Abstract
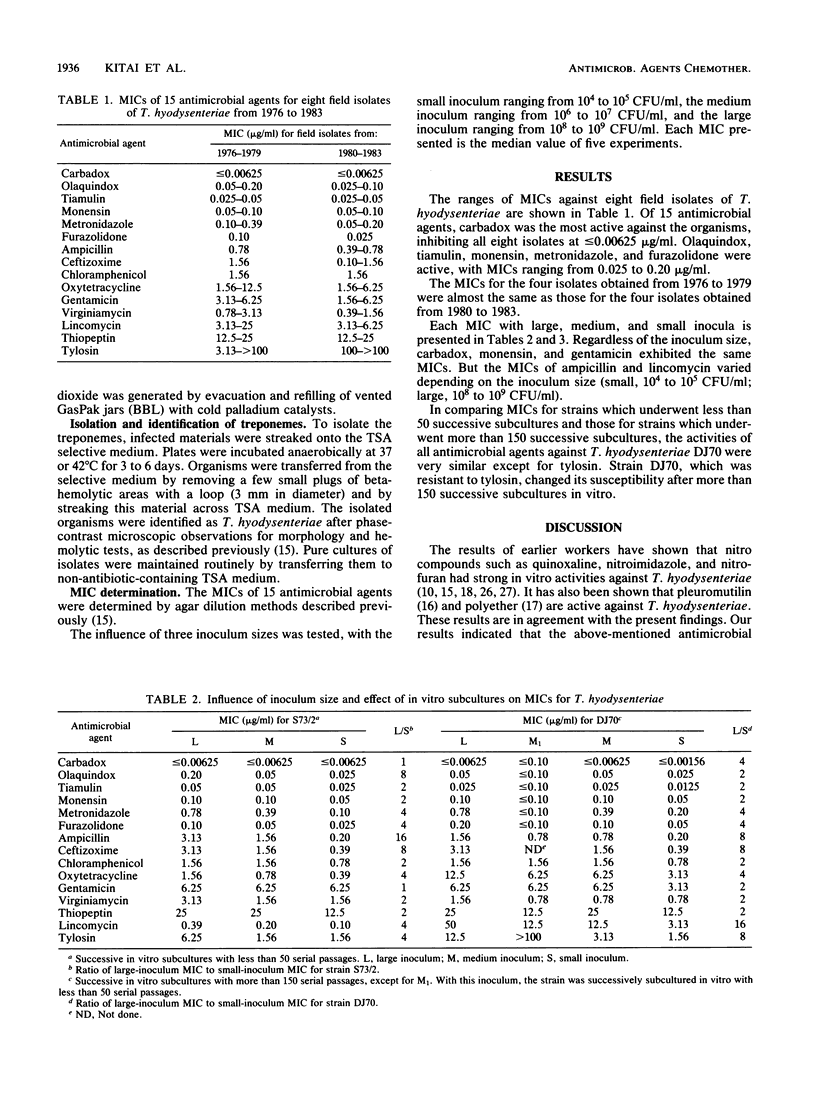
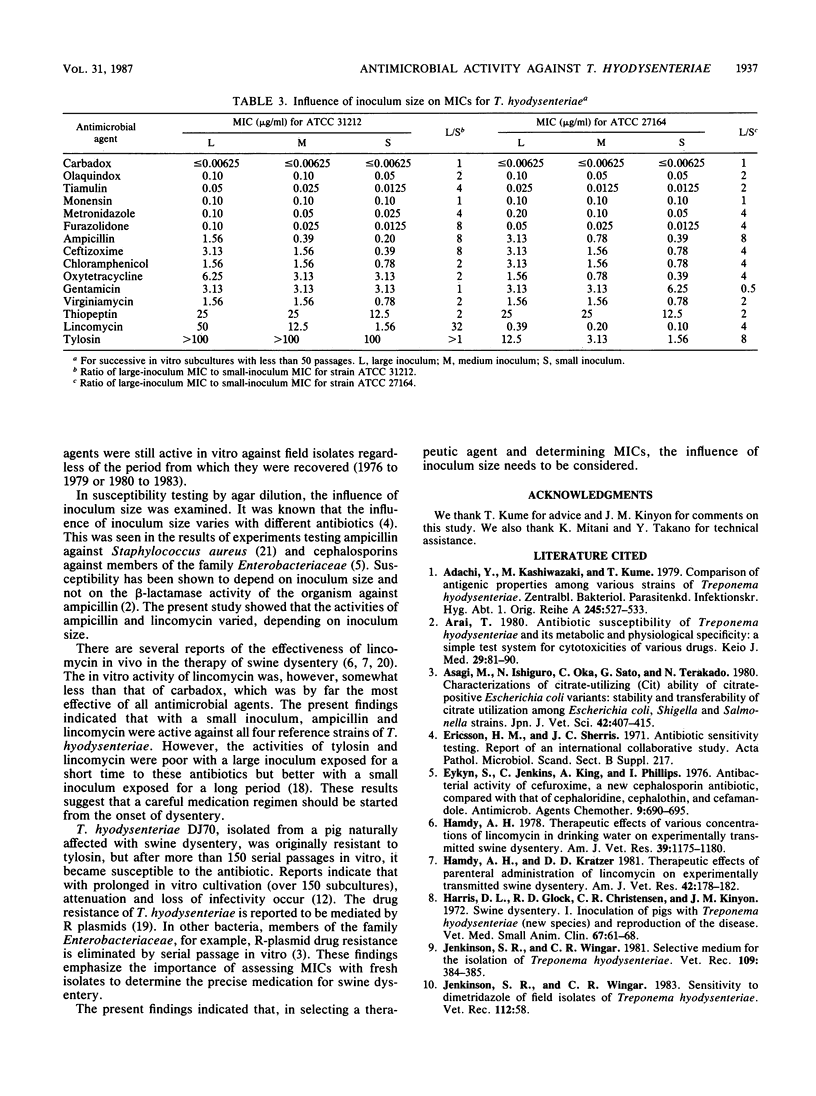
The in vitro susceptibilities of eight isolates of Treponema hyodysenteriae from pigs naturally infected with swine dysentery between 1976 and 1983 were determined by an agar dilution technique. Carbadox, olaquindox, tiamulin, metronidazole, furazolidone, and monensin were the most active against these field isolates regardless of the year of recovery. The influence of inoculum size on the MICs against four reference strains of T. hyodysenteriae was studied. Various degrees of activities of ampicillin and lincomycin were found, depending on the inoculum size. The effect of successive in vitro subcultures on the susceptibility of a reference strain of T. hyodysenteriae was examined. The strain resistant to tylosin became susceptible to the drug.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Kashiwazaki M., Kume T. Comparison of antigenic properties among various strains of Treponema hyodysenteriae. Zentralbl Bakteriol Orig A. 1979 Dec;245(4):527–533. [PubMed] [Google Scholar]
- Asagi M., Ishiguro N., Oka C., Sato G., Terakado N. Characterization of citrate-utilizing (Cit) ability of citrate-positive Escherichia coli variants: stability and transferability of citrate utilization among Escherichia coli, Shigella and Salmonella strains. Nihon Juigaku Zasshi. 1980 Aug;42(4):407–415. doi: 10.1292/jvms1939.42.407. [DOI] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Eykyn S., Jenkins C., King A., Phillips I. Antibacterial activity of cefuroxime, a new cephalosporin antibiotic, compared with that of cephaloridine, cephalothin, and cefamandole. Antimicrob Agents Chemother. 1976 Apr;9(4):690–695. doi: 10.1128/aac.9.4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy A. H., Kratzer D. D. Therapeutic effects of parenteral administration of lincomycin on experimentally transmitted swine dysentery. Am J Vet Res. 1981 Feb;42(2):178–182. [PubMed] [Google Scholar]
