Abstract
The low nutritional value of barley for poultry is because of the absence of an intestinal enzyme for efficient depolymerization of (1,3–1,4)-β-glucan, the major polysaccharide of the endosperm cell walls. This leads to high viscosity in the intestine, limited nutrient uptake, decreased growth rate, and unhygienic sticky droppings adhering to chickens and floors of the production cages. Consequently, the 7.5 billion broiler chickens produced annually in the United States are primarily raised on corn–soybean diets. Here we show that addition to normal barley of 6.2% transgenic malt containing a thermotolerant (1,3–1,4)-β-glucanase (4.28 μg⋅g−1 soluble protein) provides a weight gain equivalent to corn diets. The number of birds with adhering sticky droppings is drastically reduced. Intestines and excrements of chickens fed the barley control diet contained large amounts of soluble (1,3–1,4)-β-glucan, which was reduced by 75 and 50%, respectively, by adding transgenic malt to the diet. The amount of active recombinant enzyme in the small intestine corresponded to that present in the feed, whereas an 11-fold concentration of the enzyme was observed in the ceca, and a 7.5-fold concentration occurred in the excrement. Glycosylation of the β-glucanase isolated from the ceca testified to its origin from the transgenic barley. Analysis of the data from this trial demonstrates the possibility of introducing individual recombinant enzymes into various parts of the gastrointestinal tract of chickens with transgenic malt and thereby the possibility of evaluating their effect on the metabolism of a given ingredient targeted by the enzyme.
According to the United States Department of Agriculture National Agricultural Statistics Service (1998), the yearly production of broiler chickens in the United States has increased from 5 billion birds in 1987 to 7.5 billion in 1996. The per capita consumption of chicken meat has risen from 68 to 88 pounds per year, which corresponds to an overall rise from 16.9 to 23.9 billion pounds of broiler consumption over the 10-year period. Approximately 4.4 lb of feed are needed to produce 1 lb of live chicken, and the overwhelming majority of the feed used for this meat production is based on corn grain. Barley is an unacceptable component in chicken feed, because it is low in metabolizable energy. Feeding barley diets to poultry leads to a limited uptake of nutrients, slow initial growth, and sticky droppings adhering to the cloaca and down of the chicken as well as to the floors of the production cages.
The reason for the low nutritional value of barley grain was discovered when crude fungal or bacterial enzymes, or 2.5% malt, were added to barley diets (1–4). This improved weight gain and alleviated the problems with the sticky droppings. Rickes and coworkers purified the first (1,3–1,4)-β-glucanase from a strain of Bacillus subtilis, now designated as Bacillus amyloliquefaciens, and demonstrated a positive growth response in chickens when the purified enzyme was added to a barley diet (5). The bacterial enzyme depolymerized barley (1,3–1,4)-β-glucan and had the same substrate specificity as the endogenous barley enzyme (6). Supplementation of barley diets with crude enzymes from culture filtrates of Trichoderma reesei in the form of commercial preparations GV-P (Grindsted Products, Grindsted, Denmark) and Onozuka 3S (Serva) resulted in a highly significant increase in weight gain and alleviation of sticky droppings (7–10). It thus became clear that birds lack an enzyme that can depolymerize (1,3–1,4)-β-glucan, the major component of the barley endosperm cell walls.
T. reesei culture filtrates contain several hydrolases that depolymerize cellulose [(1,4)-β-glucan], (1,3)-β-glucan, and (1,3–1,4)-β-glucan (11–14). Cloning of the structural gene for T. reesei endoglucanase I (15) identified it as a cellulase by its primary structure. Heterologous expression of the gene in barley suspension culture cells leads to its secretion into the culture medium. Mashing experiments demonstrated that this enzyme depolymerizes barley (1,3–1,4)-β-glucan and displays considerable heat stability (16). The mixed linked (1,3–1,4)-β-glucan in the endosperm walls of barley consists of 90% polysaccharide chains yielding on cleavage with purified barley (1,3–1,4)-β-glucanase cellotriosyl (G4G4G) and cellotetraosyl (G4G4G4G4) residues. These (1,4)-linked blocks of β-glycosyl residues are separated by single (1,3)-linkages (17). The cellulase of Trichoderma depolymerizes the barley mixed linked β-glucan by hydrolyzing glucosyl 1–4 linkages within the cellotriosyl and cellotetraosyl blocks, whereas the barley and Bacillus (1,3–1,4)-β-glucanases specifically cleave a 1–4 linkage adjacent to a 1–3-linked glucose (14, 17, 18). This explains why all three enzymes can promote nutritional improvements of barley diets.
The (1,3–1,4)-β-glucans in the endosperm cell walls of barley consist of two fractions, one that is water soluble at 40–65°C, and one that is insoluble in water. The latter requires acid or alkaline hydrolysis or highly purified enzymes for depolymerization to smaller fragments that become water soluble and can then be analyzed. Woodward et al. determined the chemical structure and physicochemical properties of water-soluble barley (1,3–1,4)-β-glucans (17–19). These glucans consist of a population of molecules with molecular weights ranging from 104 to 107 and are responsible for the high viscosity of β-glucan solutions. The water-soluble β-glucans are the troublemakers in chicken feed, malting, and brewing (review in ref. 14). The water-insoluble fraction does not influence the viscosity. One kilogram of malting or feed barley grain contains typically about 50 g (1,3–1,4)-β-glucan when maturing under dry conditions or about 30 g when grain filling takes place under rainy conditions (20–23). The water-soluble portion amounts to, respectively, 10 and 5 g⋅kg−1 under the two conditions of ripening.
