Abstract
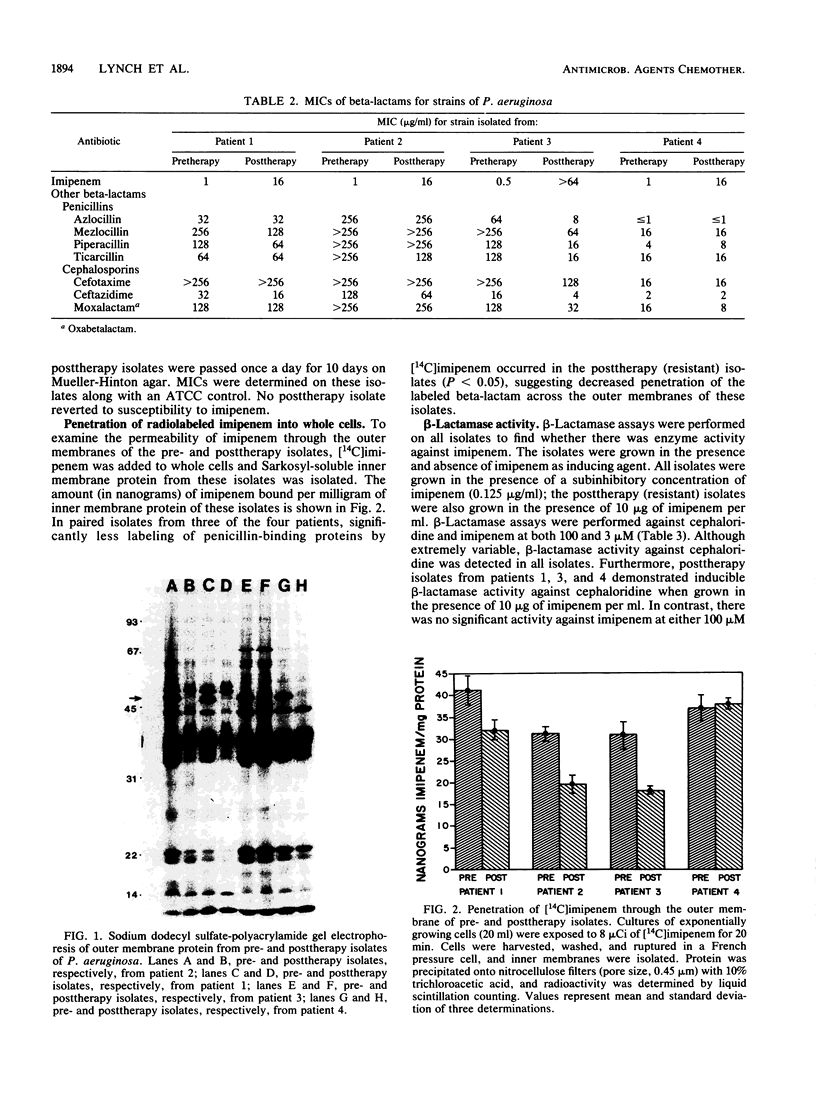
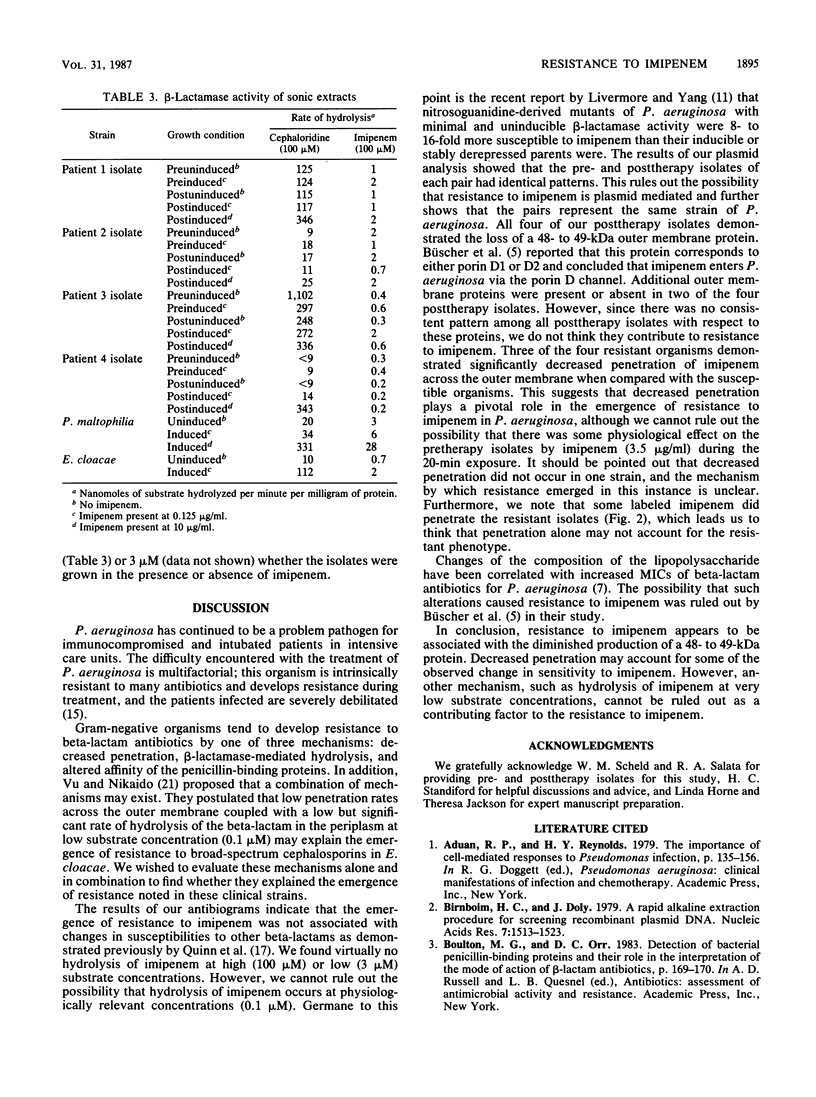
The emergence of resistance to imipenem by Pseudomonas aeruginosa was investigated with four pairs of isolates. Each pair represented pretherapy (susceptible) and posttherapy (resistant) specimens. In all cases, the imipenem-resistant isolates did not demonstrate changed susceptibilities to other beta-lactams. Agarose gel electrophoresis revealed no change in plasmid profiles between any pair of isolates. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the Sarkosyl-insoluble membrane protein revealed the loss of an outer membrane protein of apparent molecular mass 48 to 49 kilodaltons in posttherapy strains when grown with imipenem selection (5 micrograms/ml). There was no significant difference in the binding of [14C]imipenem to the penicillin-binding proteins of the pre- and posttherapy strains. Trichloroacetic acid precipitation of membranes isolated after growth in the presence of [14C]imipenem revealed that significantly less drug was bound to Sarkosyl-soluble membrane protein in three of the four posttherapy strains than the membrane proteins of the respective pretherapy strains. beta-Lactamase activity against imipenem at 100 or 3 microM was not detected in any isolate either with or without induction. These data suggest that resistance to imipenem is associated with the loss of a 48- to 49-kilodalton outer membrane protein accompanied by, in three of four cases, decreased penetration of the antibiotic across the outer membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveny I. In vitro activity of imipenem--a review. Eur J Clin Microbiol. 