Abstract
Use of cefoperazone in a patient with Aeromonas caviae in the respiratory tract selected a mutant that constitutively produced beta-lactamase. This mutant, in contrast to its parental strain with an inducible beta-lactamase, showed enhanced resistance to newer cephalosporins and aztreonam. This observation suggested that species of Aeromonas, like those of other genera with inducible beta-lactamases, may pose therapeutic problems associated with the rapid development of multiple beta-lactam resistance. Thus, a study was designed to identify the beta-lactamases in 12 strains representing four species of Aeromonas and assess their role in drug resistance. Eleven strains possessed inducible beta-lactamases. One strain showed no detectable activity. An analysis of substrate and inhibitor profiles, isoelectric points, and beta-lactam susceptibility patterns revealed the presence of at least four distinguishable inducible beta-lactamases. These enzymes were involved in the resistance of strains within the genus to penicillins, cephalosporins, aztreonam, and imipenem but not cefoxitin. Unlike most other organisms with inducible beta-lactamases, all four strains of A. caviae, one of four strains of A. sobria, and one of three strains of A. hydrophila possessed two distinct inducible beta-lactamases. Furthermore, substrate and inhibitor profiles revealed that many of these Aeromonas beta-lactamases were distinct from inducible enzymes that have been characterized in other genera of gram-negative bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altwegg M., Jöhl M. Isolation frequency of Aeromonas species in relation to patient age. Eur J Clin Microbiol. 1987 Feb;6(1):55–56. doi: 10.1007/BF02097193. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bush K. Recent developments in beta-lactamase research and their implications for the future. Rev Infect Dis. 1988 Jul-Aug;10(4):681–690. doi: 10.1093/clinids/10.4.681. [DOI] [PubMed] [Google Scholar]
