Abstract
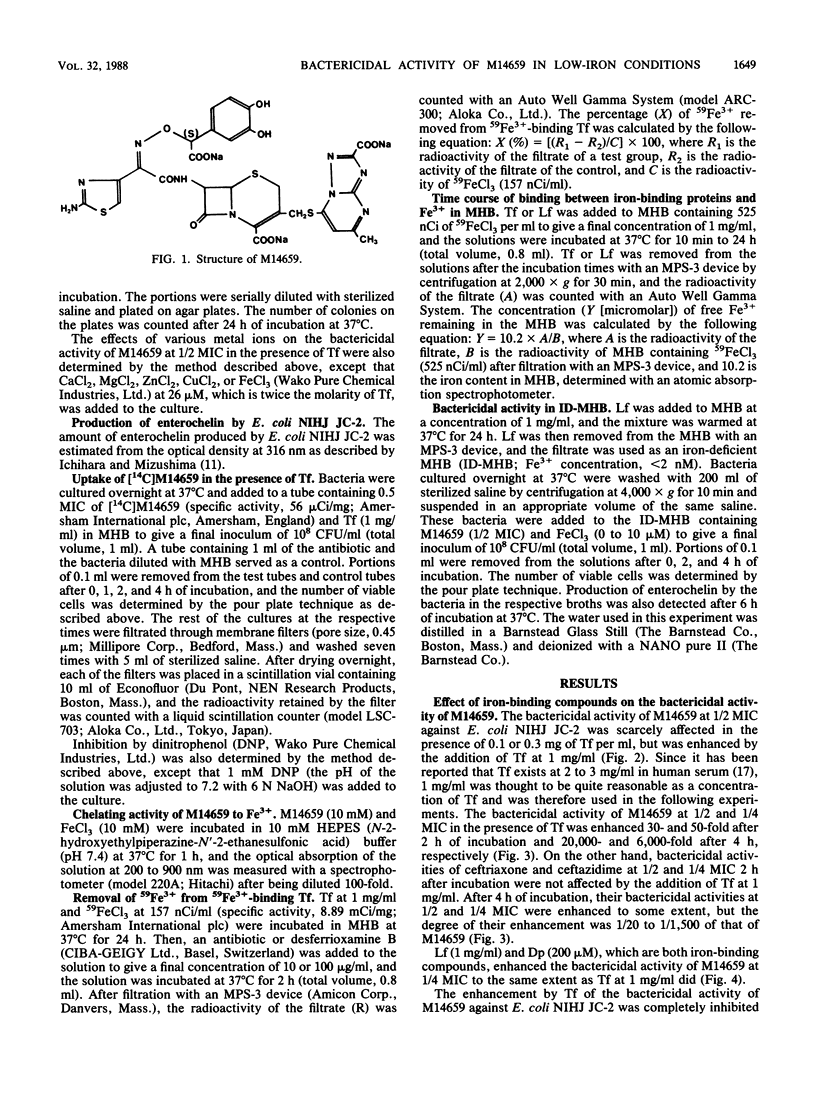
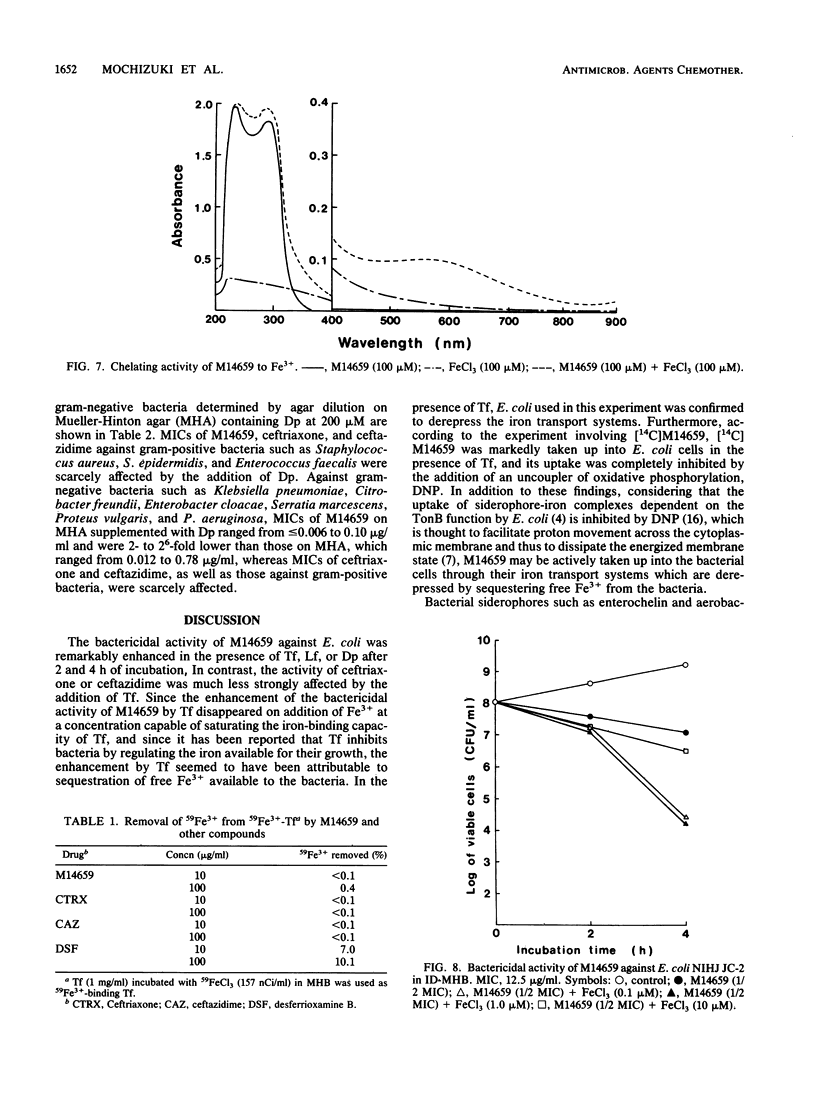
The bactericidal activity of M14659 against Escherichia coli in low-iron environments was investigated and compared with that of ceftriaxone and ceftazidime. The bactericidal activity of M14659 against E. coli in Mueller-Hinton broth was enhanced 30- to 20,000-fold by addition of transferrin, which is an iron-binding protein, whereas the activity of ceftriaxone or ceftazidime was much less strongly affected. This enhancement by transferrin was completely inhibited by saturating the iron-binding capacity of transferrin with FeCl3. M14659 was taken up markedly into bacterial cells in the presence of transferrin, and its uptake was inhibited by the protonophore dinitrophenol, which inhibits active-transport systems coupled to an energized membrane such as the iron transport systems of E. coli. The bactericidal activity of M14659, which chelates Fe3+, was also enhanced in the presence of other iron-binding compounds such as lactoferrin and alpha,alpha'-dipyridyl or in iron-deficient Mueller-Hinton broth (Fe3+ concentration, less than 2 nM) supplemented with FeCl3 at 0.1 to 1.0 microM, but not in unsupplemented iron-deficient Mueller-Hinton broth. The E. coli used in this study was confirmed to derepress iron transport systems in the presence of transferrin, lactoferrin, and alpha,alpha'-dipyridyl and in the iron-deficient Mueller-Hinton broth supplemented with FeCl3 at 0 to 1.0 microM. M14659 also showed an excellent antibacterial activity in vitro against other gram-negative bacteria in the low-iron environments. These findings indicate that M14659 may be actively taken up with Fe3+ into bacterial cells, probably through the iron transport systems under conditions of low iron and, thus, kills bacteria effectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Barclay R. The role of iron in infection. Med Lab Sci. 1985 Apr;42(2):166–177. [PubMed] [Google Scholar]
