Abstract
Bile duct ligation (BDL) causes hepatocellular oxidative stress and injury. The transcription factor nuclear factor-E2-related factor (Nrf2) induces expression of numerous genes including NAD(P)H:quinone oxidoreductase 1 (Nqo1) during periods of oxidative stress. Therefore, we hypothesized that BDL increases liver expression of mouse antioxidant genes in an Nrf2-dependent manner. BDL or sham surgeries were performed on male C57BL/6, Nrf2-null, and wild-type mice. Livers were collected at 1, 3, and 7 days after surgery for analysis of messenger ribonucleic acid (mRNA) levels of Nrf2-responsive genes as well as Nqo1 protein and activity. BDL increased mRNA expression of multiple Nrf2 genes in mouse liver, compared to sham-operated controls. Follow-up studies investigating protein expression, enzyme activity, and Nrf2 dependency were limited to Nqo1. Nqo1 protein expression and activity in mouse livers was increased 2- to 3-, and 4- to 5-fold at 3 and 7 days after BDL, respectively. Studies also showed that BDL increases Nqo1 mRNA, protein expression, and enzyme activity in livers from wild-type mice, but not in Nrf2-null mice. In conclusion, expression of Nrf2-dependent genes is increased during cholestasis. These studies also demonstrate that Nqo1 expression and activity in mouse liver are induced via an Nrf2-dependent mechanism.
INTRODUCTION
Obstructive cholestasis in humans is usually the result of physical obstruction of the biliary system at the level of the extrahepatic bile duct. Common bile duct ligation (BDL) in rodents is commonly used by researchers studying secondary liver pathology produced by cholestasis. After BDL, bile constituents such as bile acids, cholesterol, and bilirubin accumulate in serum and liver of rodents to a similar degree as seen in clinical cases. The accumulation of bile constituents following BDL causes a number of adaptive changes in hepatic gene expression. These alterations are thought to represent a concerted effort by the liver to enhance the elimination of chemicals and by-products that accumulate during cholestasis.
Oxidative stress is a potentially detrimental cellular state, during which harmful oxidative molecules are being generated in excess of the cell's capacity to detoxify them. Following BDL in rats, increases in indicators of oxidative stress, such as lipid peroxidation, deoxyribonucleic acid (DNA) adducts, and free radicals, are detected in the liver and/or circulating blood (Parola et al 1996; Tsai et al 1998; Liu et al 2001; Huang et al 2003). Previous work links the generation of oxidative stress products with exposure of hepatocytes to hydrophobic bile acids. Accumulation of oxidative stress by-products, such as 8-hydroxy-2′-deoxyguanosine and malondialdehyde, is observed in the livers of patients with primary biliary cirrhosis, a type of cholestasis (Kitada et al 2001; Vendemiale et al 2002). Interestingly, these perturbations are almost completely reversed with biliary drainage, suggesting a direct relationship between cholestasis, intrahepatic accumulation of bile constituents, and impaired redox status (Vendemiale et al 2002).
Nuclear factor-E2-related factor2 (Nrf2) is a transcription factor that is a member of the basic leucine zipper nuclear factor-E2 family of transcription factors. The basal and inducible expression of a number of hepatic genes is regulated via Nrf2. For example, Nrf2 mediates chemical induction of detoxification enzymes, such as NAD(P)H: quinone oxidoreductase 1 (Nqo1), heme oxygenase-1/ heat shock protein 32 (Ho-1), glutathione-S-transferase (Gst), and UDP-glucuronosyltransferase 1A6 (Mathers et al 2004). Nrf2 is responsible for the coordinated induction of these and other hepatic genes during oxidative stress (Chan et al 2001; Ishii et al 2002). Nrf2 dissociates from the cytoskeletal binding protein Keap1 and translocates to the nucleus in response to oxidative stress. After translocation, Nrf2 and small Maf proteins bind to antioxidant response elements and subsequently activate gene transcription. The expression of human and mouse Nqo1 genes are primarily regulated via antioxidant response element sequences in the promoter region (Venugopal and Jaiswal 1996; Nioi et al 2003).
