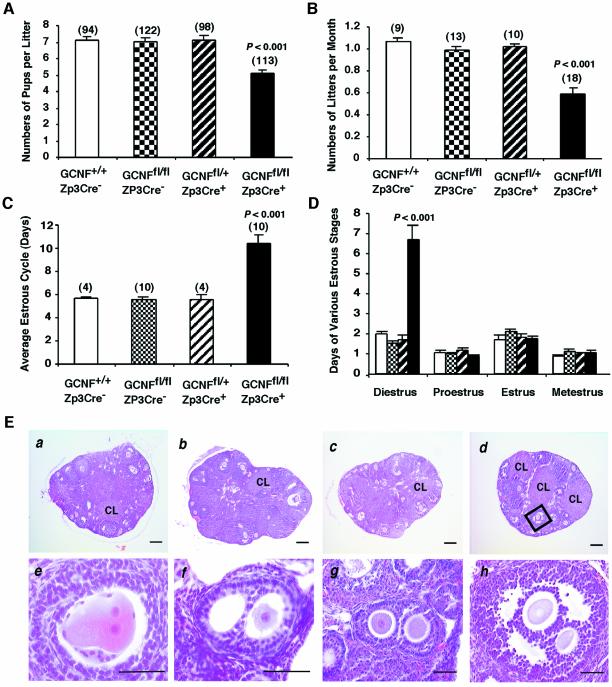
As indicated in Figure 1E, GCNFfl/flZp3Cre+ female mice are fertile. To further determine whether there is any fertility defect in these mice, the females and their control littermates of three different genotypes (GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+) were bred with stud males for up to 1 year. As shown in Figure 2A, the number of pups per litter at day 5 after birth in the knockout group (GCNFfl/flZp3Cre+) was significantly reduced (P < 0.001) compared with the three control groups (GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+). Significantly, the number of litters per month in the knockout group was reduced ∼50% compared with those in the three control groups (Figure 2B). To determine whether the reduced number of litters per month was due to defects in the estrous cycle, animals (2–4 months old) were subjected to a daily Pap smear test for two consecutive months to determine the length of the estrous cycle and the four stages of the estrous cycle (diestrus, proestrus, estrus and metestrus). As shown in Figure 2C, the average length of the estrous cycle in GCNFfl/flZp3Cre+ mice were much longer than those in the three control groups (P < 0.001). This prolonged estrous cycle was due to prolonged diestrus. No differences were observed at the other stages of the estrous cycle (Figure 2D). This prolonged diestrus in the knockout mice probably accounts for the reduced number of litters per month.
Fig. 2. Oocyte-specific GCNF knockout mice display hypofertility, abnormal estrous cycle and double-oocyte follicles. (A) Reduced numbers of pups per litter and (B) reduced numbers of litters per month from the breeding of GCNFfl/flZp3Cre+ females with wild-type males for 1 year. (C) Prolonged length of the estrous cycle in the GCNFfl/flZp3Cre+ females. (D) Prolonged diestrus of the estrous cycle in the GCNFfl/flZp3Cre+ females. (E) Normal ovarian histology except the presence of double oocyte follicles in the GCNFfl/flZp3Cre+ females. (a) GCNF+/+, (b) GCNFfl/fl, (c) GCNFfl/+Zp3Cre+ and (d) GCNFfl/flZp3Cre+ ovaries were 3-month-old littermates. CL, corpus lutea. (e–h) The presence of double-oocyte follicles at the primary (e and f), secondary (g) and antral (h) stages in GCNFfl/flZp3Cre+ ovaries. High magnification of the box in (d) is shown in (h). The bar scale in (a)–(d) is 200 µm. In (e)–(h) the bar scale is 50 µm.
Presence of double oocyte follicles in the GCNFfl/flZp3Cre+ females
There was no difference in the size and weight of ovaries among all four animal groups [GCNF+/+, 0.487 ± 0.031 ovary weight (mg)/body weight (g), n = 3; GCNFfl/fl, 0.424 ± 0.046, n = 3; GCNFfl/+Zp3Cre+, 0.447 ± 0.027, n = 3; and GCNFfl/flZp3Cre+, 0.432 ± 0.038, n = 3]. Histological studies showed that follicles at different stages and corpora lutea were present in the GCNFfl/flZp3Cre+ mice, similar to the three control groups (GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+) (Figure 2E, a–d). Careful analysis of the ovarian histology revealed the presence of double oocytes within some follicles in the oocyte-specific GCNF knockout mice (Figure 2E, d–h). Histological studies on serial ovarian sections showed that no animals in the control groups, GCNF+/+ (seven mice), GCNFfl/fl (six mice) and GCNFfl/+Zp3Cre+ (five mice) had any follicles containing double oocytes. However, in the GCNFfl/flZp3Cre+ mice, six of seven mice at diestrus had one to three follicles containing double oocytes. Among these six mice, four mice at the age ranging from 1 to 12 months had only one double-oocyte follicle. A 3-month-old GCNFfl/flZp3Cre+ mouse had two double-oocyte follicles, while another GCNFfl/flZp3Cre+ mouse at the same age had three double-oocyte follicles. These double-oocyte follicles may be derived from a single oocyte, because two nucleus-like structures were observed in one oocyte (Figure 2E, e). The double-oocyte follicles continued to develop from the primary stage to the antral stage, and no obvious defects in the histology of somatic cells of these double-oocyte follicles were observed (Figure 2E, f–h).