Burnett (24) suggested that the high viscosity per se rather than a limiting sucrose availability is the cause of the inhibitory action of barley β-glucans in poultry feed, and this suggestion has been investigated by White et al. (9, 10). Addition of barley β-glucan to a corn-based diet increased the viscosity of the intestinal fluid 3-fold, an increase that could be reduced to control levels by supplementation with cellulases. Addition of hydroxyethyl cellulose to a basal corn diet depressed growth and feed efficiency, but added cellulase eliminated growth depression. Relative viscosity in intestinal fluid increased from 1.67 to 11.2 centipoise in the diets with hydroxyethyl cellulose, whereas further addition of cellulase resulted in a value of 3.2. If the cellulase was added to a barley diet, the body weight of the chickens at the end of the 3-week feeding period was 308 g, compared with 235 g for the control. Determination of the β-glucan and glucose in the small intestine led to the conclusion that the weight gain cannot result from the limited amount of glucose generated by enzymatic depolymerization of the β-d-glucan and supported the hypothesis that the high viscosity of the soluble β-glucans interferes with the diffusion of digestive enzymes and the transport of nutrients through the unstirred water layer adjacent to the mucosal surface.
Hesselman and Åman (25) have followed digestion of starch and (1,3–1,4)-β-glucans in different sections of the intestines of chickens after treatment of the barley diet with enzymes depolymerizing (1,3–1,4)-β-glucans. Starch digestion was impeded by the barley diet in the upper sections of the small intestine but was very extensive when the diet was treated with enzyme. No (1,3–1,4)-β-glucan was found in the cecum of chickens with or without pretreatment of the diet with enzyme. It was suggested that this absence was because of an enhanced aggressive microbial flora that also led to the increased dry matter content of the colon. It is speculated that the products of the cecal microflora are responsible for the sticky droppings rather than the (1,3–1,4)-β-glucans.
In a previous communication (26), we have reported on two sets of transgenic barley plants that express a synthetic codon-optimized gene encoding a protein-engineered thermotolerant (1,3–1,4)-β-glucanase. One set produces the recombinant enzyme with the aid of an endosperm-specific gene promoter in the developing grain, the other, with an aleurone-specific α-amylase gene promoter during germination/malting for secretion into the endosperm (27, 28). The thermotolerant (1,3–1,4)-β-glucanase H(A12-M)ΔY13 (H, hybrid; A, amino acids from B. amyloliquefaciens; M, amino acids from Bacillus macerans) is a hybrid enzyme derived from the enzymes of the two species. It exhibits a half life of >4 h at 70°C (29). Its primary three-dimensional structure is very different from the endogenous barley enzyme, and its specific activity and Vmax are ≈200-fold higher than the barley enzyme. Its pH optimum is between 6 and 7 and thus is highly suitable for depolymerization of β-glucans in the duodenum, ileum, rectum, and ceca of chicken in which the pH of the contents ranges from 5.7 to 7.0 (30). But the enzyme is also efficient at the lower pH values of 4.5 to 5.0, characteristic of the contents in the crop, glandular stomach, and gizzard of the bird.
In the present investigation, we have studied the growth of chickens fed a barley diet supplemented with malt containing the recombinant heat-stable (1,3–1,4)-β-glucanase and have compared it to a standard corn-based diet, a barley diet without added malt, and a barley diet with addition of normal malt. The frequency of sticky droppings was recorded and β-glucans analyzed (31, 32) in the different portions of the gastrointestinal tract as well as in excrement. The presence of recombinant enzyme in the glandular stomach, intestine, ceca, and excrement was traced by activity measurements and diagnostic Western blots.
Materials and Methods
Experimental Animals and Conditions.
The chicken trial was performed with 240 Hubbard High Yield broilers (Fors Farms, Puyallup, WA). One-day-old chicks were transferred to electrically heated Petersime Brood units with raised floors (Petersime Incubator, Gettysburgh, OH). Each of the four experimental diets was randomly distributed among 12 pens and 5 birds randomly assigned to a pen. Feed and water were available ad libitum, and 16-hr daylight was maintained. All experiments with the animals were carried out with approval from the Washington State University Animal Care and Use Committee. The Committee follows the guidelines established by the Canadian Council on Animal Care of 1980 (33).
Diet Composition and Preparation.
Chickens were fed four ground diets with the composition given in Table 1: corn basal, barley basal (cv. Golden Promise), barley diet with malt of Golden Promise containing no β-glucanase, and barley diet with malt of transgenic line 5607 containing heat-stable β-glucanase. Malt was prepared by using microscale malting equipment available at the Department of Crop and Soil Sciences, Washington State University. Kilning was performed at 45°C for 6 h and 80°C for 4 h to deactivate the endogenous β-glucanase present in germinated barley. Malt was added at the expense of barley in the amount of 6.2% of the total diet.
Table 1.