The purpose of this study was to measure for the first time the expression of a number of mouse Nrf2-responsive genes following BDL. Nqo1 was selected as a prototypical Nrf2 gene for additional analysis of protein and activity changes in wild-type (WT) and Nrf2-null mice.
MATERIALS AND METHODS
Chemicals
2,6-Dichlorophenol-indophenol (DCPIP), dicumarol, sucrose, Tris-hydrochloride, were all obtained from Sigma (St Louis, MO, USA).
Animals and surgeries
Adult male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Adult male wild-type and Nrf2-null mice were bred at the University of Kansas Medical Center Laboratory Animal Resources facility on a mixed C57BL/6 and AKR background. Under pentobarbital-induced anesthesia (50 mg/kg, ip), the abdominal cavity was opened and the common bile duct was ligated with 4-0 surgical silk, with the gall bladder intact. The abdominal muscle was sutured with Ethicon 4-0 dissolvable suture material (Roboz, Gaithersburg, MD, USA), and the wound was closed with surgical staples (Roboz). Sham surgeries were performed by the same method, but without BDL. Livers were collected 1, 3, and 7 days after BDL, and livers from wild-type and Nrf2-null mice were collected at 1 and 3 days after BDL. Experiments using wild-type and Nrf2-null mice were limited to 1 and 3 days in order to investigate the early activation of Nrf2-mediated stress pathways. Tissues were snap-frozen in liquid nitrogen and stored at −70°C until analysis. All animal studies were conducted according to Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Ribonucleic acid isolation
Total ribonucleic acid (RNA) was isolated with RNAzol B reagent (Tel-Test, Friendswood, TX, USA) using the manufacturer's instructions. RNA concentration was assessed by ultraviolet absorbance at 260 nm, and integrity was confirmed by formaldehyde gel electrophoresis.
Branched DNA signal amplification assay
Mouse Nqo1 and Ho-1 messenger RNA (mRNA) was measured using the branched DNA signal amplification assay (QuantiGene High Volume bDNA Signal Amplification kit; Genospectra, Fremont, CA, USA) as previously described (Hartley and Klaassen 2000; Aleksunes et al 2005). Probe sets for mouse Gst isoforms a1, a4, m1, m2, and m3 are listed in
.
Western analysis of Nqo1 expression
Cytosolic fractions of frozen liver were obtained as described (Aleksunes 2006). Protein concentration was determined using the Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA, USA). Immunochemical detection of Nqo1 protein in cytosolic fractions was performed using anti-Nqo1 (ab2346; Novus Biological, Littleton, CO, USA). Nqo1 protein-antibody complexes were detected using an enhanced chemiluminescent kit (Amersham Biosciences, Arlington Heights, IL, USA) and exposed to Fuji medical x-ray film (Fisher Scientific, Springfield, NJ, USA). The intensity of Nqo1 protein bands was quantified using a PDI Image Analyzer (Protein and DNA ImageWare System; PDI, Huntington Station, NY, USA). Antibody specificity was confirmed using liver cytosol from Nqo1-null mice (generously provided by Frank Gonzalez, National Cancer Institute, Bethesda, MD, USA) as a negative control and recombinant human NQO1 (Sigma) as a positive control.
Nqo1 activity assay
Nqo1 activity (in nanomoles per minute per milligram of protein) was calculated by measuring the oxidation of NADPH to NADP+, using DCPIP as a substrate as previously described (Aleksunes 2006).