- Hamdy A. H. Therapeutic effects of various concentrations of lincomycin in drinking water on experimentally transmitted swine dysentery. Am J Vet Res. 1978 Jul;39(7):1175–1180. [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Jenkinson S. R., Wingar C. R. Selective medium for the isolation of Treponema hyodysenteriae. Vet Rec. 1981 Oct 24;109(17):384–385. doi: 10.1136/vr.109.17.384. [DOI] [PubMed] [Google Scholar]
- Jenkinson S. R., Wingar C. R. Sensitivity to dimetridazole of field isolates of Treponema hyodysenteriae. Vet Rec. 1983 Jan 15;112(3):58–58. doi: 10.1136/vr.112.3.58. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki M., Takohata T., Kume T. Isolation of Treponema hyodysenteriae from feces of pigs affected with swine dysentery by use of a medicated medium. Natl Inst Anim Health Q (Tokyo) 1977 Spring;17(1):29–30. [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L. Growth in Treponema hyodysenteriae in liquid medium. Vet Rec. 1974 Sep 7;95(10):219–220. doi: 10.1136/vr.95.10.219. [DOI] [PubMed] [Google Scholar]
- Kitai K., Kashiwazaki M., Adachi Y., Kume T., Arakawa A. In vitro activity of 39 antimicrobial agents against Treponema hyodysenteriae. Antimicrob Agents Chemother. 1979 Mar;15(3):392–395. doi: 10.1128/aac.15.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laber G. Activity of various compounds against a pathogenic strain of Treponema hyodysenteriae. Zentralbl Bakteriol Orig A. 1976 Oct;236(1):127–130. [PubMed] [Google Scholar]
- Liu C. M., Hermann T. E., Prosser B. L., Palleroni N. J., Westley J. W., Miller P. A. X-14766A, a halogen containing polyether antibiotic produced by Streptomyces malachitofuscus subsp. downeyi ATCC 31547. Discovery, fermentation, biological properties and taxonomy of the producing culture. J Antibiot (Tokyo) 1981 Feb;34(2):133–138. doi: 10.7164/antibiotics.34.133. [DOI] [PubMed] [Google Scholar]