Composition of diets in weight per cent
| Ingredients | Diets
|
|||
|---|---|---|---|---|
| Corn
|
Barley
|
Barley +
GP* malt
|
Barley + TL†
malt
|
|
| % | ||||
| Corn | 62.00 | |||
| Barley | 62.00 | 55.80 | 55.80 | |
| GP malt | 6.20 | |||
| TL malt | 6.20 | |||
| Soymeal | 24.00 | 24.00 | 24.00 | 24.00 |
Additional ingredients: fishmeal, 5%; beef tallow, 5%; dicalcium phosphate, 1.60%; limestone, 1.70%; iodized sodium chloride, 0.20; dl-methionine, 0.20%; vitamin premix, 0.25; trace mineral mix, 0.05%, in each diet.
Golden Promise.
Transgenic Line 5607 (26).
Data Collection During Trial.
The 5 birds in each pen were weighed on days 0, 6, 13, and 20. Feed consumption by individual pen was determined as difference between initial feed weight for each pen and feed weight on day of determination. Cumulative feed efficiency per chicken was calculated as ratio of weight gained to feed consumed. Dry matter of excreta was determined on days 5, 8, 11, 14, 17, and 20 by collecting droppings and drying them for 6 h at 105°C. The number of chickens with sticky droppings adhering to down of the cloaca area was noted on the same days as excreta collection.
Collection of Intestinal Contents.
On day 21 of the experiment, 192 birds were euthanized with CO2, and revival was prevented by cervical dislocation. After about 2 min, the abdomen was opened, and various parts of the chick gastrointestinal tract were excised from 4 chickens per pen. Pooled contents of the glandular stomach, intestine, and ceca were flush frozen in liquid N2 and stored at −20°C.
Analyses of Experimental Diets and Intestinal Contents.
These analyses were carried out according to Association of Official Analytical Chemists methods (34) and are published as supplemental data on the PNAS web site, www.pnas.org.
Statistical Analyses.
ANOVA was conducted by using the General Linear Models procedure of sas software (35), and treatment means were compared by least significant difference. Differences were considered significant at P < 0.05.
Results
Analyses of Diets.
The protein contents of the corn diet, the diet with Golden Promise malt, and that with malt from transgenic line 5607 containing the heat-stable (1,3–1,4)-β-glucanase were very similar, about 22% (Table 3, supplemental data). The barley diet had a 3% higher protein content. The two malt additives contained 12 and 10% protein. The fiber fractions consist of the nonstarch polysaccharides and Klason lignin. Nonstarch polysaccharides of barley are (1,3–1,4)-β-d-glucans, arabinoxylans, and cellulose. Corn contains pentosans instead of (1,3–1,4)-β-glucans. In the barley diet, considerable more fiber was extractable with neutral and acid detergent than in the corn diet, and the difference was accentuated by the addition of malt. The amount of heat-stable (1,3–1,4)-β-glucanase in the malt of the transgenic line was 4.28 μg⋅g−1 soluble protein, which resulted in a content of 0.47 μg⋅g−1 soluble protein in the barley diet with the transgenic malt. No heat-stable β-glucanase was detected in the diet containing Golden Promise malt.
Weight Gain, Feed Consumption, Feed Efficiency, and Dry Matter of Excrement.
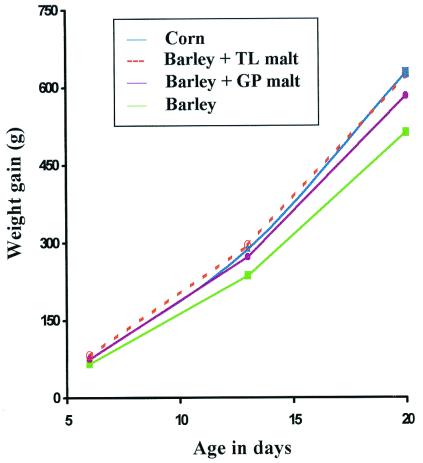
The increase in weight of the chickens over the 20-day period is presented in Fig. 1. Each point represents the average weight of 60 birds. ANOVA revealed that at day 20, the weight of broiler chickens fed barley diet with addition of malt containing heat-stable (1,3–1,4)-β-glucanase was not significantly different from the weight of broilers fed the corn diet. The weight gain of the birds fed the barley diet was significantly slower. Chickens fed the barley diet with addition of malt containing some endogenous barley (1,3–1,4)-β-glucanase had a growth rate intermediate between that of the birds fed the corn diet and those given the barley diet. Actual growth data are provided in Table 4, supplemental data. Chickens fed barley diet or barley diet with Golden Promise malt addition consumed in the period from 0–20 days less feed than those on corn diet and on barley diet with added transgenic malt. Feed efficiency or gain-to-feed ratio over the 20-day period was the same for chickens fed corn, barley with added transgenic malt, or barley with added Golden Promise malt (0.68, 0.68, 0.66). The chickens fed control barley diet had a significantly lower feed efficiency (0.60). In general, feed efficiencies of 0.8 and 0.7 are considered within the normal range. The dry matter of the excrement (Table 4, supplemental data) increased on all diets as the broiler chickens grew, with limited differences among treatments at a given day.
Figure 1.