Histopathology and morphometric analysis
Liver samples were fixed in 10% neutral-buffered formalin prior to routine processing and paraffin embedding. Five-micrometer liver sections were stained with hematoxylin and eosin. Sections were examined by light microscopy and photographed on an Olympus BX50 microscope equipped with a QImaging MicroPublisher 3.3 RTV camera. For morphometric analysis, two 4× magnification images of nonoverlapping areas for each liver section were obtained with QCapture Pro software. The 2 combined images accounted for approximately 60–70% of the entire liver section. We then determined the number of necrotic foci and measured the area of each foci. The individual areas of each foci were then added together, and this value was divided by the total area of the image to yield the total percentage of necrotic area. To determine the average percentage size for each necrotic foci, we divided the total percentage of necrotic area by the number of necrotic foci counted. Finally, the total percentage of necrotic area, number of necrotic foci, and average percentage foci size from each of the 2 images were averaged together for each liver.
Alanine aminotransferase activity
Serum alanine aminotransferase (ALT) activity was determined as a biochemical indicator of hepatocellular necrosis. Infinity ALT Liquid Stable Reagent (Thermotrace, Melbourne, Australia) was used according to the manufacturer's protocol.
Hepatic bile acid levels
Total bile acids in mouse liver were quantified as previously described (DeMeo et al 1998). Briefly, livers were extracted with t-butanol:water (1:1) and centrifuged at 10 000 × g for 20 minutes. The extracted supernatant was dried, resuspended in saline, and assayed for 3-hydroxy bile acids (DeMeo et al 1998). Changes in absorbance were compared to a cholic acid standard for determination of unknown concentrations of total bile acids in mouse liver.
Statistical analysis
Differences between groups were determined using a Student's t-test or 1-way analysis of variance followed by a Newman-Keuls post hoc test where appropriate. Significance was set at P < 0.05. Asterisks (*) represent a statistical difference (P < 0.05) between sham and BDL groups, and crosses (†) represent a statistical difference (P < 0.05) between wild-type and Nrf2-null mice.
RESULTS
Effect of BDL on Nqo1, Ho-1, and Gst mRNA expression in mouse liver
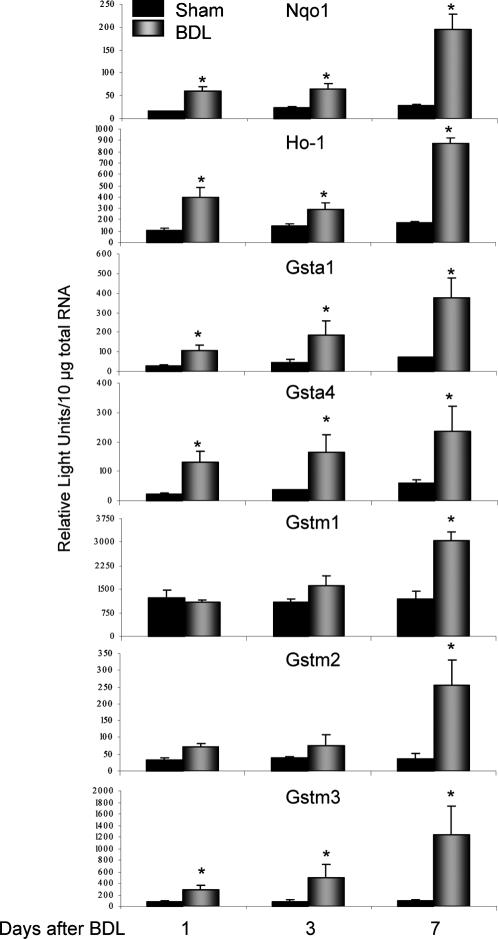
mRNA expression of multiple Nrf2-related genes is shown in Figure 1. BDL increased Nqo1 mRNA expression in mouse livers by 3-fold at 1 and 3 days, and 7-fold at 7 days. Significant elevations in mouse Ho-1 were observed at 1 day (4-fold), 3 days (2-fold), and 7 days (5-fold). Similarly, BDL induced levels of Gst transcripts with maximal hepatic changes in Gsta1 (5-fold), Gsta4 (4-fold), Gstm1 (3-fold), Gstm2 (7-fold), and Gstm3 (12-fold) occurring at 7 days. Levels of Gstm4, Gstt1, and Gstms1 mRNA were unchanged after BDL (data not shown).
