Abstract
BACKGROUND—Inflammatory bowel disease (IBD) is characterised by infiltration of inflamed mucosal regions with CD4+ T lymphocytes and other mononuclear cells. Interleukin (IL)-16 exerts a strong chemoattractant activity on CD4+ cells. Moreover, IL-16 activates expression and production of proinflammatory cytokines such as IL-1β, IL-6, IL-15, and tumour necrosis factor α (TNF-α) in human monocytes. AIM—To examine if IL-16 expression is increased in IBD patients compared with healthy controls. METHODS—Twenty one patients with IBD (10 with ulcerative colitis (UC), 11 with Crohn's disease (CD)), seven disease specificity controls (DSC), and seven healthy controls were studied. Biopsies were taken during colonoscopies and IL-16 mRNA as well as protein expression were investigated by reverse transcriptase-polymerase chain reaction, ELISA, western blot, and immunohistochemistry. RESULTS—IL-16 mRNA and protein expression in the colonic mucosa of IBD patients were increased twofold compared with healthy controls, DSC, or IBD patients under steroid treatment. Most of the detected IL-16 protein was in its bioactive 17 kDa form and was predominantly expressed in eosinophils. Increased IL-16 expression in UC patients appeared to be mainly restricted to the inflamed regions of the colonic mucosa. Levels of caspase 3, which processes the 68 kDa IL-16 precursor molecule into the biological active 17 kDa form, were not increased. CONCLUSIONS—Our results provide evidence that IL-16 expression is significantly increased in the inflamed colonic mucosa of IBD patients but not in control individuals, DSC, or patients under steroid treatment. Therefore, upregulation of IL-16 expression seems to be specific for chronic intestinal inflammation and could lead to increased secretion of other proinflammatory cytokines in IBD. Keywords: interleukin-16; T lymphocytes; eosinophils; Crohn's disease; ulcerative colitis; inflammatory bowel disease
Full Text
The Full Text of this article is available as a PDF (655.1 KB).
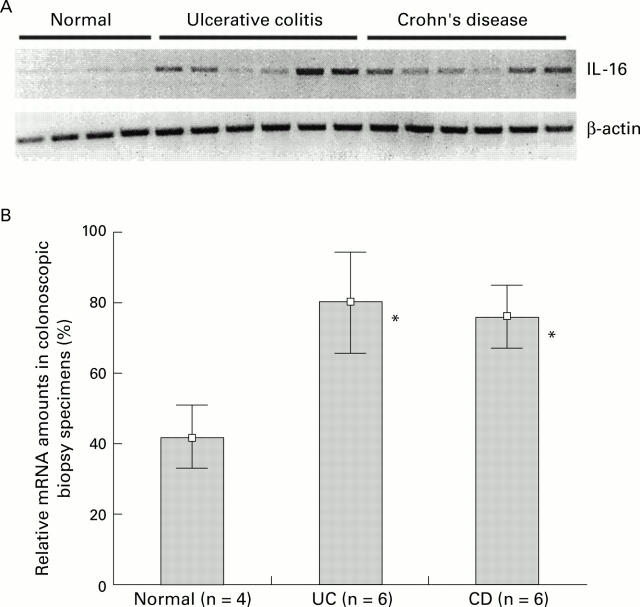
Figure 1 .
(A) Total RNA was extracted from biopsies from four normal control individuals and 12 patients with IBD (six Crohn's disease and six ulcerative colitis patients). Interleukin 16 (IL-16) mRNA was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) and analysed on an agarose gel (top panel). The use of equal amounts of total RNA was confirmed in a parallel experiment by amplification of β-actin mRNA (bottom panel). PCR products were analysed on a 1% agarose gel. (B) Densitometric quantification of IL-16 specific PCR products. Values are given as mean (SD) percentage of intensity of the highest detected densitometric strength. *p<0.05 v controls.