Addition of 6.2% transgenic malt to barley diet provides a weight gain equivalent to presently used corn diet. TL, transgenic line; GP, Golden Promise.
Number of Chickens with Adhering Sticky Droppings.
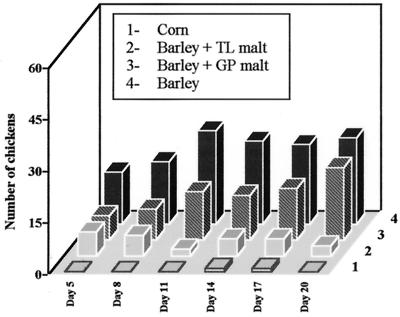
An analysis of the number of chickens with sticky droppings adhering to their down (Fig. 5, supplemental data) among the 60 on a given diet over the trial period is presented in the bar graph of Fig. 2. Only two chickens on corn diet had sticky droppings on days 14 and 17, respectively. On the barley diet, between 15 and 17 chickens per diet had sticky droppings adhering to their cloaca region throughout the trial. Floor bonitations confirmed the extensive sticky droppings by the chickens in the pens feeding on barley diet. A significant reduction of the droppings adhering to the down is observed by supplementing the barley diet with normal barley malt. The transgenic malt-addition barley reduced the occurrence of the sticky droppings to a frequency of 2:7 among the 60 chickens on this diet at a given day. A further increase in the amount of transgenic malt added is likely to eliminate the undesirable droppings completely.
Figure 2.
Number of chickens with adhering sticky droppings.
Contents of (1,3–1,4)-β-Glucans in Diets, Malts, and the Gastrointestinal Tract.
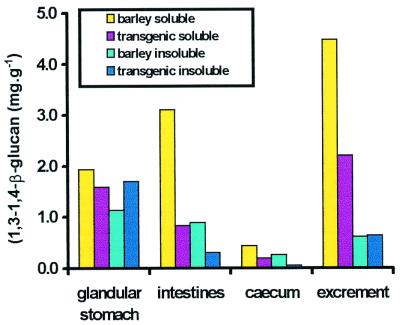
The (1,3–1,4)-β-glucan content of the barley cv. Golden Promise used in the feed trial amounted to ≈30 mg⋅g−1 flour. About 8 mg⋅g−1 grain was extractable with water at 65°C (Table 5, supplemental data). Diets with Golden Promise and transgenic malt contained a somewhat higher amount of soluble β-glucans. The two malts had a low content of water-extractable β-glucans. The effect of the heat-stable (1,3–1,4)-β-glucanase on the content of (1,3–1,4)-β-glucans in different parts of the gastrointestinal tract (Fig. 6, supplemental data) and in the excrements of chickens raised on barley diet with added transgenic malt is compared in Fig. 3 with the corresponding contents in the birds fed the barley diet without this addition. On barley diet, the intestines and the excrements contained 3.1 ± 0.8 and 4.5 ± 0.7 mg⋅g−1 wet-weight water-extractable (1,3–1,4)-β-glucans at day 20. This was reduced to 0.8 ± 0.2 and 2.2 ± 0.4 mg⋅g−1 in the chickens raised on barley diet with the transgenic malt addition. A reduction of the limited amount of soluble β-glucans by the enzyme is also seen in the glandular stomach and the cecum. The amount of insoluble β-glucans in the digesta from the glandular stomach and intestine and in the excrements of the chickens on barley diet is low (1.2, 0.9, and 0.6 mg⋅g−1). An effect of the enzyme is evident only in the intestine. The amount of β-glucans in the cecum of the broilers on barley diet is below 1 mg⋅g−1, but the enzyme addition in the malt decreased both soluble and insoluble β-glucan content.
Figure 3.
Water-soluble (100°C) and insoluble (1,3–1,4)-β-glucans in different parts of the gastrointestinal tract and in the excrements of chickens on barley diet and on diet with added transgenic malt containing heat-stable (1,3–1,4)-β-glucanase.
Analysis of Heat-Stable (1,3–1,4)-α-Glucanase in the Gastrointestinal Tract and Excrement.
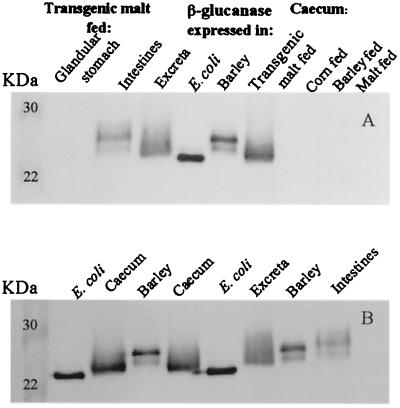
As illustrated (Fig. 7, supplemental data), the amount of enzyme activity in the intestine was 0.40 μg⋅g−1 soluble protein, corresponding to the amount of enzyme present in the diet with transgenic malt (0.47 μg⋅g−1 soluble protein). Enzyme activity in the glandular stomach was 0.11 μg⋅g−1 because of the limited amount of feed that was present in this stomach. The ceca, which are enlarged in broilers on barley diet compared with the size seen in the chickens on corn diet, concentrate the enzyme to an activity of 5.2 μg⋅g−1, and the excrement accumulate high amounts of active heat-stable (1,3–1,4)-β-glucanase. This matches with a strong reduction of the β-glucans in the ceca and excrements (Fig. 3). The heat-stable β-glucanase was characterized by SDS/PAGE followed by Western blotting and decoration with a specific antibody (Fig. 4 A and B). Purified unglycosylated enzyme expressed in Escherichia coli, and purified glycosylated enzyme from transgenic barley were used as standards. Glycosylated recombinant (1,3–1,4)-β-glucanase was present in the extracts from the intestine, excrement, and ceca but was absent in the ceca of birds fed corn, barley, or barley with Golden Promise malt. The limited amount of enzyme present in the glandular stomach was not revealed in the Western blot. The presence of the glycosylated enzyme in the ceca testifies to its origin from the transgenic barley and excludes the possibility that the (1,3–1,4)-β-glucanase is produced by the uric acid decomposing anaerobic bacteria of the ceca.