Fig 1.
Total hepatic RNA was isolated from mice that underwent sham or BDL surgery at days 1, 3, and 7 and analyzed by the bDNA assay for Nqo1, Ho-1, Gst a1, a4, m1, m2, m3 mRNA expression. The data are presented as mean relative light units ± standard error of the mean (n = 3–6 animals). Asterisks (*) represent a statistical difference (P < 0.05) between sham and BDL groups
Effect of BDL on Nqo1 protein and activity in mouse liver
Because BDL increased Nqo1 mRNA in mouse liver, Western blots using anti-Nqo1 antibodies were performed to determine whether corresponding increases in Nqo1 protein levels occurred following BDL (Fig 2A). An immunoreactive band for mouse Nqo1 in liver cytosol migrated to 28–29 kDa, which is identical to the migration pattern of recombinant human Nqo1 protein (data not shown). No band was observed in liver cytosol from Nqo1-null mice (data not shown). BDL induced Nqo1 protein expression by 60%, 160%, and 327% after 1, 3, and 7 days, compared to expression in livers from sham-operated controls (Fig 2B). In livers from sham-operated mice, Nqo1 activity ranged from 30 to 42 nmol reduced DCPIP/min/mg protein (Fig 2C). One day after BDL, Nqo1 activity in livers from BDL mice did not differ from that in sham-operated controls. However, Nqo1 activity increased by 100% and 400% at 3 and 7 days after BDL, compared to sham controls. There was no significant change in hepatic Nqo1 activity in sham animals over the 7-day period.
Fig 2.
Nqo1 protein expression and activity in liver cytosolic fractions from mice 1, 3, and 7 days after sham or BDL surgery. (A) Representative Western blot of liver cytosolic fractions from mice that underwent sham surgery or BDL surgery (days 1, 3, and 7) stained with anti-Nqo1 antibodies. (B) Quantification of Nqo1 protein levels in liver cytosolic fractions from mice that underwent sham or BDL surgery. The data are presented as relative protein expression ± standard error of the mean (SEM) (n = 5–13 animals). (C) The data are presented as nanomoles of reduced DCPIP per minute per milligram of protein ± SEM (n = 5–6 animals). Asterisks (*) represent a statistical difference (P < 0.05) between sham and BDL groups
Effect of BDL on Nqo1 mRNA expression in liver from wild-type and Nrf2-null mice
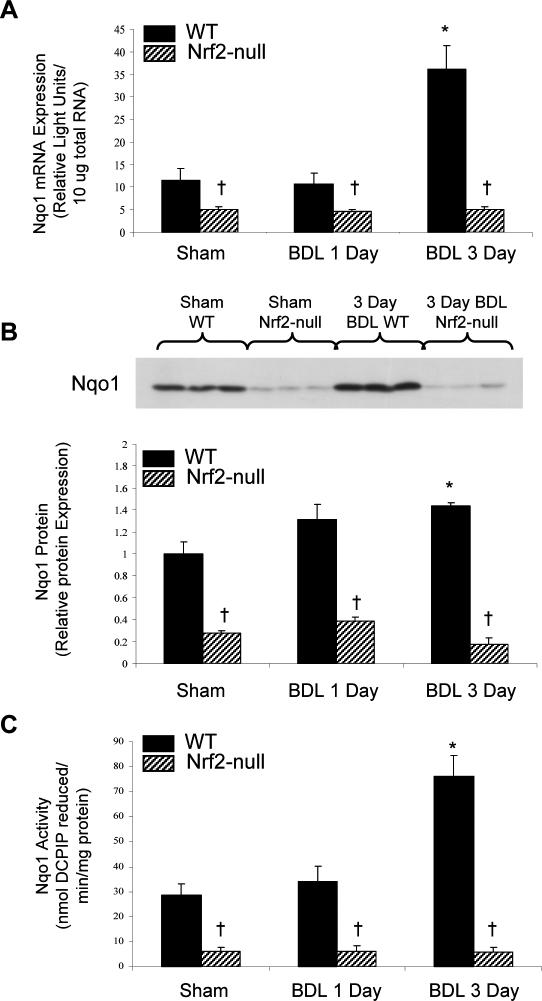
Because Nqo1 mRNA levels were increased after BDL, it indicates that the observed induction of Nqo1 mRNA, protein, and activity in liver is initiated through a transcription-mediated process. Previous studies have demonstrated that Nqo1 basal expression and induction after chemical treatment is mediated through activation of the transcription factor Nrf2 (Ramos-Gomez et al 2001). Therefore, to determine whether Nrf2 was directly responsible for Nqo1 induction during cholestasis, sham and BDL surgeries were performed on wild-type and Nrf2-null mice, and livers were collected at 1 and 3 days. Nqo1 mRNA expression was reduced by 50% in livers from sham Nrf2-null compared to sham wild-type mice (Fig 3A). There was no change in Nqo1 mRNA expression in livers from either genotype 1 day after BDL. However, 3 days after BDL, Nqo1 mRNA expression was tripled in livers from wild-type, but not Nrf2-null mice.