Reduced steroid hormone levels in theGCNFfl/flZp3Cre+ mice at diestrus
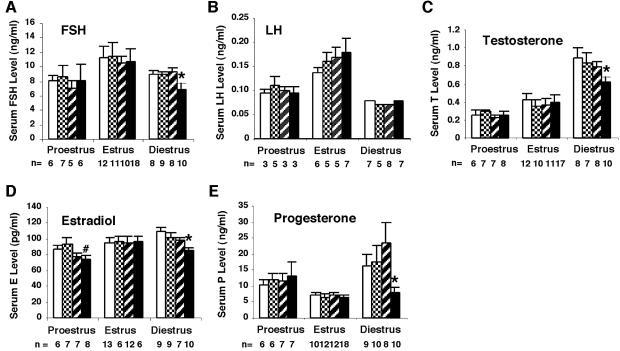
The prolonged estrus cycle indicated that there could be alterations in the levels of circulating gonadotropins and steroid hormones in the GCNFfl/flZp3Cre+ mice. Using standard radioimmunoassays, hormone levels of 2- to 3-month-old mice at various stages of the estrous cycle were determined in a double-blind test. As shown in Figure 3 and Supplementary table, no significant changes in follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone and progesterone levels were observed in the GCNFfl/flZp3Cre+ mice at proestrus and estrus. No significant changes in estradiol levels were observed in the GCNFfl/flZp3Cre+ females at estrus (Figure 3D). Although estradiol levels in the GCNFfl/flZp3Cre+ females at proestrus were lower than those of GCNF+/+ and GCNFfl/fl controls, there was no significant change in the estradiol levels between GCNFfl/flZp3Cre+ and GCNFfl/+Zp3Cre+ controls (Figure 3D). However, at diestrus, gonadotropin FSH, but not LH levels, and steroid hormones including testosterone, estradiol and progesterone levels were significantly reduced in the GCNFfl/flZp3Cre+ mice, when compared with the three control groups (GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+) (P < 0.05). The reduced steroid hormone levels at diestrus could account for the abnormal estrous cycle observed in the GCNFfl/flZp3Cre+ mice.
Fig. 3. Serum levels of gonadotropins and steroid hormones in the GCNFfl/flZp3Cre+ females during the estrous cycle. (A) FSH, (B) LH, (C) testosterone, (D) estradiol and (E) progesterone levels in the GCNFfl/flZp3Cre+ (filled bar), and three control groups, GCNF+/+ (open bar), GCNFfl/fl (grid bar) and GCNFfl/+Zp3Cre+ (striped bar), are presented as means ± SEs among the indicated numbers of samples in each group in the bar graph. *P < 0.05, when compared with three control groups at diestrus. #P < 0.05, when compared with GCNF+/+ or GCNFfl/fl at estrus.
Mis-expression of key steroidogenic genes in the GCNFfl/flZp3Cre+ mice at diestrus
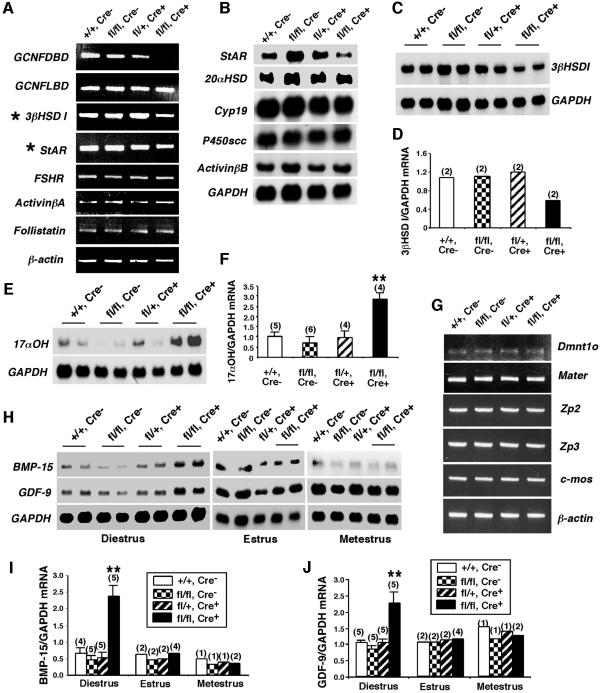
Since steroid hormone levels in the GCNFfl/flZp3Cre+ females at diestrus were reduced (Figure 3), the expression of key enzymatic and regulatory genes involved in steroidogenesis in the somatic cells of the ovary was analyzed. As determined by RT–PCR or northern blot analyses (Figure 4A–D), the expression levels of steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase I (3βHSD I) were reduced in the GCNFfl/flZp3Cre+ovaries at diestrus compared with control animals. In contrast, the expression of 17-α hydroxylase (17αOH) was increased compared with control animals (Figure 4E and F). Quantitative RT–PCR analyses confirmed the above results (Supplementary figure 1). Other ovarian steroidogenic enzymatic genes including cholesterol side chain cleavage cytochrome P450 protein (P450scc), Cyp19 (aromatase) and 20α-hydroxysteroid dehydrogenase (20αHSD) were not affected in the GCNFfl/flZp3Cre+ ovaries, neither were FSHR, activin βA, activin βB, follistatin, LHR and inhibin α, which are expressed in the somatic cells of the ovary (Figure 4A and B, and Supplementary figure 1). Therefore, inactivation of the GCNF gene in the oocytes indirectly caused mis-expression of key components of the steroidogenic pathway in somatic cells, such as StAR, 3βHSD I and 17αOH, which leads to reduced steroid hormone levels in the GCNFfl/flZp3Cre+ females at diestrus. Indeed, we have searched the StAR, 3βHSD I and 17αOH gene promoters for DR0 elements and found none, which, in conjunction with no expression of GCNF or Cre activity in the somatic cells of the ovary in the oocyte-specific GCNF knockouts (Figure 1), supports the notion that the effects of loss of GCNF in the oocyte on somatic gene expression is indirect.
Fig. 4. Expression of ovarian marker genes in the GCNFfl/flZp3Cre+ ovary. (A–F) Expression of key ovarian genes expressed in somatic cells of GCNF+/+ (+/+, Cre–), GCNFfl/fl (fl/fl, Cre–), GCNFfl/+Zp3Cre+ (fl/+, Cre+) and GCNFfl/flZp3Cre+ (fl/fl, Cre+) ovaries at diestrus (day 1–2). (A) RT–PCR showing the reduced StAR and 3βHSD I expression in the GCNFfl/flZp3Cre+ mice. (B–F) Nothern blot analyses showing the mis-expression of (B) StAR, (C and D) 3βHSD I and (E and F) 17αOH in GCNFfl/flZp3Cre+ mice. Quantitative 3βHSD I and 17αOH mRNA levels in the northern blots are shown in the bar graph in (D) and (F), respectively. (G–J) Expression of oocyte marker genes. (G) RT–PCR showing expression of oocyte genes, Dmnt1o, Mater, Zp2, Zp3 and c-mos, in ovaries. Experiments were repeated twice using two individual animals. (H) Representative radiographs of northern blot analyses showing the BMP-15 and GDF-9 expression. (I and J) Quantitative ovarian (I) BMP-15 and (J) GDF-9 mRNA levels in northern blots. For (F), (I) and (J), relative mRNA levels (normalized to GAPDH mRNA levels) are presented as means ± SEs from various numbers of animal samples (indicated by alphabetic numbers) of each genotype. **P < 0.01 compared with three other groups at diestrus.
