Abstract
Isoprene (2-methyl-1,3-butadiene) emission varies diurnally in different species. In poplar (Populus spp.), it has recently been shown that the gene encoding the synthesizing enzyme for isoprene, isoprene synthase (ISPS), displays diurnal variation in expression. Working on shoot cultures of Grey poplar (Populus × canescens) placed under a different light regime in phytochambers, we showed that these variations in PcISPS gene expression, measured by quantitative real-time polymerase chain reaction, are not only due to day-night changes, but also are linked to an internal circadian clock. Measurement of additional selected isoprenoid genes revealed that phytoene synthase (carotenoid pathway) displays similar fluctuations, whereas 1-deoxy-d-xylulose 5-phosphate reductoisomerase, possibly the first committed enzyme of the 1-deoxy-d-xylulose 5-phosphate pathway, only shows light regulation. On the protein level, it appeared that PcISPS activity and protein content became reduced under constant darkness, whereas under constant light, activity and protein content of this enzyme were kept high. In contrast, isoprene emission rates under continuous irradiation displayed circadian changes as is the case for gene expression of PcISPS. Furthermore, binding assays with Arabidopsis (Arabidopsis thaliana) late elongated hypocotyl, a transcription factor of Arabidopsis involved in circadian regulation, clearly revealed the presence of circadian-determining regulatory elements in the promoter region of PcISPS.
Rhythms in tune with the day-night cycle of earth are observed in each organism, in metabolism, physiology, or even behavior. In plants, such rhythms have been detected at the physiological level for numerous processes, such as closing of flowers or leaves, chloroplast movements, photosynthetic capacity, stomatal conductance, cell division, and many others (for review, see Johnson, 2001). These events are not simple responses to the external environment and continue under constant conditions. Such phenomena with approximately 24-h periodicity are controlled by an endogenous oscillator, the circadian clock. It allows organisms to anticipate daily changes in the environment, providing them with an adaptive advantage.
Circadian clocks have often been divided into three major components: input (resetting the clock), the oscillator itself, and output (physiological phenomena; Somers, 1999; Devlin, 2002). Underlying the physiological rhythms, genes encoding diverse enzymes or regulatory and structural proteins show circadian rhythm in their expression (Johnson et al., 1984; Piechulla, 1993). However, a few genes at a time were characterized until two groups, using microarrays, each representing around 8,000 Arabidopsis (Arabidopsis thaliana) genes, gave a global idea of clock-controlled genes (Harmer et al., 2000; Schaffer et al., 2001), thus confirming that many genes involved in photosynthetic processes and in carbon allocation are circadian regulated.
However, if these primary metabolic processes are relatively well characterized for rhythms, secondary metabolites still must be analyzed. Concerning terpenoids, reports on circadian biosynthetic rhythms are sparse and have been limited mainly to in planta chemical analysis and emission profiles (Helsper et al., 1998; Dudareva et al., 2003, 2005). However, some studies about circadian rhythms also exist on a molecular level: Lu et al. (2002) showed cyclic expression of a β-pinene synthase gene in leaves of Artemisia annua under continuous conditions; Simkin et al. (2004) a carotenoid cleavage dioxygenase implicated in β-ionone synthesis in petunia (Petunia hybrida) leaves and flowers; and Dudareva et al. (2003) β-ocimene and myrcene synthases in snapdragon (Antirrhinum majus) petals. However, no information exists as to whether biosynthesis of isoprene (2-methyl-1,3-butadiene), the simplest isoprenoid (terpenoid) compound, is regulated by circadian rhythm. Isoprene is a highly volatile organic compound (VOC) naturally emitted from many tree species (Kesselmeier and Staudt, 1999) with significant influence on atmospheric chemistry (Thompson, 1992; Biesenthal et al., 1997; Derwent et al., 1998). For the plant itself, isoprene emission is thought to prevent leaf metabolic processes from thermal (Sharkey and Loreto, 1993; Singsaas et al., 1997; Loreto et al., 2001) and oxidative stress (Loreto and Velikova, 2001; Affek and Yakir, 2002) or serve as an overflow mechanism for excess carbon intermediates (Rosenstiel et al., 2004) or photosynthetic energy (Sharkey and Yeh, 2001).
Variations of isoprene emissions during the day can be explained by synthesis of isoprene synthase (ISPS) substrates, mainly originating from recently fixed CO2 (Schnitzler et al., 2004), and by the temperature dependence of ISPS activity and other enzymes of the plastidic 1-deoxy-d-xylulose 5-P (DOXP) pathway (Brüggemann and Schnitzler, 2002; Wolfertz et al., 2003), thus reflecting the temperature response of isoprene emission (Monson et al., 1992).
Sparse information is present on the regulation of genes related to isoprene biosynthesis. There are indications that light and temperature stimulate gene expression of DOXP synthase (DXS), the starting enzyme of the DOXP pathway (Sprenger et al., 1997; Eisenreich et al., 2001), DOXP reductoisomerase (DXR), the first committed enzyme of this pathway (Takahashi et al., 1998), proposed to be one of its key regulators (Carretero-Paulet et al., 2002), and ISPS (Carretero-Paulet et al., 2002; Hsieh and Goodman, 2005; Sasaki et al., 2005). A recent study in poplar (Populus spp.) shows partial correlation of ISPS gene expression and enzyme activity during the season (Mayrhofer et al., 2005). Diurnal variations of PcISPS and PcDXR (Mayrhofer et al., 2005) gene expression were also reported, expression peaking in the morning for PcISPS and in the afternoon for PcDXR. However, no information exists on the factors responsible for these diurnal changes and whether circadian components are involved in that regulation.