Figure 4.
Identification of heat-stable (1,3–1,4)-β-glucanase with specific antibodies in different parts of the gastrointestinal tract and excrements by Western blots. The enzyme expressed in E. coli is not glycosylated (Mr = 24,000). The enzyme expressed in barley is glycosylated (Mr = 28,000) and therefore has a lower electrophoretic mobility.
Viscosity of Digesta in the Gastrointestinal Tract.
Measurements confirm that a barley diet leads to higher viscosity in the glandular stomach and intestine than does a corn diet (Table 2). The addition of barley or transgenic malt reduces the viscosity in these two parts of the digestive tract. Corn diet resulted in higher viscosity of cecum contents than barley diet and barley diet with an addition of normal malt. Transgenic malt increased the viscosity of cecum contents toward and above that observed for corn diet.
Table 2.
Viscosity of contents in the digestive tract of chicken fed different diets
| Diets | Viscosity,
centipoise
|
||
|---|---|---|---|
| Glandular stomach | Intestine | Cecum | |
| Corn | 0.9 ± 0.3 | 1.24 ± 0.1 | 4.18 ± 1.5 |
| Barley | 1.22 ± 0.3 | 2.55 ± 0.9 | 3.21 ± 1.1 |
| Barley + GP* malt | 0.96 ± 0.2 | 1.79 ± 0.4 | 3.32 ± 1.6 |
| Barley + TL† malt | 1.01 ± 0.3 | 1.97 ± 0.4 | 7.17 ± 4.0 |
Golden Promise.
Transgenic Line.
Discussion
The chicken-feed trial presented in this communication demonstrates that inclusion of 6.2% transgenic malt containing a protein-engineered thermotolerant (1,3–1,4)-β-glucanase (4.6 μg⋅g−1 soluble protein) provides a weight gain of Hubbard High Yield Broilers indistinguishable from that of presently used corn diets. The gene encoding the enzyme is expressed with an α-amylase gene promoter in the aleurone and synthesized with a signal peptide for secretion into the endosperm of the germinating grain. The enzyme concentration in the diet was 0.47 μg⋅g−1 soluble protein. The excellent growth and survival of the 60 broiler chickens receiving this diet show the transgenic malt not to be toxic. The chickens did not develop the extensive unhygienic sticky droppings characteristic of chickens fed barley diets. Advantages in using the transgenic malt containing the thermostable (1,3–1,4)-β-glucanase for chicken feed are several. The required malt corresponding in amount to feed ingredients such as fish meal, beef tallow, or dicalcium phosphate can be added to normal barley in areas that have to import grain corn and can constitute the major basis of the feed in those areas. The feed with added transgenic malt provides an alternative to the use of corn grain, which is more extensively used and is needed more as food for humans than barley. Corn grain is also 30–50% more expensive. Only 10% of the barley harvest in the United States is used as malt for beer and less than 1% for production of ingredients in human food (Washington Agricultural Statistics 1997–1998). As an example, the State of Washington produces annually 40 million broilers with imported corn grain. Using barley for raising this number of broiler chickens would require 3,400 tons of presently available transgenic malt and 280,000 tons of normal barley, i.e., ≈1/3 of the barley harvest of the state. Barley is needed in Washington agriculture for crop rotation. When the transgenic barley plants that produce larger amounts of enzyme during grain maturation become available (26), mature grain can be used as additive, and a level corresponding to the present vitamin mix will suffice. The thermostability of the enzyme allows it to survive the heat generated by pressing barley into feed pellets and pasteurization of the feed required to prevent infection of the chickens by Salmonella typhimurium. Further trials are necessary to determine the optimal amount of transgenic malt or grain that have to be added to the diet.
