Abstract
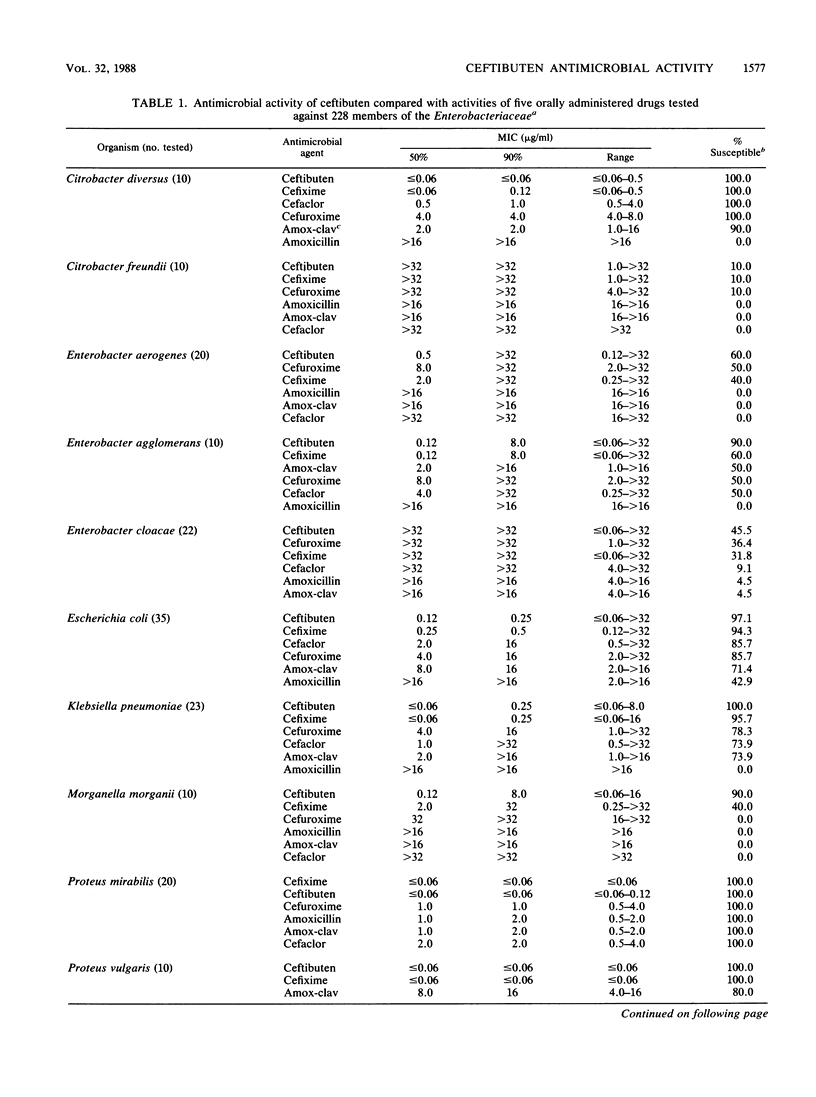
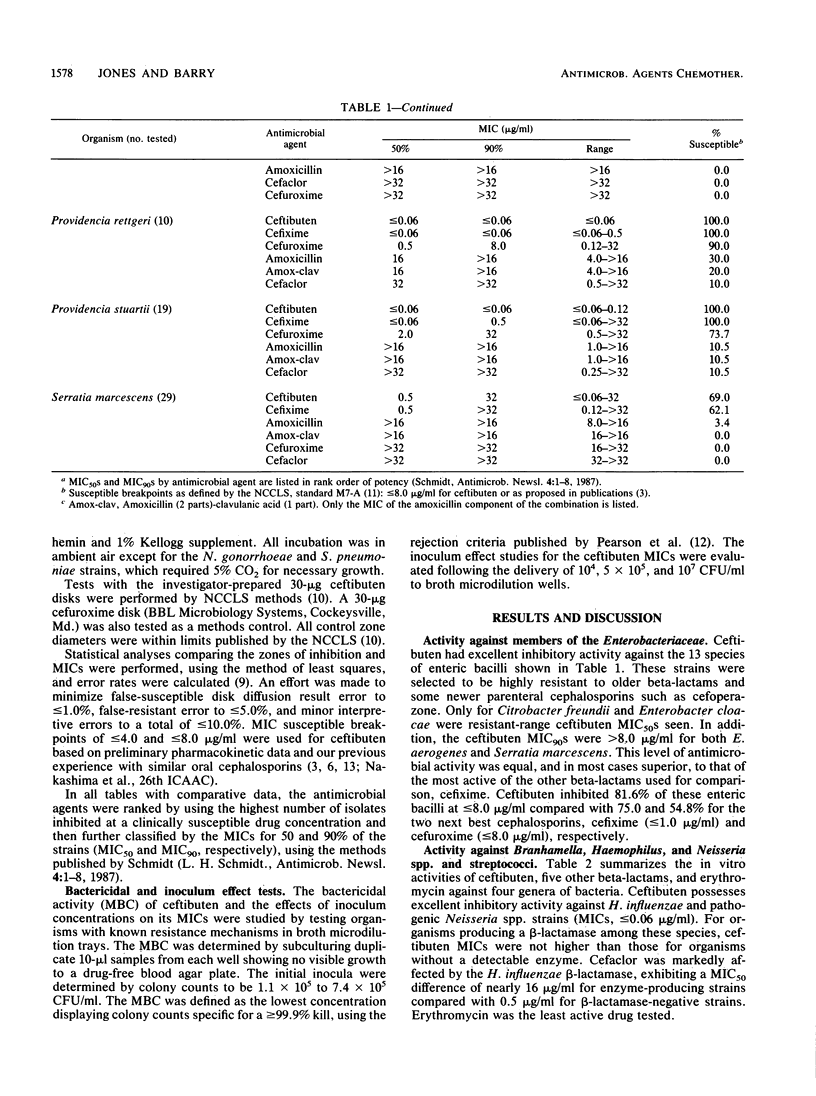
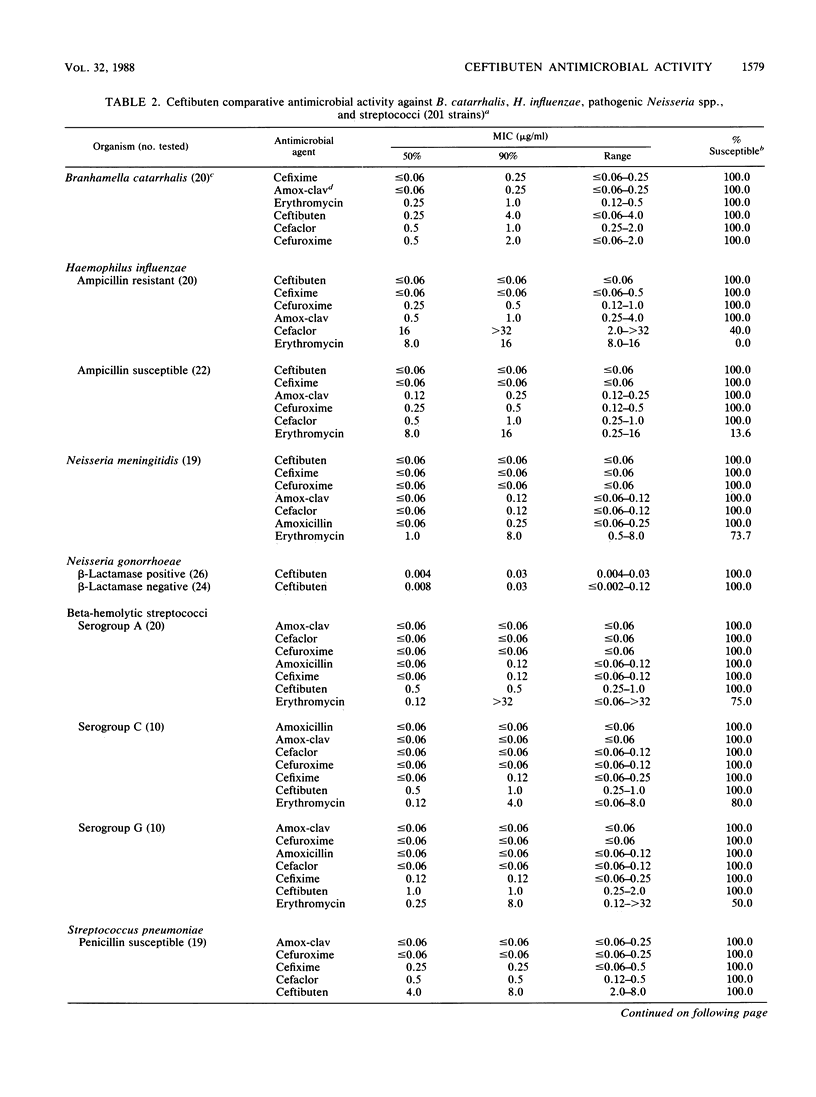
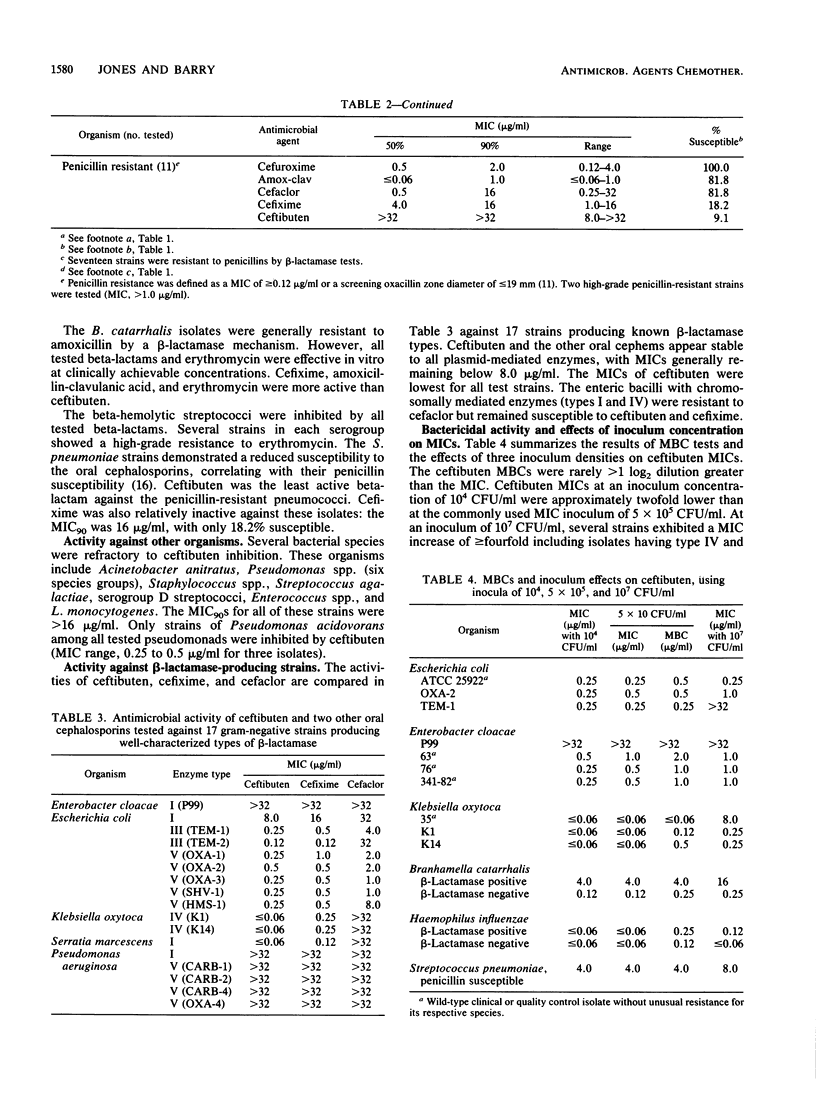
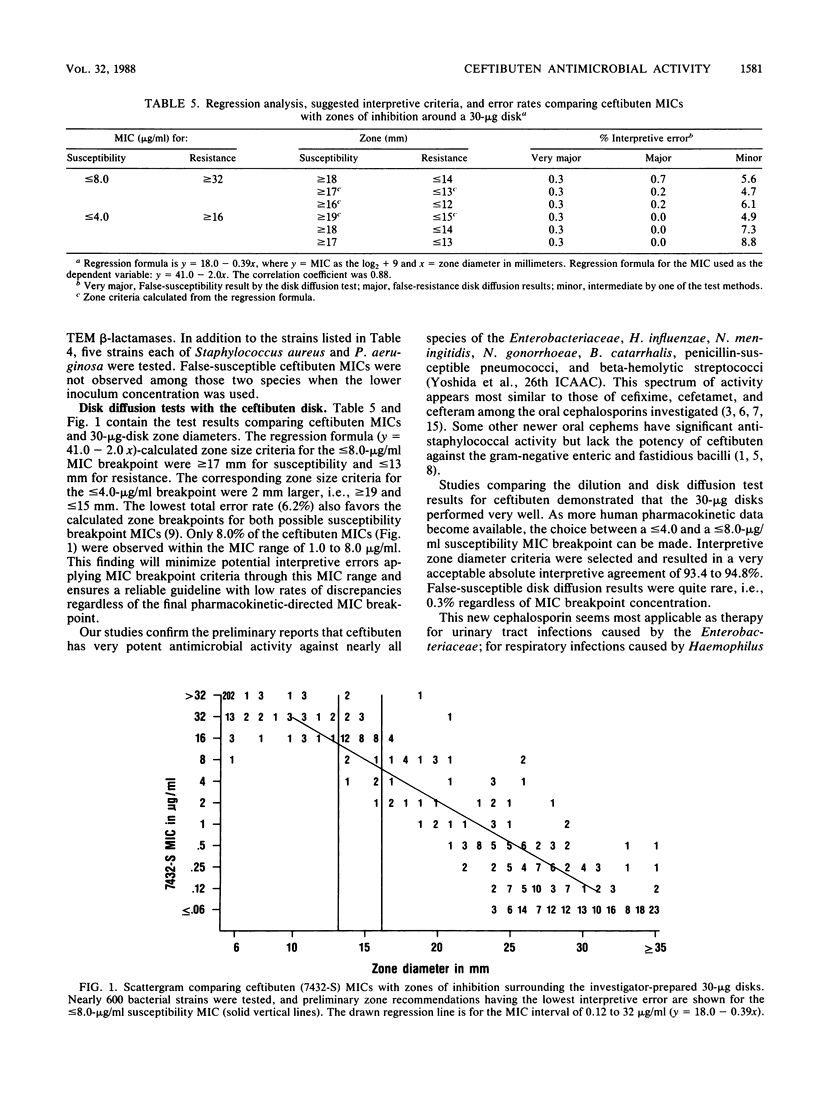
The antimicrobial activity and spectrum of ceftibuten (7432-S; SCH 39720) was determined on a wide variety of bacterial species selected for resistance to oral and parenteral beta-lactam antimicrobial agents. Ceftibuten was found to be the most active beta-lactam tested against members of the family Enterobacteriaceae, inhibiting 81.6% of strains at less than or equal to 8.0 micrograms/ml compared with 75.0 and 54.8% of strains inhibited by cefixime and cefuroxime, respectively. All strains of Haemophilus influenzae (MIC for 90% of strains [MIC90], less than or equal to 0.06 microgram/ml), Branhamella catarrhalis (MIC90, 3.0 micrograms/ml), and pathogenic Neisseria spp. (MIC90, less than or equal to 0.06 and 0.019 microgram/ml) were susceptible to ceftibuten. Beta-hemolytic