Fig 3.
Expression and activity of Nqo1 in liver cytosolic fractions from wild-type and Nrf2-null mice 1 and 3 days after sham or BDL surgery. (A) Total RNA was isolated from livers of WT and Nrf2-null mice that underwent sham or BDL surgery 1 or 3 days previously, and analyzed by the bDNA assay for Nqo1 mRNA expression. Data from sham animals at 1 and 3 days were not statistically different and were pooled and denoted as sham. The data are presented as mean relative light units ± standard error of the mean (SEM) (n = 4–7 animals). (B) Upper panel: Representative Western blot of liver cytosolic fractions from mice 3 days after sham or BDL surgery (n = 4–7 animals) stained with anti-Nqo1 antibodies. Lower panel: Quantification of Nqo1 protein levels in liver cytosolic fractions from mice 1 and 3 days after sham or BDL surgery. The data are presented as relative protein expression ± SEM (n = 4–7 animals). (C) Analysis of Nqo1 activity in liver cytosolic fractions from WT and Nrf2-null mice that underwent sham or BDL surgery 1 or 3 days previously. The data are presented as nanomoles of DCPIP reduced per minute per milligram of protein ± SEM (n = 4–7 animals). Asterisks (*) represent a statistical difference (P < 0.05) between WT sham and WT BDL groups, and crosses (†) represent a statistical difference between WT and Nrf2-null mice undergoing the same type of surgery
Effect of BDL on Nqo1 protein expression in liver from wild-type and Nrf2-null mice
Western blots using the anti-Nqo1 antibody were performed to confirm that the changes in Nqo1 mRNA expression observed after BDL in livers from wild-type and Nrf2-null mice corresponded to similar protein changes (Fig 3B). Nqo1 protein levels from sham Nrf2-null mice were more than 50% lower than that observed in sham wild-type mice. This correlates with reduced Nqo1 mRNA basal expression seen in knockout mice. BDL did not increase Nqo1 protein levels in wild-type or Nrf2-null mice 1 day after BDL (Fig 3B). However, 3 days after BDL, Nqo1 protein levels were increased by 40% in wild-type, but not in Nrf2-null mice (Fig 3B).
Effect of BDL on Nqo1 activity in liver from wild-type and Nrf2-null mice
Nqo1 activity in livers from wild-type and Nrf2-null mice was quantified to determine whether differences in Nqo1 mRNA and protein expression between genotypes translated into differences in Nqo1 activity. Nqo1 activity detected in liver cytosolic fractions from sham Nrf2-null mice was about 80% lower than that in sham wild-type mice (Fig 3C). BDL increased hepatic Nqo1 activity in wild-type mice 150% 3 days after BDL, with no significant change at 1 day. In contrast, BDL did not increase Nqo1 activity in Nrf2-null mice at either time point.