Overexpression of oocyte-specific GDF-9 and BMP-15 genes in the GCNFfl/flZp3Cre+ mice at diestrus
Since GCNF is exclusively expressed in oocytes (Chen et al., 1994; Katz et al., 1997) and functions as a transcription factor to regulate gene expression (Fuhrmann et al., 2001), the target genes for GCNF in the ovary must be expressed in the oocytes. These potential GCNF target gene products can be secreted from the oocyte, and directly or indirectly regulate steroidogenesis in the somatic cells of the ovary. Oocyte-secreted transforming growth factor-β (TGF-β) family members, GDF-9 and BMP-15, are two candidate GCNF target genes, as GDF-9 and BMP-15 have been shown to be involved in paracrine signaling to adjacent somatic cells during steroidogenesis (Dong et al., 1996; Elvin et al., 1999a<//em>,b, 2000; Otsuka et al., 2000, 2001; Solovyeva et al., 2000; Vitt et al., 2000a,b). To gain insight into the molecular mechanisms of the ovarian defects in GCNFfl/flZp3Cre+ females, the expression levels of several known oocyte-specific genes, including BMP-15 and GDF-9 in GCNFfl/flZp3Cre+ females, were determined. As shown by RT–PCR (Figure 4G), the expression of zona pellucida protein 2 (Zp2), Zp3, c-mos, maternal antigen that embryos require (Mater) and oocyte-specific DNA methyltransferase-1 (Dnmt1o) were not altered in the GCNFfl/flZp3Cre+ ovaries at diestrus. However, the expression levels of BMP-15 and GDF-9 were increased >2-fold in the GCNFfl/flZp3Cre+ ovaries at diestrus but not at estrus or metestrus, compared with the levels in GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+ ovaries, as determined by northern blot analyses (Figure 4H–J). These results indicate that BMP-15 and GDF-9 are two potential target genes whose expression can be negatively regulated by GCNF within the oocyte at diestrus.
Expression of BMP-15 and GDF-9 is directly regulated by GCNF
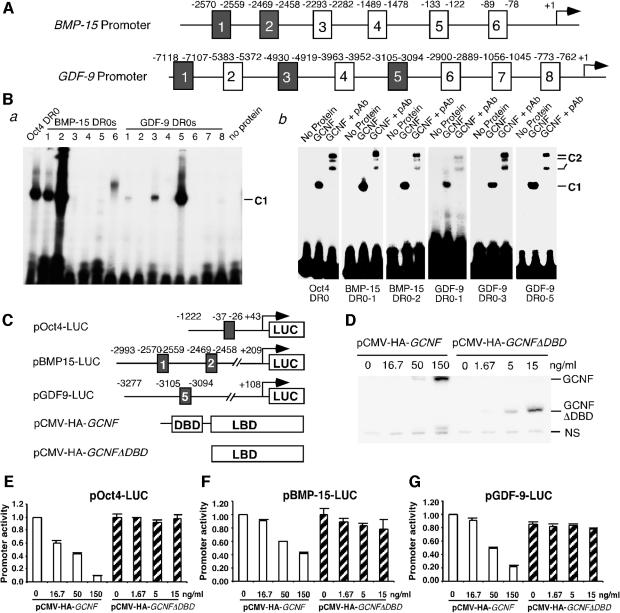
The increased expression of BMP-15 and GDF-9 genes in GCNFfl/flZp3Cre+ ovaries at diestrus (Figure 4H–J) prompted us to test whether these two genes can be directly regulated by GCNF. We searched the 5′ DNA sequences of mouse BMP-15 and GDF-9 genes in the Celera mouse genome database, and found that there are multiple putative DR0 elements in these two promoters with 1–4 bp mismatches from the consensus DR0 sequence (Figure 5A). Electrophoretic mobility shift assays (EMSAs) were then performed to determine whether GCNF can bind to these putative DR0 elements (Figure 5B). Radiolabeled DR0 oligonucleotides from the Oct4 promoter, as previously reported (Fuhrmann et al., 2001), were used as a positive control for EMSAs. We found that two BMP-15 DR0s (BMP-15 DR0-1 and BMP15 DR0-2) and three GDF-9 DR0s (GDF-9 DR0-1, GDF-9 DR0-3 and GDF-9 DR0-5) can bind in vitro translated GCNF, forming a GCNF–DR0 DNA complex (Figure 5B, a). Addition of specific anti-GCNF antibodies (Lan et al., 2003) reduced the mobility of these GCNF–DR0 DNA complexes (Figure 5B, b). Although a DNA–protein complex was observed in EMSA using BMP15 DR0-6 as a probe (Figure 5B, a), addition of anti-GCNF antibodies did not super-shift this complex (our unpublished data), indicating that this complex does not contain GCNF. Our results suggest that GCNF can bind to multiple DR0 elements in the BMP-15 and GDF-9 promoters, forming GCNF–DR0 DNA complexes.
Fig. 5. Direct regulation of the BMP-15 and GDF-9 expression by GCNF. (A) Schematic representation of putative DR0s in the mouse BMP-15 and GDF-9 promoters. (B) EMSAs showing the binding of in vitro translated GCNF to multiple DR0 elements in the BMP-15 and GDF-9 promoters. (a) GCNF was incubated with different 32P-labeled oligonucleotide probes containing the putative DR0 elements. (b) Retarded DNA-protein complexes in EMSAs by anti-GCNF antibodies (pAb). C1, GCNF–DR0 DNA complexes; C2, antibody retarded GCNF–DR0 DNA complexes. (C) Schematic representation of GCNF expression plasmids and luciferase reporter plasmids containing Oct4, BMP-15 or GDF-9 promoters. (D) Western blot analysis showing the expression levels of full-length GCNF protein and GCNF mutant protein (GCNFΔDBD) in transfected CHO cells using anti-HA antibodies. NS, non-specific protein. (E, G) Dose-dependent repression of the (E) Oct4, (F) BMP-15 and (G) GDF-9 expression by GCNF, not GCNFΔDBD, in CHO cells. Promoter activities are presented as means ± SDs of the percentages of the total promoter activities (without pCMV-HA-GCNF plasmid) and represent three independent measurements.