The barley feed used in this study contained 8 mg⋅g−1 water-soluble and 22 mg⋅g−1 insoluble (1,3–1,4)-β-glucan (Table 5, supplemental data). The barley diet containing the transgenic malt had a somewhat higher soluble (12 mg⋅g−1) and a lower insoluble (14 mg⋅g−1) (1,3–1,4)-β-glucan content. In the glandular stomach, the intestines and the excrements increasing amounts of water-soluble (1,3–1,4)-β-glucans have been determined for birds fed barley diet without transgenic malt, whereas the insoluble content stayed more or less constant (Fig. 3). For the interpretation of these values, one has to consider the time solid-phase food spends in various parts of the digestive tract (mean retention time). For 1,800-g broilers on corn–canola or corn–soybean diet, this is 39 min for the gizzard and glandular stomach, 191 min for the intestine, 119 min for the ceca, and 56 min for the rectum (36). In the gizzard and glandular stomach, the feed is retained a relative short time. The concentration of the insoluble and soluble (1,3–1,4)-β-glucans in the glandular stomach was reduced to 5 and 25% of that in the barley diet, respectively. A reduction to 13% was also registered in chickens fed the diet with transgenic malt. This reduction is possibly effected by the HCl secreted with 93 mM⋅l−1 in the stomach together with pepsinogen (30, 37). The pH of gastric secretions in the gizzard and glandular stomach is between 2 and 3, although the contents of the stomach usually have a higher pH because of the presence of ingesta (30). During the ≈3-h retention of the feed in the intestine, our data indicate a strong increase in the concentration of soluble β-glucans (Fig. 3). This results from an ongoing conversion of insoluble to soluble (1,3–1,4)-β-glucans and accumulation of the soluble part. The soluble (1,3–1,4)-β-glucan in the intestine of the chickens on barley diet was measured to be 3.1 ± 0.8 mg⋅g−1 (n = 6 pens). This value was reduced to 0.83 ± 0.2 mg⋅g−1 (n = 6 pens) in the chickens on barley diet containing 6.2% transgenic malt (Fig. 3). The depolymerization of the soluble (1,3–1,4)-β-glucan was carried out by the heat-stable (1,3–1,4)-β-glucanase present in the intestine with an activity corresponding to that in the diet. Collection of excrements over a 48-h period yielded a content of 4.6 mg⋅g−1 soluble (1,3–1,4)-β-glucans (Fig. 3). The stickiness of the droppings is because of the accumulation of this large amount of soluble β-glucans. The activity of the heat-stable β-glucanase effects the reduction of the soluble β-glucans in the excreta of chickens on barley diet with transgenic malt. The reduction was from 4.6 mg⋅g−1 (n = 6 pens) to 2.3 mg⋅g−1 (n = 6 pens). Active heat-stable enzyme had accumulated in the excrement to a 7.5-fold higher concentration than in the feed (Fig. 7, supplemental data).
Development of longer ceca is observed in birds on high-fiber diets (38). In agreement therewith, larger ceca were observed in chickens on barley diet with higher fiber content than in birds on corn diet with lower fiber content. The main function of ceca in birds is nutritional. These ceca take part in the digestion of fine particulate matter and food fiber and in the production of volatile fatty acids, mainly acetate, propionate, and butyrate (39, 40). Large amounts of uric acid and intestinal and urethral water are moved by peristaltis and antiperistalsis into the ceca (41–43). Volatile fatty acids, which can reach a concentration of 125 mM, water, and ions are reabsorbed through the cecal wall into the bloodstream. A large number of different anaerobic bacteria, including Clostridium species, are present in the ceca at titers of 108–1010⋅g−1 cecal material (44). They decompose uric acid to ammonium and ferment polysaccharides to acetate, propionate, and butyrate. The production of volatile fatty acids in the ceca can meet 11–18% of the energy needs for the bird's basal metabolism (45). Cecectomy significantly reduces metabilization of feed, volatile fatty acid absorption, and digestibility of polysaccharides (dietary fiber) if the birds are preconditioned to a high-fiber diet (46). The amount of soluble and insoluble (1,3–1,4)-β-glucan in the cecum of chickens on barley diet is low but is significantly lowered by addition of transgenic malt. The 11-fold concentration of the heat-stable (1,3–1,4)-β-glucanase in the cecum (Fig. 7, supplemental data) is remarkable and shows that potentially other recombinant enzymes in chicken feed can be concentrated in the cecum and used to probe enhancement of utilization of fibers other than (1,3–1,4)-β-glucans.
Barley diets containing high amounts of β-glucans produce lower amounts of cholesterol in the low-density lipoprotein of the chicken blood plasma than corn diets (47–49). Supplementation of barley diet with β-glucanase improved average daily chicken weight gain and increased low-density lipid cholesterol concentration as well as digestibility of lipids. In one feeding trial on barley diets with and without β-glucanase supplement, soluble and insoluble β-glucan concentrations were determined in digesta of the small and large intestines and in excrements (49). The six-row waxy hull-less cultivar of the feed contained more than twice as much total β-glucans and soluble β-glucans as the two-row barley used in the present investigation. Insoluble β-glucan concentrations in the small intestine, the large intestine, and excrements of chickens on the barley diet were 101, 56, and 87 mg⋅g−1. The enzyme supplement decreased these values to 84, 44, and 77 mg⋅g−1. On the other hand, soluble β-glucans increased in the two intestine sections and excreta from the chickens on the enzyme-supplemented diet, i.e., from 23 to 64 mg⋅g−1 in the small intestine, from 8 to 31 mg⋅g−1 in the large intestine, and from 30 to 58 mg⋅g−1 in the excrement. We have interpreted our results by two sequential processes: the insoluble β-glucans were first converted into soluble β-glucans, and these were subsequently depolymerized into saccharides. In the experiment of Wang et al. (49), the enzyme addition had effected conversion of insoluble to soluble β-glucans but not yet a significant reduction of the accumulated soluble β-glucans. This may be because of a lower efficiency of the enzyme supplement or the different conditions of sampling. In our trial, the gastrointestinal track was sampled without starvation of the chickens. In the experiment of Wang et al. (49), food was withheld overnight, and the chickens were allowed free access to diets for 1 h followed by 2 h without food before analysis.