Effect of BDL on liver histology, plasma ALT activity, and hepatic bile acid levels from wild-type and Nrf2-null mice
Histologic examination of liver sections from sham-operated wild-type and Nrf2-null mice demonstrated normal histology (Fig 4A,B, respectively). At 3 days after BDL, both genotypes had multifocal hepatocellular degeneration, multifocal coagulative necrosis, and scattered hepatocellular hyperplasia. Using morphometric analysis, there was no difference in the total percentage of necrotic area in sections from wild-type (2.7 ± 0.8%) vs Nrf2-null (2.0 ± 0.3%) mice. However, the number and size of necrotic foci differed between genotypes. Quantitative analysis demonstrated a lower number of necrotic foci in wild-type mice (4.8 ± 1.6) (Fig 4C,E) in relation to Nrf2-null mice (9.0 ± 2.1) (Fig 4D,F). In contrast, the area of each necrotic foci was greater in wild-type mice (1.1 ± 0.02%) compared to Nrf2-null mice (0.2 ± 0.02%). Both genotypes had similar mild to moderate biliary hyperplasia accompanied by neutrophil and lymphocyte accumulation after BDL.
Fig 4.
Histologic examination of liver sections and analysis of hepatic bile acid accumulation from wild-type and Nrf2-null mice 3 days after BDL. Portions of liver from wild-type and Nrf2-null mice 3 days after sham or BDL surgery were fixed in formalin and stained with hematoxylin and eosin. Sham-operated wild-type (A) and Nrf2-null (B) mouse liver demonstrated normal tissue histology (4× magnification). BDL resulted in multifocal hepatocellular necrosis (arrows) in wild-type (C, E) and Nrf2-null (D, F) mice. Hepatocyte degeneration and necrosis is seen at 4× (C, D) and 10× (E, F) magnification. (G) Determination of liver bile acid concentration (nanomoles per gram of tissue). Values are expressed as mean ± standard error of the mean. Asterisks (*) represent a statistical difference (P < 0.05) between sham and BDL groups for a respective genotype. Crosses (†) represent a statistical difference (P < 0.05) between wild-type and Nrf2-null mice undergoing BDL
Bile duct ligation of WT and Nrf2-null mice resulted in hepatocellular injury as measured by serum ALT levels. Increases in serum ALT activity in both genotypes were noted 3 days after BDL. Slightly greater ALT levels were seen in BDL WT (mean, 646 ± 240 U/L) compared to Nrf2-null (mean, 257 ± 87 U/L) mice, relative to sham-operated mice (WT mean, 18 ± 3 U/L; Nrf2-null mean, 24 ± 11 U/L).
Recognizing that accumulation of bile acids in the liver is the primary cause of cellular injury during cholestasis, total hepatic bile acid concentrations in Nrf2-null and wild-type mice were quantified (Fig 4G). Surprisingly, bile acid accumulation was significantly lower in Nrf2-null mice at 3 days after BDL, with approximately 2-fold lower levels than in wild-type mice (Fig 4G). There were reduced basal levels of bile acids in the livers of sham-operated Nrf2-null compared to wild-type mice, although this difference was not statistically significant.
DISCUSSION
Oxidative stress results from an imbalance in radical-generating and radical-scavenging activities. Shifts in cellular redox status reflect perturbations in the relative ratios of oxidative and toxic products (eg, bile acids, superoxide, hydroxyl radical, lipid peroxides) and antioxidants during cholestatic liver disease. Although the precise mechanism is not completely known, it is generally accepted that oxidative stress is involved in the progression of hepatic damage during human cholestasis (Parola et al 1996; Aboutwerat et al 2003).