Having established that GCNF can bind to DR0 elements in the BMP-15 and GDF-9 promoters, we next performed promoter reporter analysis to determine whether GCNF can directly regulate the transcription of the BMP-15 and GDF-9 genes using transient transfection techniques in Chinese hamster ovary (CHO) cells. Promoter reporter plasmids pBMP-15-LUC, pGDF-9-LUC or pOct4-LUC were transfected into cultured CHO cells along with pCMV-HA-GCNF or pCMV-HA-GCNFΔDBD (Figure 5C). The expression levels of GCNF and GCNFΔDBD were determined by western blot analysis using anti-HA antibodies. As shown in Figure 5D, the amounts of GCNF protein in CHO cells transfected with 16.7, 50 and 150 ng/ml of pCMV-HA-GCNF were comparable to the levels of GCNFΔDBD in CHO cells transfected with 1.67, 5 and 15 ng/ml of pCMV-HA-GCNFΔDBD, respectively. In CHO cells transfected with pOct4-LUC plasmid, GCNF repressed Oct4 reporter activity in a dose-dependent manner, whereas the deletion mutant of GCNF (GCNFΔDBD) did not have any effect on the Oct4 promoter activity (Figure 5E). These results confirm our previous observation that the DBD of GCNF is essential for GCNF to repress Oct4 expression in somatic cells of early mouse embryos and that the LBD of GCNF does not have DNA binding-independent activities during early mouse development (Lan et al., 2002). In cells transfected with pBMP-15-LUC, the BMP-15 promoter activity was also repressed by GCNF, but not by GCNFΔDBD, in a dose-dependent manner (Figure 5F). Similarly, the GDF-9 reporter activity in transfected CHO cells was repressed by GCNF, but not by GCNFΔDBD, in a dose-dependent manner (Figure 5G). As GCNF bound to BMP-15 DR0-1 and BMP-15 DR0-2 in the BMP-15 promoter and GDF-9 DR0-5 in the GDF-9 promoter (Figure 5B), and GCNFΔDBD did not have DNA binding activity to the consensus DR0 elements (our unpublished data), the repression of BMP-15 and GDF-9 reporter activity by GCNF might be due to the binding of GCNF to the DR0 elements via the DBD of GCNF. These results support our in vivo observation that the expression levels of GDF-9 and BMP-15 were increased in GCNFfl/flZp3Cre+ ovaries at diestrus (Figure 4H–J), indicating that GDF-9 and BMP-15 are two direct target genes of GCNF in the oocytes at diestrus.
Discussion
The oocyte-specific GCNF expression pattern suggests that GCNF may play a role in female reproduction (Chen et al., 1994; Katz et al., 1997). The embryonic lethality of the GCNF knockout mice prevented us from analyzing its functional role during female reproduction (Chung et al., 2001; Lan et al., 2002). In this study, we successfully generated floxed GCNF mice that can by-pass early embryonic lethality using a Cre/loxP targeting strategy (Figure 1A). Using Zp3Cre transgenic mice (de Vries et al., 2000), we were able to generate oocyte-specific GCNF knockout mice, in which the GCNF gene was inactivated specifically in oocytes, not in granulosa cells, or in other organs such as pituitary, hypothalamus or uterus (Figure 1). Oocyte-specific GCNF knockout mice display reduced fertility (Figure 2), which is likely to be due to the prolonged estrous cycle, as the reduced numbers of estrous cycles per month (54% of the means of the three control groups) were almost the same as the numbers of litters per month (57% of the means of the three control groups) in the oocyte-specific GCNF knockout mice (Figure 2B and C). The prolonged estrous cycle observed in the oocyte-specific GCNF knockout mice appears to result from defects in ovarian somatic cells such as granulosa, thecal and luteal cells. In fact, circulating steroid hormones including estradiol, progesterone and testosterone levels, and gonadotropins FSH, but not LH, levels were reduced significantly at the diestrus stage of the estrous cycle in the GCNFfl/flZp3Cre+ mice (Figure 3). The reduced steroid hormone and FSH levels (Figure 3) indicate that there is a defect in steroidogenesis in the somatic cells within the ovaries of the GCNFfl/flZp3Cre+ females at diestrus. To support this notion, ovarian somatic cell marker genes, StAR, 3βHSD I and 17αOH, which are involved in steroidogenesis, were found to be mis-expressed in the ovary at diestrus (Figure 4A–F, and Supplementary figure 1). The reduced expression of StAR (Figure 4A and B, and Supplementary figure 1), which mediates the rate-limiting step in steroidogenesis (Stocco, 2001), alone may well cause the reduced estradiol, progesterone and testosterone levels in the GCNFfl/flZp3Cre+ females at diestrus. The reduced expression of 3βHSD I (Figure 4A, C and D, and Supplementary figure 1), an enzyme involved in the conversion of pregnenolone to progesterone (Bain et al., 1991), in combination with the increased expression of 17αOH (Figure 4E and F, and Supplementary figure 1), an enzyme involved in the conversion of progesterone to 17α-hydroxypregnenolone (Lieberman and Warne, 2001), could well explain why progesterone levels are reduced more markedly than either estradiol or testosterone levels in the oocyte-specific GCNF knockout mice at diestrus (Figure 3). The reduced steroidogenesis could also be due to the reduced FSH levels in the GCNFfl/flZp3Cre+ mice at diestrus (Figure 3), as FSH/FSHR signaling is required for female fertility, normal folliculogenesis and steroidogenesis (Kumar et al., 1997; Dierich et al., 1998; Danilovich et al., 2002; Eimerl and Orly, 2002). However, the mechanism by which inactivation of GCNF in oocytes causes reduction in serum FSH levels in the GCNFfl/flZp3Cre+ mice at diestrus remains to be elucidated. Taken together, the oocyte-specific GCNF knockout mice have defects in steroidogenesis of the ovary at the diestrus stage of the estrous cycle, and these defects in steroidogenesis are likely the cause of the abnormal estrous cycle, leading to female subfertility.