- Chang B. J., Bolton S. M. Plasmids and resistance to antimicrobial agents in Aeromonas sobria and Aeromonas hydrophila clinical isolates. Antimicrob Agents Chemother. 1987 Aug;31(8):1281–1282. doi: 10.1128/aac.31.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily O. P., Joseph S. W., Coolbaugh J. C., Walker R. I., Merrell B. R., Rollins D. M., Seidler R. J., Colwell R. R., Lissner C. R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981 Apr;13(4):769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. A., 2nd, Kane J. G., Garagusi V. F. Human aeromonas infections: a review of the literature and a case report of endocarditis. Medicine (Baltimore) 1978 May;57(3):267–277. [PubMed] [Google Scholar]
- Dworzack D. L., Clark R. B., Padgitt P. J. New causes of pneumonia, meningitis, and disseminated infections associated with immersion. Infect Dis Clin North Am. 1987 Sep;1(3):615–633. [PubMed] [Google Scholar]
- Hedges R. W., Medeiros A. A., Cohenford M., Jacoby G. A. Genetic and biochemical properties of AER-1, a novel carbenicillin-hydrolyzing beta-lactamase from Aeromonas hydrophila. Antimicrob Agents Chemother. 1985 Apr;27(4):479–484. doi: 10.1128/aac.27.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman-Brenner F. W., MacDonald K. L., Steigerwalt A. G., Fanning G. R., Brenner D. J., Farmer J. J., 3rd Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J Clin Microbiol. 1987 May;25(5):900–906. doi: 10.1128/jcm.25.5.900-906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Reitano M., Bottone E. J. Biotyping of Aeromonas isolates as a correlate to delineating a species-associated disease spectrum. J Clin Microbiol. 1984 Jan;19(1):44–47. doi: 10.1128/jcm.19.1.44-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Finan M., Yousuf M. In-vitro antagonism by N-formimidoyl thienamycin and cefoxitin of second and third generation cephalosporins in Aeromonas hydrophila and Serratia marcescens. J Antimicrob Chemother. 1983 Apr;11(4):311–318. doi: 10.1093/jac/11.4.311. [DOI] [PubMed] [Google Scholar]