Hepatic gene expression is dramatically altered in response to cholestatic liver disease. These compensatory changes include a reduction in bile acid synthesis and induction of basolateral transport proteins involved in efflux of bile acids and oxidative stress by-products (Wagner et al 2003). These responses appear to be a concerted effort by the liver to lessen injury by limiting the intracellular accumulation of potentially toxic substances. Because of the presence of substances that can activate antioxidant responses after BDL, we hypothesized that the transcription factor Nrf2 is activated during cholestasis as a means for the liver to coordinately regulate oxidative stress–responsive genes in order to limit the progression of hepatic injury. This is the first report investigating the involvement of Nrf2 signaling in cholestasis. The data presented in this manuscript conclusively demonstrate that extrahepatic cholestasis, modeled by BDL, induces expression of numerous genes regulated by Nrf2 including Nqo1, Ho-1, and Gst (isoforms a1, a4, m1, m2, m3). Transcriptional up-regulation of Nqo1 in mouse liver after BDL corresponded with induction of both protein and activity via an Nrf2-dependent mechanism.
An important question is the significance of the strong induction of Nqo1 in response to BDL. Although Nqo1 is a 2-electron reductase enzyme with a broad substrate profile including quinones, nitro, and azo compounds, Nqo1 likely plays a more general role in reducing the extent of oxidative stress in liver after BDL. This is supported by recent data documenting the ability of Nqo1 to scavenge the reactive oxidative product, superoxide, and recycle endogenous antioxidants (Ross 2004; Siegel et al 2004).
Histopathological analysis of liver sections from BDL wild-type and Nrf2-null mice demonstrated a similar extent of total hepatocellar necrosis. The anticipated outcome of this study was that Nrf2-null mice would exhibit more severe hepatic injury during cholestasis in light of their heightened sensitivity to a number of other oxidative-type pathological conditions (Chan et al 2001;Ramos-Gomez et al 2001; Cho et al 2002). A likely explanation for the lack of difference in total hepatocellular necrosis between genotypes is provided by our results showing that Nrf2-null mice had 2-fold lower accumulation of liver bile acids after BDL compared to wild-type mice (Fig 4G). Reduced accumulation of hepatic bile acids could be related to lower basal production, decreased de novo synthesis, and/or changes in enterohepatic recycling and excretion in Nrf2-null mice after BDL. BDL is known to reduce the expression of a number of bile acid synthesis genes including Cyp7b1 and Cyp8b1 to compensate for impaired excretion of bile acids into bile (Stedman et al 2005). Interestingly, we observed a more profound reduction in hepatic levels of Cyp7b1 and Cyp8b1 after BDL in Nrf2-null compared to wild-type mice in preliminary studies (data not shown). A greater decrease in the expression of these enzymes in livers of Nrf2-null mice after BDL corresponds with lower bile acid accumulation and lesser than anticipated liver injury. Additional studies are needed to further document these changes in Cyp7b1 and Cyp8b1 expression and to investigate the relationship between reduced bile acid accumulation in Nrf2-null mice after BDL with cholestatic liver injury.
Induction of Nqo1 mRNA is also seen during chemical-induced hepatocellular injury in rodents treated with a single dose of the pro-oxidants acetaminophen, carbon tetrachloride, and bromobenzene (Heijne et al 2004; Aleksunes et al 2005). These observations suggest that up-regulation of Nqo1 represents a general adaptive response to oxidative damage rather than a specific response activated only during obstructive cholestasis. Data have been generated demonstrating that patients with primary biliary cirrhosis or acetaminophen-induced liver injury have increased Nqo1 protein expression and activity, suggesting activation of antioxidant response elements in human liver in response to oxidative damage (Aleksunes 2006). Together with the data in this manuscript, the coordinated up-regulation of Nqo1 and additional Nrf2-responsive genes in mouse and human liver is likely a compensatory mechanism that attempts to hinder progression of hepatic disease. Although these adaptive changes turned out to be ineffective at fully preventing acute cholestatic liver injury following BDL, these responses could be important in more chronic forms of hepatic diseases with an oxidative stress component.