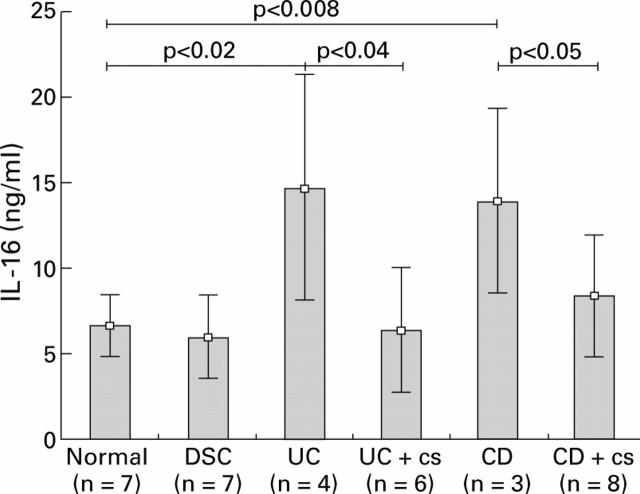
Figure 2 .
Quantification of interleukin 16 (IL-16) protein in colonic mucosal biopsy specimens from 21 patients with inflammatory bowel disease (10 ulcerative colitis (UC) patients (6/10 under corticosteroid (cs) therapy), 12 Crohn's disease (CD) patients (8/12 under cs treatment)), seven disease specificity controls (DSC), and seven normal individuals. Tissues were lysed and adjusted to a protein concentration of 0.5 mg/ml: 50 µl of each sample were used to detect IL-16 content by IL-16 specific ELISA. Values are mean (SD).
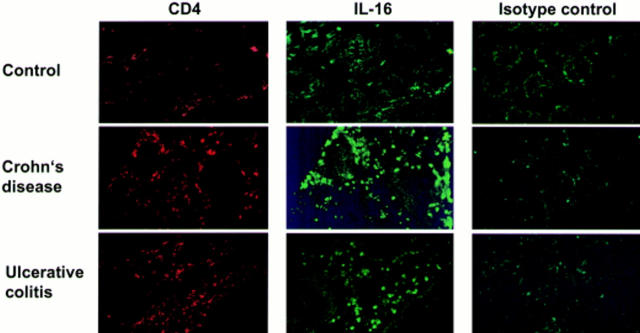
Figure 3 .
Detection of CD4 and interleukin 16 (IL-16) by immunofluorescence staining in biopsies from control individuals and patients with inflammatory bowel disease (ulcerative colitis and Crohn's disease). The specificity of the IL-16 antibody was confirmed by an irrelevant isotype antibody (biotin conjugated rabbit IgG). CD4 infiltration was visualised with a Cy3 conjugated antibody (red) while IL-16 protein was detected by an FITC labelled antibody (green).
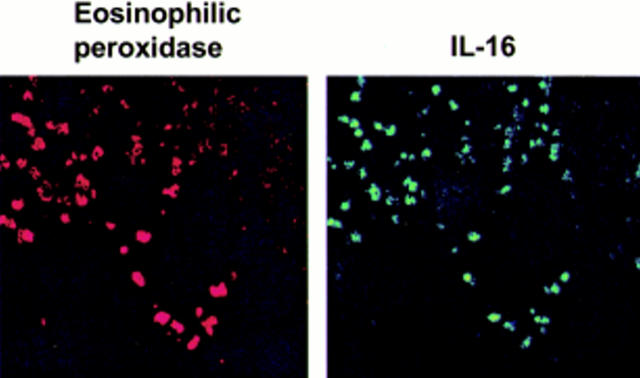
Figure 4 .
Staining of biopsies from Crohn's disease patients with anti-eosinophilic peroxidase antibody (left) and anti-interleukin 16 (IL-16) antibody (panel).
Figure 5 .