Importantly, because GCNF is expressed in germ cells (Chen et al., 1994; Katz et al., 1997) and functions to repress gene transcription (Fuhrmann et al., 2001), the aberrant steroidogenesis observed in the GCNFfl/flZp3Cre+ females is likely due to mis-regulated oocyte paracrine signaling molecules. There is a large body of evidence indicating that the oocyte plays an active role in orchestrating and coordinating the development of follicles by secreting a factor that affects granulosa cell differentiation, steroidogenesis and proliferation (Vanderhyden and Tonary, 1995; Eppig, 2001; Eppig et al., 2002; Matzuk et al., 2002). Two TGF-β family members, GDF-9 and BMP-15, are oocyte-secreted factors involved in the regulation of adjacent somatic cell function in the ovary (Eppig, 2001; Matzuk et al., 2002). GDF-9–/– mice are infertile with defects in follicular development beyond the primary stage and thecal cell development (Dong et al., 1996; Elvin et al., 1999b). Elevated progesterone and FSH levels and mis-expression of ovarian steroidogenic genes in somatic cells such as 17αOH in thecal cells have been reported in GDF-9–/– mice (Dong et al., 1996; Elvin et al., 1999b). Regulation of steroidogenesis by recombinant GDF-9 has also been documented, not only in granulosa cells (Elvin et al., 1999a, 2000; Vitt et al., 2000a), but also in thecal cells (Solovyeva et al., 2000; Vitt et al., 2000b). BMP-15, another oocyte-secreted TGF-β family member, also modulates follicular development and steroidogenesis in ovarian somatic cells (Otsuka et al., 2000, 2001; Yan et al., 2001). Therefore, reduced steroid hormone levels and mis-expression of StAR, 3βHSD I and 17αOH in the GCNFfl/flZp3Cre+ ovary at diestrus (Figures 3 and 4A–F, and Supplementary figure 1) are likely due to aberrant expression of BMP-15 and GDF-9 in the oocyte. In fact, we found that BMP-15 and GDF-9 expression levels were up-regulated in the GCNFfl/flZp3Cre+ ovary at diestrus (Figure 4H–J). As ovarian size and weight of GCNFfl/flZp3Cre+ females and ovarian morphology were similar to those of control littermates (GCNF+/+, GCNFfl/fl and GCNFfl/+Zp3Cre+ mice) (Figure 2E), and there were no changes in expression levels of oocyte-specific genes, Dnmt1o, Mater, Zp2, Zp3 and c-mos (Figure 4G), the increased expression levels of BMP-15 and GDF-9 at diestrus (Figure 4H–J) are not due to increased oocyte numbers within the ovary nor oocyte size. Rather, our results indicate that the expression of the BMP-15 and GDF-9 genes can be negatively regulated by GCNF within the oocyte at diestrus. Using EMSAs, we were able to show that GCNF can bind to multiple DR0 elements in the BMP-15 and GDF-9 promoters (Figure 5B). In cultured CHO cells, both the BMP-15 and GDF-9 promoter activities were repressed by GCNF (Figure 5F and G). These results demonstrate that BMP-15 and GDF-9 are direct target genes of GCNF in oocytes. The diestrus stage-specific overexpression of BMP-15 and GDF-9 (Figure 4H–J) correlates well with the reduced steroid hormone levels (Figure 3) and mis-expression of StAR, 3βHSD I and 17αOH in the GCNFfl/flZp3Cre+ mice at diestrus (Figure 4A–F, and Supplementary figure 1). Therefore, overexpression of GDF-9 and BMP-15 in oocytes is a primary defect in the oocyte-specific GCNF knockout mice, and might be the cause of aberrant expression of ovarian steroidogenic genes, StAR, 3βHSD I and 17αOH in the somatic cells, which leads to reduced circulating steroid hormone levels at the diestrus stage of the estrous cycle.
BMP-15–/– mice are subfertile with reduced litter sizes and reduced numbers of litters per month and have multiple oocyte follicles, and introducing a mutant GDF-9 allele into BMP-15–/– mice further reduces female fertility (Yan et al., 2001), whereas the GDF-9 knockout mice are infertile (Dong et al., 1996). Ablation of BMPRIB/ALK6, a putative BMP-15/GDF-9 receptor that is expressed in the granulosa cells of the ovary, also causes prolonged diestrus of the estrous cycle with reduced estradiol biosynthesis in the mouse ovary (Yi et al., 2001). These loss-of-function studies show that BMP-15/GDF-9 signaling in the ovary is important for proper follicular development and efficient female fertility. In this study, we found that the inactivation of the GCNF gene in oocytes, which directly caused overexpression of BMP-15 and GDF-9 (gain-of-function) at the diestrus stage of the estrous cycle (Figure 4H–J), also led to female hypofertility, with reduced litter sizes, reduced numbers of litters per month, a prolonged estrous cycle and the formation of double oocyte follicles (Figure 2). Therefore, it appears that the maintenance of precise expression levels of BMP-15 and GDF-9 in oocytes is essential for efficient female fertility and proper follicular development. The presence of regulatory feedback systems between the oocyte BMP-15/GDF-9 and granulosa cell kit ligand characterized recently (Elvin et al., 1999b; Joyce et al., 2000; Otsuka and Shimasaki, 2002) could be the mechanism of maintaining the appropriate levels of BMP-15 and GDF-9 in oocytes required to exert their physiological functions.
Similar to BMP-15–/–, GDF-9+/–BMP-15–/– (Yan et al., 2001), FSHR+/– (Danilovich and Sairam, 2002), genistein- or diethylstibestrol-treated (Iguchi et al., 1990; Jefferson et al., 2002), and rat inhibin α transgenic (McMullen et al., 2001) mice, some double-oocyte follicles were observed in the oocyte-specific GCNF knockout (Figure 2E). This phenotype is unlikely to be due to the aberrant ovarian expression of FSHR and inhibin α expression in the somatic cells, as expression of these two genes were not affected in GCNFfl/flZp3Cre+ mice at diestrus (Figure 4, and Supplementary figure 1). Rather, it could be due to defects in the granulosa cells (e.g. steroidogenesis and estradiol-induced estrogen receptor expression) at diestrus caused by the abnormal GDF-9 and BMP-15 signaling from the oocyte, which was resulted from the inactivation of GCNF in the oocyte.