- Molnár L., Szent-Iványi T. Sensitivity to different chemotherapeutics of Treponema hyodysenteriae strains isolated in Hungary. Acta Vet Acad Sci Hung. 1981;29(2):111–118. [PubMed] [Google Scholar]
- Ohmae K., Yonezawa S., Terakado N. Epizootiological studies on R plasmid with carbadox resistance. Nihon Juigaku Zasshi. 1983 Apr;45(2):165–170. doi: 10.1292/jvms1939.45.165. [DOI] [PubMed] [Google Scholar]
- Rainier R. H., Harris D. L., Glock R. D., Kinyon J. M., Brauer M. A. Carbadox and lincomycin in the treatment and carrier state control of swine dysentery. Am J Vet Res. 1980 Sep;41(9):1349–1356. [PubMed] [Google Scholar]
- Sherris J. C., Rashad A. L., Lighthart G. A. Laboratory determination of antibiotic susceptibility to ampicillin and cephalothin. Ann N Y Acad Sci. 1967 Sep 27;145(2):248–267. doi: 10.1111/j.1749-6632.1967.tb50223.x. [DOI] [PubMed] [Google Scholar]
- Songer J. G., Kinyon J. M., Harris D. L. Selective medium for isolation of Treponema hyodysenteriae. J Clin Microbiol. 1976 Jul;4(1):57–60. doi: 10.1128/jcm.4.1.57-60.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Simmons J. R., Laird H. M. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Vet Rec. 1980 Apr 12;106(15):326–332. doi: 10.1136/vr.106.15.326. [DOI] [PubMed] [Google Scholar]
- Williams B. J., Babcock W. E. In vitro susceptibility of Treponema hyodysenteriae to carbadox, virginiamycin, and tylosin. Vet Med Small Anim Clin. 1976 Jul;71(7):957–959. [PubMed] [Google Scholar]
- Williams B. J., Shively J. E. In vitro antitreponemal activities of carbadox, virginiamycin, olaquindox, and tylosin as indices of their effectiveness for preventing swine dysentery. Vet Med Small Anim Clin. 1978 Mar;73(3):349–351. [PubMed] [Google Scholar]