(A) Interleukin 16 (IL-16) protein expression was analysed by western blotting in biopsies taken from four control individuals (lanes 1-4), four ulcerative colitis (UC) patients (lanes 5-8), and four Crohn's disease (CD) patients (lanes 9-12). The experiment revealed the predominant existence of the bioactive 17 kDa form of IL-16 in IBD patients compared with controls. (B) Biopsies from four UC and three CD patients were taken from non-inflamed (—) or inflamed (+) regions of the colon and tested for IL-16 protein expression in a western blot assay. (C) Extracts of the same biopsies as in (A) were used to detect caspase 3 protein expression. A lysate from unstimulated human A431 cells supplied with the anti-caspase 3 antibody served as a control (WB, western blot).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baier M., Bannert N., Werner A., Lang K., Kurth R. Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5273–5277. doi: 10.1073/pnas.94.10.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini A., Yoshimura H., Vittori E., Marini M., Mattoli S. Bronchial epithelial cells of patients with asthma release chemoattractant factors for T lymphocytes. J Allergy Clin Immunol. 1993 Sep;92(3):412–424. doi: 10.1016/0091-6749(93)90120-5. [DOI] [PubMed] [Google Scholar]
- Berman J. S., Cruikshank W. W., Center D. M., Theodore A. C., Beer D. J. Chemoattractant lymphokines specific for the helper/inducer T-lymphocyte subset. Cell Immunol. 1985 Oct 1;95(1):105–112. doi: 10.1016/0008-8749(85)90299-0. [DOI] [PubMed] [Google Scholar]
- Berrebi D., Besnard M., Fromont-Hankard G., Paris R., Mougenot J. F., De Lagausie P., Emilie D., Cezard J. P., Navarro J., Peuchmaur M. Interleukin-12 expression is focally enhanced in the gastric mucosa of pediatric patients with Crohn's disease. Am J Pathol. 1998 Mar;152(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Center D. M., Cruikshank W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982 Jun;128(6):2563–2568. [PubMed] [Google Scholar]
- Chupp G. L., Wright E. A., Wu D., Vallen-Mashikian M., Cruikshank W. W., Center D. M., Kornfeld H., Berman J. S. Tissue and T cell distribution of precursor and mature IL-16. J Immunol. 1998 Sep 15;161(6):3114–3119. [PubMed] [Google Scholar]
- Cruikshank W. W., Center D. M., Nisar N., Wu M., Natke B., Theodore A. C., Kornfeld H. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5109–5113. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank W. W., Kornfeld H., Center D. M. Signaling and functional properties of interleukin-16. Int Rev Immunol. 1998;16(5-6):523–540. doi: 10.3109/08830189809043007. [DOI] [PubMed] [Google Scholar]
- Cruikshank W., Center D. M. Modulation of lymphocyte migration by human lymphokines. II. Purification of a lymphotactic factor (LCF). J Immunol. 1982 Jun;128(6):2569–2574. [PubMed] [Google Scholar]
- Krautwald S. IL-16 activates the SAPK signaling pathway in CD4+ macrophages. J Immunol. 1998 Jun 15;160(12):5874–5879. [PubMed] [Google Scholar]
- Kusugami K., Fukatsu A., Tanimoto M., Shinoda M., Haruta J., Kuroiwa A., Ina K., Kanayama K., Ando T., Matsuura T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995 May;40(5):949–959. doi: 10.1007/BF02064182. [DOI] [PubMed] [Google Scholar]
- Laberge S., Cruikshank W. W., Beer D. J., Center D. M. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996 Jan 1;156(1):310–315. [PubMed] [Google Scholar]
- Laberge S., Cruikshank W. W., Kornfeld H., Center D. M. Histamine-induced secretion of lymphocyte chemoattractant factor from CD8+ T cells is independent of transcription and translation. Evidence for constitutive protein synthesis and storage. J Immunol. 1995 Sep 15;155(6):2902–2910. [PubMed] [Google Scholar]
- Laberge S., Ernst P., Ghaffar O., Cruikshank W. W., Kornfeld H., Center D. M., Hamid Q. Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am J Respir Cell Mol Biol. 1997 Aug;17(2):193–202. doi: 10.1165/ajrcmb.17.2.2750. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. G., Wan H. C., Bozza P. T., Resnick M. B., Wong D. T., Cruikshank W. W., Kornfeld H., Center D. M., Weller P. F. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol. 1996 Apr 1;156(7):2566–2570. [PubMed] [Google Scholar]