Streptococcus spp. (serogroups A, B, C, and G) were also inhibited by ceftibuten, but penicillin-resistant pneumococci were generally resistant to cefixime and ceftibuten. The activity and spectrum of ceftibuten seem most applicable to infections of the respiratory and urinary tract plus those infections caused by pathogenic Neisseria spp. Ceftibuten disks (30 micrograms) were evaluated and found to have an acceptable correlation (r = 0.88) with ceftibuten MICs. Preliminary zone size interpretive criteria for MIC breakpoints of less than or equal to 4.0 and less than or equal to 8.0 micrograms/ml were calculated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Thornsberry C., Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H. Cefuroxime, an in vitro Comparison with Six Other Cephalosporins. Proc R Soc Med. 1977;70(Suppl 9):63–71. [PMC free article] [PubMed] [Google Scholar]
- Buck R. E., Price K. E. Cefadroxil, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1977 Feb;11(2):324–330. doi: 10.1128/aac.11.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N. Cefixime disk susceptibility test criteria. J Clin Microbiol. 1986 Oct;24(4):647–649. doi: 10.1128/jcm.24.4.647-649.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay D. R., Meatherall R. C., Harding G. K., Brown G. R. Pharmacokinetics of cefixime (CL 284,635; FK 027) in healthy subjects and patients with renal insufficiency. Antimicrob Agents Chemother. 1986 Sep;30(3):485–490. doi: 10.1128/aac.30.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Preliminary antimicrobial susceptibility interpretive criteria for cefetamet (Ro 15-8074) and cefteram (Ro 19-5247) disk tests. J Clin Microbiol. 1987 Sep;25(9):1796–1799. doi: 10.1128/jcm.25.9.1796-1799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura T., Kojo H., Matsumoto Y., Mine Y., Goto S., Kuwahara S. In vitro and in vivo antibacterial properties of FK 027, a new orally active cephem antibiotic. Antimicrob Agents Chemother. 1984 Jan;25(1):98–104. doi: 10.1128/aac.25.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Pursiano T. A., Buck R. E., Tsai Y. H., Chisholm D. R., Misiek M., Desiderio J. V., Kessler R. E. BMY 28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Feb;31(2):238–243. doi: 10.1128/aac.31.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston D. A., Jones R. N., Barry A. L., Thornsberry C. Comparison of the antibacterial spectra of cephalexin and cefaclor with those of cephalothin and newer cephalosporins: reevaluation of the class representative concept of susceptibility testing. J Clin Microbiol. 1983 Jun;17(6):1156–1158. doi: 10.1128/jcm.17.6.1156-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Piddock L. J. In vitro activity of Ro 15-8074 and Ro 19-5247, two orally administered cephalosporin metabolites. Antimicrob Agents Chemother. 1986 Jun;29(6):1067–1072. doi: 10.1128/aac.29.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]