Acknowledgments
Portions of this work were presented at the Annual Meeting of the Society of Toxicology (March 6–10, 2005, New Orleans, LA, USA). This work was supported by National Institutes of Health Grants ES-09716, ES-07079, and ES-10093. L.M.A. is a Howard Hughes Medical Institute Predoctoral Fellow.
REFERENCES
- Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon F, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 2003;1637:142–150. doi: 10.1016/s0925-4439(02)00225-9.0006-3002(2003)1637[0142:OSIASF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Goedken MJ, Manautou JE. Up-regulation of NAD(P)H quinone oxidoreductase 1 during human liver injury. World J Gastroenterol. 2006;12:1937–1940. doi: 10.3748/wjg.v12.i12.1937.1007-9327(2006)012[1937:UONQOD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83:44–52. doi: 10.1093/toxsci/kfi013.1096-0929(2005)083[0044:DEOMHT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098.1091-6490(2001)098[4611:AIFONI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501.1044-1549(2002)026[0175:RONIPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- DeMeo M, Kolli S, and Keshavarzian A. et al. 1998 Beneficial effect of a bile acid resin binder on enteral feeding induced diarrhea. Am J Gastroenterol. 93:967–971. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Klaassen CD. Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab Dispos. 2000;28:608–616.0090-9556(2000)028[0608:DOCDEO]2.0.CO;2 [PubMed] [Google Scholar]
- Heijne WH, Slitt AL, and van Bladeren PJ. et al. 2004 Bromobenzene-induced hepatotoxicity at the transcriptome level. Toxicol Sci. 79:411–422. [DOI] [PubMed] [Google Scholar]
- Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stress-related changes in the livers of bile-duct-ligated rats. J Biomed Sci. 2003;10:170–178. doi: 10.1007/BF02256052.1021-7770(2003)010[0170:OCITLO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5.0076-6879(2002)348[0182:RONIAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kitada T, Seki S, Iwai S, Yamada T, Sakaguchi H, Wakasa K. In situ detection of oxidative DNA damage, 8-hydroxydeoxyguanosine, in chronic human liver disease. J Hepatol. 2001;35:613–618. doi: 10.1016/s0168-8278(01)00171-4.0168-8278(2001)035[0613:ISDOOD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor κB. Ann Clin Lab Sci. 2001;31:383–390.0091-7370(2001)031[0383:FRHIOE]2.0.CO;2 [PubMed] [Google Scholar]
- Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, and McLellan LI 2004 Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp 157–176. [DOI] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754.0264-6021(2003)374[0337:IOANNA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20:351–359. doi: 10.1016/0891-5849(96)02055-2.0891-5849(1996)020[0351:OTROLP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798.1091-6490(2001)098[3410:STCIIA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev. 2004;36:639–654. doi: 10.1081/dmr-200033465.0360-2532(2004)036[0639:QRMITM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238.0026-895X(2004)065[1238:NORAAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stedman CA, Liddle C, and Coulter SA. et al. 2005 Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 102:2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LY, Lee KT, Liu TZ. Evidence for accelerated generation of hydroxyl radicals in experimental obstructive jaundice of rats. Free Radic Biol Med. 1998;24:732–737. doi: 10.1016/s0891-5849(97)00330-4.0891-5849(1998)024[0732:EFAGOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vendemiale G, Grattagliano I, Lupo L, Memeo V, Altomare E. Hepatic oxidative alterations in patients with extra-hepatic cholestasis. Effect of surgical drainage. J Hepatol. 2002;37:601–605. doi: 10.1016/s0168-8278(02)00234-9.0168-8278(2002)037[0601:HOAIPW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960.1091-6490(1996)093[14960:NANPAC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Fickert P, and Zollner G. et al. 2003 Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 125:825–838. [DOI] [PubMed] [Google Scholar]