- MacDonald T. T., Hutchings P., Choy M. Y., Murch S., Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990 Aug;81(2):301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Ceska M., Effenberger F., Kurlak L., Lindley I., Hawkey C. J. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci (Lond) 1992 Mar;82(3):273–275. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- Mathy N. L., Bannert N., Norley S. G., Kurth R. Cutting edge: CD4 is not required for the functional activity of IL-16. J Immunol. 2000 May 1;164(9):4429–4432. doi: 10.4049/jimmunol.164.9.4429. [DOI] [PubMed] [Google Scholar]
- Mathy N. L., Scheuer W., Lanzendörfer M., Honold K., Ambrosius D., Norley S., Kurth R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000 May;100(1):63–69. doi: 10.1046/j.1365-2567.2000.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli L., Hauser C., Zgraggen K., Wagner H. E., Hess M. W., Laissue J. A., Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996 Feb;178(2):201–206. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Biancone L., Marasco R., Morrone G., Marasco O., Luzza F., Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997 Apr;112(4):1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- Müller S., Lory J., Corazza N., Griffiths G. M., Z'graggen K., Mazzucchelli L., Kappeler A., Mueller C. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol. 1998 Jan;152(1):261–268. [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Watanabe M., Yamazaki M., Yajima T., Hayashi T., Ishii H., Mukai M., Yamada T., Watanabe N., Jameson B. A. A synthetic mimetic of CD4 is able to suppress disease in a rodent model of immune colitis. Eur J Immunol. 1999 Jan;29(1):355–366. doi: 10.1002/(SICI)1521-4141(199901)29:01<355::AID-IMMU355>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Parada N. A., Center D. M., Kornfeld H., Rodriguez W. L., Cook J., Vallen M., Cruikshank W. W. Synergistic activation of CD4+ T cells by IL-16 and IL-2. J Immunol. 1998 Mar 1;160(5):2115–2120. [PubMed] [Google Scholar]
- Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989 Jan 14;298(6666):82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler G., Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998 Apr;22(4):382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- Rumsaeng V., Cruikshank W. W., Foster B., Prussin C., Kirshenbaum A. S., Davis T. A., Kornfeld H., Center D. M., Metcalfe D. D. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J Immunol. 1997 Sep 15;159(6):2904–2910. [PubMed] [Google Scholar]
- Stronkhorst A., Radema S., Yong S. L., Bijl H., ten Berge I. J., Tytgat G. N., van Deventer S. J. CD4 antibody treatment in patients with active Crohn's disease: a phase 1 dose finding study. Gut. 1997 Mar;40(3):320–327. doi: 10.1136/gut.40.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Durant D. A., Potter J. W. Migration of human helper/inducer T cells in response to supernatants from Con A-stimulated suppressor/cytotoxic T cells. J Immunol. 1983 Aug;131(2):697–700. [PubMed] [Google Scholar]
- Wu D. M., Zhang Y., Parada N. A., Kornfeld H., Nicoll J., Center D. M., Cruikshank W. W. Processing and release of IL-16 from CD4+ but not CD8+ T cells is activation dependent. J Immunol. 1999 Feb 1;162(3):1287–1293. [PubMed] [Google Scholar]
- Zhang Y., Center D. M., Wu D. M., Cruikshank W. W., Yuan J., Andrews D. W., Kornfeld H. Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem. 1998 Jan 9;273(2):1144–1149. doi: 10.1074/jbc.273.2.1144. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J. Review article: Chemokine production by intestinal epithelial cells: a therapeutic target in inflammatory bowel disease? Aliment Pharmacol Ther. 1997 Dec;11 (Suppl 3):116–121. doi: 10.1111/j.1365-2036.1997.tb00816.x. [DOI] [PubMed] [Google Scholar]