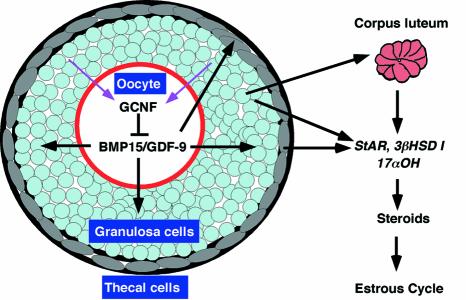
Based on our results, we propose a model for the function of GCNF in the ovary during female reproduction (Figure 6). In the oocyte, GCNF binds to DR0 elements in the GDF-9 and BMP-15 promoters and represses their transcription to maintain appropriate levels of GDF-9 and BMP-15 at diestrus. The loss of repression activity of GCNF causes abnormal BMP-15/GDF-9 expression in oocytes, leading to aberrant BMP-15 and GDF-9 signaling to the somatic cells of the ovary. Mis-expression of StAR, 3βHSD I and 17αOH in the somatic cells of the ovary, caused by abnormal BMP-15/GDF-9 signaling, results in reduced steroid hormone levels, leading to an aberrant estrous cycle that affects female fertility. Somatic cells may play a feedback role in regulating oocyte GCNF activity (pink arrows in Figure 6) in a ligand-dependent or -independent manner.
Fig. 6. A model for GCNF regulation of BMP-15/GDF-9 signaling during female reproduction. GCNF represses BMP-15/GDF-9 expression, which in turn affects steroidogenesis and female fertility.
In summary, we have successfully generated an oocyte-specific GCNF knockout mouse model that displays hypofertility, abnormal estrous cycle, aberrant steroidogenesis and double-oocyte follicles in the ovaries. These reproductive defects are secondary to the primary defect, which is up-regulation of the TGF-β family members BMP-15 and GDF-9 in oocytes at diestrus. We show that GCNF can directly repress expression of the BMP-15 and GDF-9 genes, an effect mediated via direct interactions of GCNF with multiple DR0 elements in the promoters of both genes. The oocyte-specific GCNF knockout mouse model has uncovered a new regulatory pathway in ovarian function. We conclude that a paracrine regulatory pathway exists among oocytes and adjacent somatic cells to modulate the estrous cycle during female reproduction, and this function is mediated by BMP-15 and GDF-9, whose expression is regulated by GCNF in oocytes.
Materials and methods
Generation of GCNFfl mice and mouse genotyping
GCNFfl mice were generated by homologous recombination in the AB1.2 ES cell line using the Cre/loxP system (Figure 1A). GCNFfl/+ ES clones were obtained after GCNF3lox ES cells (Lan et al., 2002) were transiently transfected with a Cre expression vector, pOG231 (O’Gorman et al., 1997). Microinjection of GCNFfl/+ ES cells into mouse C57BL/6 blastocysts produced chimeric animals. Breeding of male chimeras (>90% agouti coat color) with C57BL/6 females produced GCNFfl/+ mice. Mouse tail genomic DNA was extracted and genotyped either by Southern blot analysis using a 5′ GCNF probe (Chung et al., 2001) or by PCR using different sets of primers listed in the Supplementary data.
RNA analysis
Total ovarian RNA was isolated using Trizol reagent (Invitrogen). RT–PCR and quantitative RT–PCR analyses are described in the Supplementary data. In situ hybridization and northern blot analyses were performed as described previously (Katz et al., 1997). Mouse cDNA probes used for northern blot analyses were as follows (nucleotides and DDBJ/EMBL/GenBank accession Nos): StAR (821–1436 of L36062), 17αOH (813–1433 of M64863), P450scc (19–696 of NM_019779), Cyp19 (64–544 of NM_007810), 20αHSD (376–1060 of AB059565), activin βB (14–548 of X83376), 3βHSD I (466–1214 of M58567), GDF-9 (17–1360 of L06444) and BMP-15 (1–2000 of AF082348). The same blots were then reprobed with glyceraldedyde-3-phosphate dehydrogenase (GAPDH) as a loading and quality control. Quantitative mRNA levels of 3βHSD I, 17αOH, BMP-15 and GDF-9 were determined by ImageQuant program, and normalized to the GAPDH mRNA levels in the northern blots.
Collection of oocytes and cumulus cells, classification of the estrous cycle and hormone assays
Oocytes and cumulus cells were collect from 1- to 2-month-old animals after PMSG/hCG treatment as described previously (Elvin et al., 2000). The estrous cycle of adult mice were determined as reported previously (Jablonka-Shariff et al., 1999; Nothnick, 2000). Each cycle length was determined as the length of time between two consecutive occurrences of estrus in the animals that had progressed through at least three consecutive estrous cycles. Serum estradiol, progesterone, testosterone, FSH and LH levels were determined by the Core Ligand and Assay Laboratory (University of Virginia) using radioimmunoassay kits.
Histological analysis
Ovaries were fixed in 4% paraformaldehyde, dehydrated and then embedded in paraffin. The paraffin-embedded tissues were serially sectioned at 7 µm thickness and stained with hematoxylin and eosin. β-galactosidase staining of tissue samples was performed according to the manufacturers protocol (Specialty Media, NJ). After staining, tissues were post-fixed, dehydrated, embedded and then sectioned followed by counter-staining with 0.1% Nuclear Fast Red.
EMSAs
EMSAs were performed as described previously (Cooney et al., 1998). Production of in vitro translated GCNF and GCNF antibodies were described previously (Lan et al., 2003). The Oct4 DR0 sequences of the double-stranded oligonucleotide probe for EMSAs were as reported previously (Fuhrmann et al., 2001). The sequences of the double-stranded oligonucleotide probes, containing putative DR0 elements in the BMP-15 and GDF-9 promoter, for EMSAs are listed in the Supplementary data.