- Bezkorovainy A. Antimicrobial properties of iron-binding proteins. Adv Exp Med Biol. 1981;135:139–154. doi: 10.1007/978-1-4615-9200-6_8. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Cunarro J., Weiner M. W. Mechanism of action of agents which uncouple oxidative phosphorylation: direct correlation between proton-carrying and respiratory-releasing properties using rat liver mitochondria. Biochim Biophys Acta. 1975 May 15;387(2):234–240. doi: 10.1016/0005-2728(75)90106-1. [DOI] [PubMed] [Google Scholar]
- Holbein B. E. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981 Oct;34(1):120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980 Sep;29(3):886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Involvement of outer membrane proteins in enterochelin-mediated iron uptake in Escherichia coli. J Biochem. 1977 Mar;81(3):749–756. doi: 10.1093/oxfordjournals.jbchem.a131513. [DOI] [PubMed] [Google Scholar]
- Letendre E. D., Holbein B. E. Mechanism of impaired iron release by the reticuloendothelial system during the hypoferremic phase of experimental Neisseria meningitidis infection in mice. Infect Immun. 1984 May;44(2):320–325. doi: 10.1128/iai.44.2.320-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre E. D., Holbein B. E. Turnover in the transferrin iron pool during the hypoferremic phase of experimental Neisseria meningitidis infection in mice. Infect Immun. 1983 Jan;39(1):50–59. doi: 10.1128/iai.39.1.50-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H., Oikawa Y., Yamada H., Kusakabe S., Shiihara T., Murakami K., Kato K., Ishiguro J., Kosuzume H. Antibacterial and pharmacokinetic properties of M14659, a new injectable semisynthetic cephalosporin. J Antibiot (Tokyo) 1988 Mar;41(3):377–391. doi: 10.7164/antibiotics.41.377. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Uptake of ferrienterochelin by Escherichia coli: energy dependent stage of uptake. J Bacteriol. 1977 Apr;130(1):26–36. doi: 10.1128/jb.130.1.26-36.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. G. Influence of iron on infection. Am J Surg. 1986 Feb;151(2):291–295. doi: 10.1016/0002-9610(86)90090-5. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