- Motyl M. R., McKinley G., Janda J. M. In vitro susceptibilities of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae to 22 antimicrobial agents. Antimicrob Agents Chemother. 1985 Jul;28(1):151–153. doi: 10.1128/aac.28.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt J. F., George W. L. Comparative in vitro activities of selected antimicrobial agents against Aeromonas species and Plesiomonas shigelloides. Antimicrob Agents Chemother. 1985 Apr;27(4):643–645. doi: 10.1128/aac.27.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. J., Robinson J. O., Wagener L. B., Burke V. In-vitro susceptibility of Aeromonas app. to antimicrobial agents. J Antimicrob Chemother. 1982 Apr;9(4):267–274. doi: 10.1093/jac/9.4.267. [DOI] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Clinical importance of inducible beta-lactamases in gram-negative bacteria. Eur J Clin Microbiol. 1987 Aug;6(4):435–438. doi: 10.1007/BF02013106. [DOI] [PubMed] [Google Scholar]
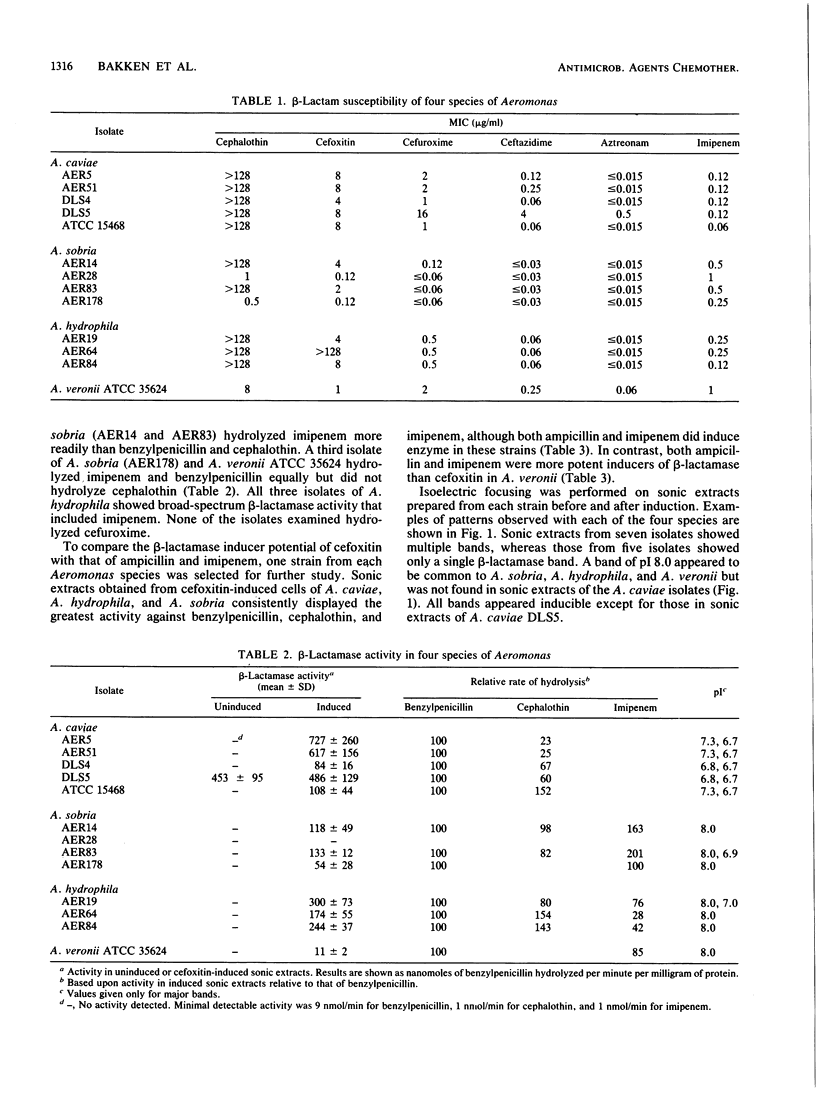
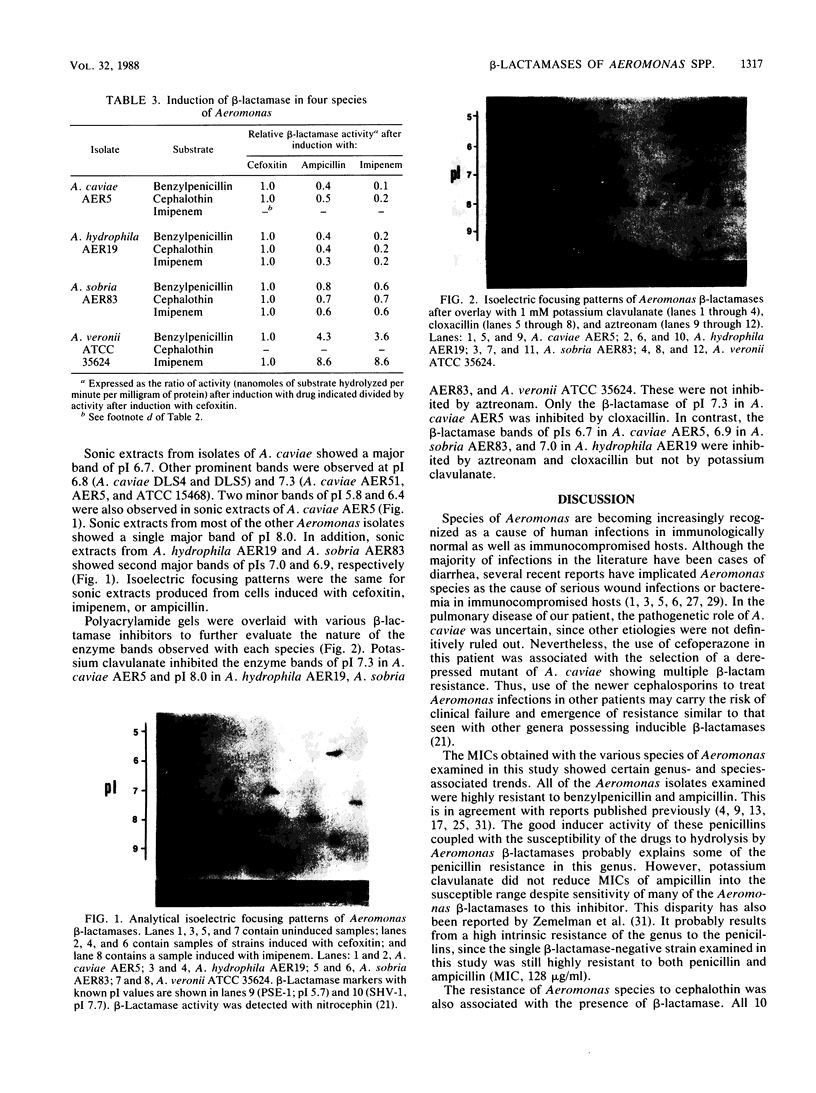
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Morioka K., Ogawa M., Yamagishi S. Inducible oxacillin-hydrolyzing penicillinase in Aeromonas hydrophila isolated from fish. Antimicrob Agents Chemother. 1976 Aug;10(2):191–195. doi: 10.1128/aac.10.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Phillips I. Beta-lactamases with high activity against imipenem and Sch 34343 from Aeromonas hydrophila. J Antimicrob Chemother. 1986 Jan;17(1):45–50. doi: 10.1093/jac/17.1.45. [DOI] [PubMed] [Google Scholar]
- Trust T. J., Chipman D. C. Clinical involvement of Aeromonas hydrophila. Can Med Assoc J. 1979 Apr 21;120(8):942–946. [PMC free article] [PubMed] [Google Scholar]
- Watson I. M., Robinson J. O., Burke V., Gracey M. Invasiveness of Aeromonas spp. in relation to biotype, virulence factors, and clinical features. J Clin Microbiol. 1985 Jul;22(1):48–51. doi: 10.1128/jcm.22.1.48-51.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman R., Gonzalez C., Mondaca M. A., Silva J., Merino C., Dominguez M. Resistance of Aeromonas hydrophila to beta-lactam antibiotics. J Antimicrob Chemother. 1984 Dec;14(6):575–579. doi: 10.1093/jac/14.6.575. [DOI] [PubMed] [Google Scholar]