Cell culture, western blot analysis, plasmid construction and transient transfection assays
CHO cells were cultured in Ham’s F12 media (Invitrogen) with 10% fetal calf serum. A BMP-15 promoter DNA fragment from –2993 to +209 nt was obtained from a BAC clone (RP24-294F7, BAC-PAC; CHORI, CA) and inserted into the KpnI and HindIII sites of the pGL-3-basic luciferase reporter vector (Promega) to generate the pBMP-15-LUC plasmid. The GDF-9 promoter reporter plasmid, pGDF-9-LUC, was generously provided by Dr M.Matzuk, in which a GDF-9 promoter DNA fragment (–3277 to +108 nt) containing GDF-9 DR0-5 was cloned into the pGL3-basic reporter vector. The Oct4 promoter reporter plasmid, pOct4-LUC (Fuhrmann et al., 2001), was used as a positive control for the transfection experiments. Expression vectors, pCMV-GCNF and pCMV-GCNFΔDBD, were constructed by inserting the full-length mouse GCNF cDNA (Chen et al., 1994) and the LBD encoding mouse GCNF cDNA sequences into the pCMV-HA vector (Promega), respectively. Cells were transiently transfected with plasmids for 48 h, using Fugene 6 (Roche) as described previously (Fuhrmann et al., 2001). Western blot analysis with anti-HA antibodies was performed as reported previously (Lan et al., 2003). Luciferase activities in the samples were determined using a firefly luciferase assay system (Promega), and normalized to the co-reporter activity of the pRL-TK Renilla luciferase control vector (Promega).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Drs B.Knowles, P.Soriano, A.Bradley, S.O’Gorman and M.Matzuk for Zp3Cre mice, floxed ROSA mice, AB1.2 ES cells and floxed NeoTK plasmid, pOG231, and pGDF-9-LUC, respectively. This research was supported by NICHD/NIH through cooperative agreement (U54 HD07495) and (U54 HD28934) as part of the Specialized Cooperative Centers Program in Reproduction Research, NIH grants HD32878 (to A.J.C.) and T32 DK07763 (to Z.-J.L.), and the Lalor Foundation (to Z.-J.L.).
References
- Agoulnik I.Y., Cho,Y., Niederberger,C., Kieback,D.G. and Cooney,A.J. (1998) Cloning, expression analysis and chromosomal localization of the human nuclear receptor gene GCNF. FEBS Lett., 424, 73–78. [DOI] [PubMed] [Google Scholar]
- Bain P.A., Yoo,M., Clarke,T., Hammond,S.H. and Payne,A.H. (1991) Multiple forms of mouse 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase and differential expression in gonads, adrenal glands, liver, and kidneys of both sexes. Proc. Natl Acad. Sci. USA, 88, 8870–8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat A.K., Zandbergen,M.A., De Vries,E., Van Der Burg,B., Bogerd,J. and Goos,H.J. (1999) Cloning and expression of the zebrafish germ cell nuclear factor. Mol. Reprod. Dev., 53, 369–375. [DOI] [PubMed] [Google Scholar]
- Chen F., Cooney,A.J., Wang,Y., Law,S.W. and O’Malley,B.W. (1994) Cloning of a novel orphan receptor (GCNF) expressed during germ cell development. Mol. Endocrinol., 8, 1434–1444. [DOI] [PubMed] [Google Scholar]
- Chung A.C., Katz,D., Pereira,F.A., Jackson,K.J., DeMayo,F.J., Cooney,A.J. and O’Malley,B.W. (2001) Loss of orphan receptor germ cell nuclear factor function results in ectopic development of the tail bud and a novel posterior truncation. Mol. Cell. Biol., 21, 663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A.J., Hummelke,G.C., Herman,T., Chen,F. and Jackson,K.J. (1998) Germ cell nuclear factor is a response element-specific repressor of transcription. Biochem. Biophys. Res. Commun., 245, 94–100. [DOI] [PubMed] [Google Scholar]
- Danilovich N. and Sairam,M.R. (2002) Haploinsufficiency of the follicle-stimulating hormone receptor accelerates oocyte loss inducing early reproductive senescence and biological aging in mice. Biol. Reprod., 67, 361–369. [DOI] [PubMed] [Google Scholar]
- Danilovich N., Javeshghani,D., Xing,W. and Sairam,M.R. (2002) Endocrine alterations and signaling changes associated with declining ovarian function and advanced biological aging in follicle-stimulating hormone receptor haploinsufficient mice. Biol. Reprod., 67, 370–378. [DOI] [PubMed] [Google Scholar]
- de Vries W.N., Binns,L.T., Fancher,K.S., Dean,J., Moore,R., Kemler,R. and Knowles,B.B. (2000) Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis, 26, 110–112. [PubMed] [Google Scholar]
- Dierich A., Sairam,M.R., Monaco,L., Fimia,G.M., Gansmuller,A., LeMeur,M. and Sassone-Corsi,P. (1998) Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl Acad. Sci. USA, 95, 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Albertini,D.F., Nishimori,K., Kumar,T.R., Lu,N. and Matzuk,M.M. (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature, 383, 531–535. [DOI] [PubMed] [Google Scholar]
- Eimerl S. and Orly,J. (2002) Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: differential responses of cytochrome p450 side-chain cleavage, steroidogenic acute regulatory protein, and 3beta-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biol. Reprod., 67, 900–910. [DOI] [PubMed] [Google Scholar]
- Elvin J.A., Clark,A.T., Wang,P., Wolfman,N.M. and Matzuk,M.M. (1999a) Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol., 13, 1035–1048. [DOI] [PubMed] [Google Scholar]
- Elvin J.A., Yan,C., Wang,P., Nishimori,K. and Matzuk,M.M. (1999b) Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol. Endocrinol., 13, 1018–1034. [DOI] [PubMed] [Google Scholar]
- Elvin J.A., Yan,C. and Matzuk,M.M. (2000) Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc. Natl Acad. Sci. USA, 97, 10288–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J.J. (2001) Oocyte control of ovarian follicular development and function in mammals. Reproduction, 122, 829–838. [DOI] [PubMed] [Google Scholar]
- Eppig J.J., Wigglesworth,K. and Pendola,F.L. (2002) The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc. Natl Acad. Sci. USA, 99, 2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G., Chung,A.C., Jackson,K.J., Hummelke,G., Baniahmad,A., Sutter,J., Sylvester,I., Scholer,H.R. and Cooney,A.J. (2001) Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev. Cell, 1, 377–387. [DOI] [PubMed] [Google Scholar]
- Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science, 265, 103–106. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Fukazawa,Y., Uesugi,Y. and Takasugi,N. (1990) Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol. Reprod., 43, 478–484. [DOI] [PubMed] [Google Scholar]
- Jablonka-Shariff A., Ravi,S., Beltsos,A.N., Murphy,L.L. and Olson,L.M. (1999) Abnormal estrous cyclicity after disruption of endothelial and inducible nitric oxide synthase in mice. Biol. Reprod., 61, 171–177. [DOI] [PubMed] [Google Scholar]
- Jefferson W.N., Couse,J.F., Padilla-Banks,E., Korach,K.S. and Newbold,R.R. (2002) Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol. Reprod., 67, 1285–1296. [DOI] [PubMed] [Google Scholar]
- Joos T.O., David,R. and Dreyer,C. (1996) xGCNF, a nuclear orphan receptor is expressed during neurulation in Xenopus laevis. Mech. Dev., 60, 45–57. [DOI] [PubMed] [Google Scholar]
- Joyce I.M., Clark,A.T., Pendola,F.L. and Eppig,J.J. (2000) Comparison of recombinant growth differentiation factor-9 and oocyte regulation of KIT ligand messenger ribonucleic acid expression in mouse ovarian follicles. Biol. Reprod., 63, 1669–1675. [DOI] [PubMed] [Google Scholar]
- Katz D., Niederberger,C., Slaughter,G.R. and Cooney,A.J. (1997) Characterization of germ cell-specific expression of the orphan nuclear receptor, germ cell nuclear factor. Endocrinology, 138, 4364–4372. [DOI] [PubMed] [Google Scholar]
- Kumar T.R., Wang,Y., Lu,N. and Matzuk,M.M. (1997) Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet., 15, 201–204. [DOI] [PubMed] [Google Scholar]
- Lan Z.J., Chung,A.C., Xu,X., DeMayo,F.J. and Cooney,A.J. (2002) The embryonic function of germ cell nuclear factor is dependent on the DNA binding domain. J. Biol. Chem., 277, 50660–50667. [DOI] [PubMed] [Google Scholar]
- Lan Z.J., Gu,P., Xu,X.P. and Cooney,A.J. (2003) Expression of the orphan nuclear receptor, germ cell nuclear factor, in mouse gonads and pre-implantation embryos. Biol. Reprod., 68, 282–289. [DOI] [PubMed] [Google Scholar]
- Lieberman S. and Warne,P.A. (2001) 17-Hydroxylase: an evaluation of the present view of its catalytic role in steroidogenesis. J. Steroid Biochem. Mol. Biol., 78, 299–312. [DOI] [PubMed] [Google Scholar]
- Matzuk M.M., Burns,K.H., Viveiros,M.M. and Eppig,J.J. (2002) Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science, 296, 2178–2180. [DOI] [PubMed] [Google Scholar]
- McMullen M.L., Cho,B.N., Yates,C.J. and Mayo,K.E. (2001) Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology, 142, 5005–5014. [DOI] [PubMed] [Google Scholar]
- Nothnick W.B. (2000) Disruption of the tissue inhibitor of metalloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol. Reprod., 63, 905–912. [DOI] [PubMed] [Google Scholar]
- Nuclear Receptor Nomenclature Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell, 97, 161–163. [DOI] [PubMed] [Google Scholar]
- O’Gorman S., Dagenais,N.A., Qian,M. and Marchuk,Y. (1997) Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl Acad. Sci. USA, 94, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F. and Shimasaki,S. (2002) A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc. Natl Acad. Sci. USA, 99, 8060–8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F., Yao,Z., Lee,T., Yamamoto,S., Erickson,G.F. and Shimasaki,S. (2000) Bone morphogenetic protein-15. Identification of target cells and biological functions. J. Biol. Chem., 275, 39523–39528. [DOI] [PubMed] [Google Scholar]
- Otsuka F., Yamamoto,S., Erickson,G.F. and Shimasaki,S. (2001) Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J. Biol. Chem., 276, 11387–11392. [DOI] [PubMed] [Google Scholar]
- Solovyeva E.V., Hayashi,M., Margi,K., Barkats,C., Klein,C., Amsterdam,A., Hsueh,A.J. and Tsafriri,A. (2000) Growth differentiation factor-9 stimulates rat theca-interstitial cell androgen biosynthesis. Biol. Reprod., 63, 1214–1218. [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet., 21, 70–71. [DOI] [PubMed] [Google Scholar]
- Stocco D.M. (2001) Tracking the role of a star in the sky of the new millennium. Mol. Endocrinol., 15, 1245–1254. [DOI] [PubMed] [Google Scholar]
- Susens U., Aguiluz,J.B., Evans,R.M. and Borgmeyer,U. (1997) The germ cell nuclear factor mGCNF is expressed in the developing nervous system. Dev. Neurosci., 19, 410–420. [DOI] [PubMed] [Google Scholar]
- Vanderhyden B.C. and Tonary,A.M. (1995) Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol. Reprod., 53, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Vitt U.A., Hayashi,M., Klein,C. and Hsueh,A.J. (2000a) Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol. Reprod., 62, 370–377. [DOI] [PubMed] [Google Scholar]
- Vitt U.A., McGee,E.A., Hayashi,M. and Hsueh,A.J. (2000b) In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology, 141, 3814–3820. [DOI] [PubMed] [Google Scholar]
- Yan C. et al. (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol., 15, 854–866. [DOI] [PubMed] [Google Scholar]
- Yan Z.H., Medvedev,A., Hirose,T., Gotoh,H. and Jetten,A.M. (1997) Characterization of the response element and DNA binding properties of the nuclear orphan receptor germ cell nuclear factor/retinoid receptor- related testis-associated receptor. J. Biol. Chem., 272, 10565–10572. [DOI] [PubMed] [Google Scholar]
- Yi S.E., LaPolt,P.S., Yoon,B.S., Chen,J.Y., Lu,J.K. and Lyons,K.M. (2001) The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl Acad. Sci. USA, 98, 7994–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.L., Akmal,K.M., Tsuruta,J.K., Shang,Q., Hirose,T., Jetten,A.M., Kim,K.H. and O’Brien,D.A. (1998) Expression of germ cell nuclear factor (GCNF/RTR) during spermatogenesis. Mol. Reprod. Dev., 50, 93–102. [DOI] [PubMed] [Google Scholar]