A significant conclusion can be derived from the results of the present chicken trial. It is possible to introduce an individual recombinant enzyme into various parts of the gastrointestinal tract of chickens with transgenic malt and, by inference, transgenic mature grain together with the chosen diet. It was thus possible to determine how much recombinant (1,3–1,4)-β-glucanase accumulated in active form in the various parts of the digestive tract and what effect it had on the metabolism of (1,3–1,4)-β-glucans. Analogously, it will be possible to study the effects of transgenic grain expressing the hybrid enzyme that combines the catalytic activity of heat-stable (1,3–1,4)-β-glucanase with a cellulase from Erwinia carotovora. This multienzyme has been shown to depolymerize the consecutive (1,4)-β-linked glucose units that result from the action of the (1,3–1,4)-β-glucanase on mixed linked barley β-glucan (50). The importance of arabinoxylans in chicken diets can be appropriately probed with the recombinantly expressed hybrid enzyme combining the activity of (1,3–1,4)-β-glucanase with that of (1,4)-β-xylanase (51).
Supplementary Material
Acknowledgments
We thank Mr. Frank E. Hagie and Dr. H. Horvath for useful advice and moral support. Financial support by the Washington Technology Center (Grant No. 99 B-1) and by Applied Phytologics, Sacramento, CA, is gratefully acknowledged. This is scientific paper CSS 010906 from the College of Agriculture and Home Economics Research Center, Washington State University, Pullman, WA.
References
- 1.Jensen L, Fry R E, Allred J B, McGinnis J. Poultry Sci. 1957;36:919–921. [Google Scholar]
- 2.Laerdal O A, Bird H R, Sunde M L, Phillips P H. Poultry Sci. 1959;38:1221. [Google Scholar]
- 3.Rose R J, Arscott G H. Poultry Sci. 1962;41:124–130. [Google Scholar]
- 4.Willingham H E, Jensen L S, McGinnis J. Poultry Sci. 1959;38:539–544. [Google Scholar]
- 5.Rickes E L, Ham E A, Moscatelli E A, Ott W H. Arch Biochem Biophys. 1962;96:371–375. doi: 10.1016/0003-9861(62)90422-8. [DOI] [PubMed] [Google Scholar]
- 6.Moscatelli E A, Ham E A, Rickes E L. J Biol Chem. 1961;236:2858–2862. [PubMed] [Google Scholar]
- 7.Hesselman K, Elwinger K, Nilsson M, Thomke S. Poultry Sci. 1981;60:2664–2671. [Google Scholar]
- 8.Hesselman K, Elwinger K, Thomke S. Anim Feed Sci Technol. 1982;7:351–358. [Google Scholar]
- 9.White W B, Bird H R, Sunde M L, Prentice N, Burger W C, Marlett J A. Poultry Sci. 1981;60:1043–1048. doi: 10.3382/ps.0601043. [DOI] [PubMed] [Google Scholar]
- 10.White W B, Bird H R, Sunde M L, Marlett J A, Prentice N A, Burger W C. Poultry Sci. 1983;62:853–862. doi: 10.3382/ps.0601043. [DOI] [PubMed] [Google Scholar]
- 11.Bamforth C W, Martin H L, Wainwright T. J Inst Brew. 1979;85:334–338. [Google Scholar]
- 12.Forrest I S, Wainwright T. Eur Brew Conv Proc Congr. 1977;1977:401–413. [Google Scholar]
- 13.Prentice N, Babler S, Faber S. Cereal Chem. 1980;57:198–202. [Google Scholar]
- 14.Stone B A, Clark E A. Chemistry and Biology of (1–3)-β-Glucans. Bundoora, Australia: La Trobe Univ. Press; 1992. pp. 1–803. [Google Scholar]
- 15.Penttilä M, Lehtovaara P, Nevalainen H, Bhikhabhai R, Knowles J. Gene. 1986;45:253–263. doi: 10.1016/0378-1119(86)90023-5. [DOI] [PubMed] [Google Scholar]
- 16.Aspegren K, Mannonen L, Ritala A, Puupponen-Pimiä R, Kurtén U, Salmenkallio-Marttila M, Kauppinen V, Teeri T H. Mol Breeding. 1995;1:91–99. [Google Scholar]
- 17.Woodward J R, Fincher G B, Stone B A. Carbohydr Polym. 1983;3:207–225. [Google Scholar]
- 18.Woodward J R, Fincher G B. Brew. Dig. 1983. , 28–32. [Google Scholar]
- 19.Woodward J R, Phillips D R, Fincher G B. Carbohydr Polym. 1983;3:143–156. [Google Scholar]
- 20.Aastrup S. Carlsberg Res Commun. 1979;44:381–394. [Google Scholar]
- 21.Jørgensen K G. Carlsberg Res Commun. 1988;53:277–285. [Google Scholar]