1984 Oct;3(5):456–462. doi: 10.1007/BF02017375. [DOI] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra G. B., Hesney M., Grad C. A multiclinic randomized study of the comparative efficacy, safety and tolerance of imipenem/cilastatin and moxalactam. Eur J Clin Microbiol. 1984 Oct;3(5):478–487. doi: 10.1007/BF02017380. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner S. A., Dudek E. J., Boisvert W. E., Berndt K. D. Effect of highly potent antipseudomonal beta-lactam agents alone and in combination with aminoglycosides against Pseudomonas aeruginosa. Rev Infect Dis. 1984 Sep-Oct;6 (Suppl 3):S678–S688. doi: 10.1093/clinids/6.supplement_3.s678. [DOI] [PubMed] [Google Scholar]
- Livermore D. M., Yang Y. J. Beta-lactamase lability and inducer power of newer beta-lactam antibiotics in relation to their activity against beta-lactamase-inducibility mutants of Pseudomonas aeruginosa. J Infect Dis. 1987 Apr;155(4):775–782. doi: 10.1093/infdis/155.4.775. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Comparative in vitro activity of N-formimidoyl thienamycin against gram-positive and gram-negative aerobic and anaerobic species and its beta-lactamase stability. Antimicrob Agents Chemother. 1982 Jan;21(1):180–187. doi: 10.1128/aac.21.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols L., Gudmundsson S., Maki D. G. Experience with cefsulodin therapy for lower respiratory tract infections caused by Pseudomonas aeruginosa in adults without cystic fibrosis or granulocytopenia. Rev Infect Dis. 1984 Sep-Oct;6 (Suppl 3):S711–S720. doi: 10.1093/clinids/6.supplement_3.s711. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Gebhart R. L., Palmer D. L., Wade B. H., Scheld W. M., Groschel D. H., Wenzel R. P., Mandell G. L., Duma R. J. Pneumonia treated with imipenem/cilastatin. Am J Med. 1985 Jun 7;78(6A):104–109. doi: 10.1016/0002-9343(85)90110-x. [DOI] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]