- 22.Jørgensen K G, Aastrup S. Carlsberg Res Commun. 1988;53:287–296. [Google Scholar]
- 23.Jørgensen K G, Aastrup S. Mod. Methods Plant Anal. New Series. Vol. 7. 1988. pp. 88–108. [Google Scholar]
- 24.Burnett G S. Br Poultry Sci. 1966;7:55–75. [Google Scholar]
- 25.Hesselman K, Åman P. In: New Approaches to Research on Cereal Carbohydrates. Hill R D, Munck L, editors. Amsterdam: Elsevier; 1984. pp. 363–372. [Google Scholar]
- 26.Horvath H, Huang J, Wong O, Kohl E, Okita T, Kannangara C G, von Wettstein D. Proc Natl Acad Sci USA. 2000;97:1914–1919. doi: 10.1073/pnas.030527497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen L G, Olsen O, Kops O, Wolf N, Thomsen K K, von Wettstein D. Proc Natl Acad Sci USA. 1996;93:3487–3491. doi: 10.1073/pnas.93.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen L G, Politz O, Olsen O, Thomsen K K, von Wettstein D. Hereditas (Lund, Swed) 1998;129:215–225. [Google Scholar]
- 29.Politz O, Simon O, Olsen O, Borriss R. Eur J Biochem. 1993;216:829–834. doi: 10.1111/j.1432-1033.1993.tb18204.x. [DOI] [PubMed] [Google Scholar]
- 30.Denbow M. In: Sturkie's Avian Physiology. 5th Ed. Whittow G C, editor. New York: Academic; 2000. pp. 299–325. [Google Scholar]
- 31.McCleary V B, Mugford D C. J AOAC Int. 1997;80:580–583. [Google Scholar]
- 32.McCleary V B, Glennie-Holmes M. J Inst Brew. 1985;91:285–295. [Google Scholar]
- 33.Canadian Council on Animal Care. Guide to Care and Use of Experimental Animals. Vol. 1. Ottawa, ON: Canadian Council on Animal Care; 1980. [Google Scholar]
- 34.Association of Official Analytical Chemists. Official Methods of Analysis. 15th Ed. Gaithersburg, MD: Association of Official Analytical Chemists; 1990. [Google Scholar]
- 35.SAS Institute. SAS/STAT: Guide for Personal Computers. Cary, NC: SAS Institute; 1986. [Google Scholar]
- 36.Shires A, Thompson J R, Turner B V, Kennedy P M, Goh Y K. Poultry Sci. 1987;66:289–298. doi: 10.3382/ps.0660289. [DOI] [PubMed] [Google Scholar]
- 37.Long J F. Am J Physiol. 1967;212:1303–1307. doi: 10.1152/ajplegacy.1967.212.6.1303. [DOI] [PubMed] [Google Scholar]
- 38.McLelland J. J Exp Zool Suppl. 1989;3:2–9. doi: 10.1002/jez.1402520503. [DOI] [PubMed] [Google Scholar]
- 39.Braun E J, Duke G E. J Exp Zool Suppl. 1989;3:1–130. [Google Scholar]
- 40.Goldstein D L, Skadhauge E. In: Sturkie's Avian Physiology. 5th Ed. Whittow G C, editor. New York: Academic; 2000. pp. 265–297. [Google Scholar]
- 41.Skadhauge E. Osmoregulation in Birds. New York: Springer; 1981. [Google Scholar]
- 42.Skadhauge E. Adv Comp Environ Physiol. 1993;16:67–93. [Google Scholar]
- 43.Braun E. In: New Insights in Vertebrate Kidney Function. Brown J A, Balment R J, Rankin J C, editors. Cambridge: Cambridge Univ. Press; 1993. pp. 167–188. [Google Scholar]
- 44.Barnes E M, Impey C S. J Appl Bacteriol. 1974;37:393–409. doi: 10.1111/j.1365-2672.1974.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 45.Annison E F, Hill K J, Kenworthy R. Br J Nutr. 1968;22:207–216. doi: 10.1079/bjn19680026. [DOI] [PubMed] [Google Scholar]
- 46.Chaplin S B. J Exp Zool Suppl. 1989;3:81–86. doi: 10.1002/jez.1402520514. [DOI] [PubMed] [Google Scholar]
- 47.Fadel J G, Newman R K, Newman C W, Barnes A E. Nutr Rep Int. 1987;35:1049–1058. [Google Scholar]
- 48.Newman R K, Newman C W, Hofer P J, Barnes A E. Plant Foods Hum Nutr. 1991;41:371–380. doi: 10.1007/BF02310630. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Newman R K, Newman C W, Hofer P J. J Nutr. 1992;122:2292–2297. doi: 10.1093/jn/122.11.2292. [DOI] [PubMed] [Google Scholar]
- 50.Olsen O, Thompson K K, Weber J, Duus J Ø, Svndsen I, Wegener C, von Wettstein D. Biotechnology. 1996;14:71–76. doi: 10.1038/nbt0196-71. [DOI] [PubMed] [Google Scholar]
- 51.Aÿ J, Götz F, Borriss R, Heinemann U. Proc Natl Acad Sci USA. 1998;95:6613–6618. doi: 10.1073/pnas.95.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.