In this work, we tried to answer the questions of (1) whether diurnal variations of isoprene emission and PcISPS expression are due to circadian regulation or simply related to light cycle-fluctuating day-night conditions and (2) whether other isoprenoid biosynthesis-related genes follow a similar expression pattern. Hence, we measured isoprene emission, ISPS activity, and protein content, as well as gene transcript levels of PcISPS, PcDXR, and phytoene synthase (PSY) in Grey poplar (Populus × canescens) shoot cultures under different light regimes. These activities were accompanied by isolation and analysis of the PcISPS promoter where two regions, which are targets for circadian regulatory proteins, were identified.
RESULTS
Expression of Isoprenoid Biosynthesis-Related Genes under Continuous Light and Darkness
Transcript levels of the three genes were determined at different time points during the day over 3 d for shoot culture plants exposed to long-day (LD) conditions (Figs. 1A, 2A, and 3A for ISPS, DXR, and PSY, respectively) in the climate chamber. It is striking that all gene expressions fluctuate, being at low levels during the night and at higher levels during the day. Diurnal fluctuations were also detected under short-day conditions (8-light/16-h dark; data not shown). Each gene presents a distinct pattern.
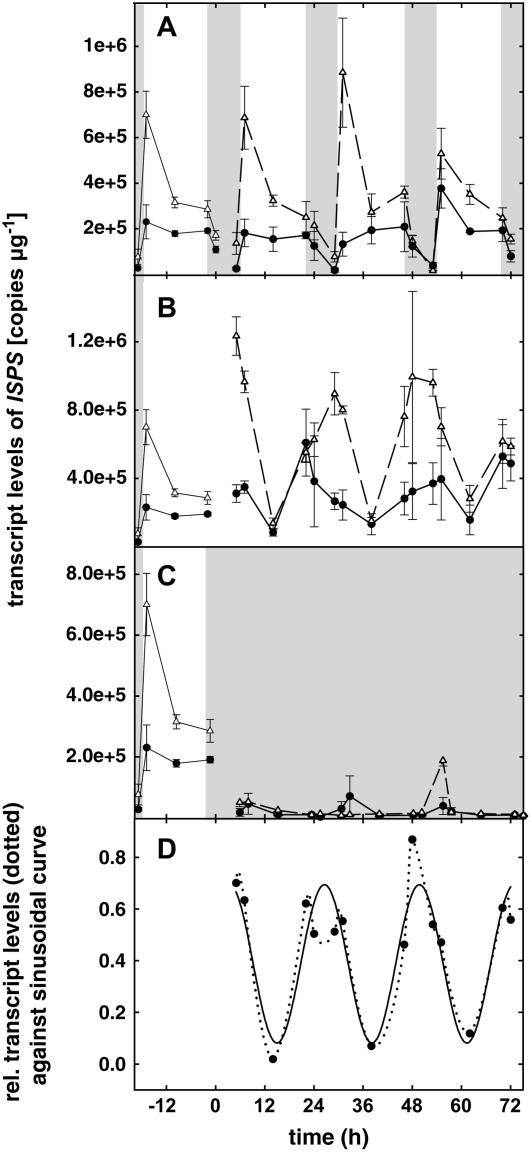
Figure 1.
PcISPS gene expression under LD (16-h light/8-h dark) conditions (A), under LL conditions (B), and under DD conditions (C). In each graph, the two curves represent two independent experiments (n = 3 ± se) and the curves shown in the first 24 h of each graph represent the mean of 3-d expression levels under LD conditions. D, Sinusoidal (y = y0 + a sin [2πx/b + c]; with a = 0.3058; b = 23.8008; c = 1.3433; y0 = 0.3920) regression on relative data under LL conditions (mean of both experiments; adjusted R2 = 0.9690, P < 0.05). Darkness is represented as shaded areas. Transcript levels are related to RNA quantity.
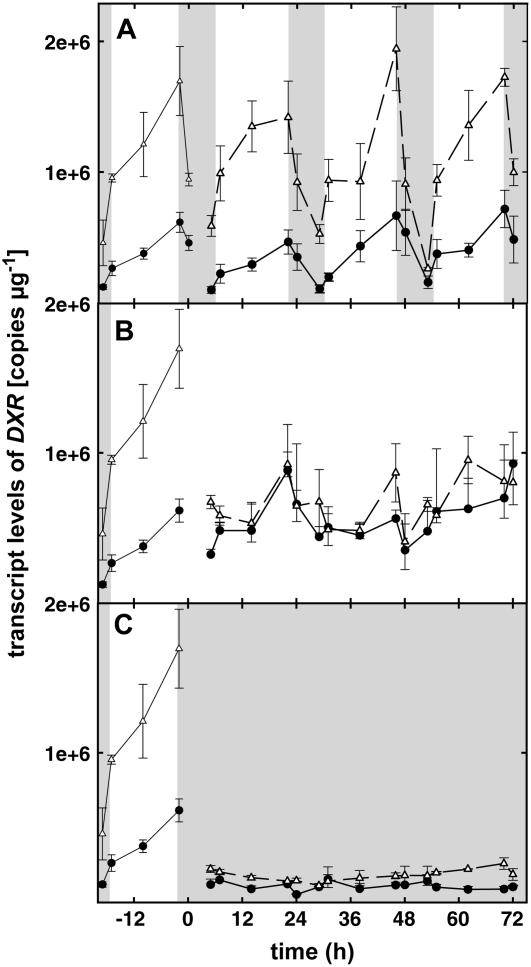
Figure 2.
PcDXR gene expression under LD (16-h light/8-h dark) conditions (A), under LL conditions (B), and under DD conditions (C). In each graph, the two curves represent two independent experiments (n = 3 ± se) and the curves shown in the first 24 h of each graph represent the mean of 3-d expression levels under LD conditions. Darkness is represented as shaded areas. Transcript levels are related to RNA quantity.
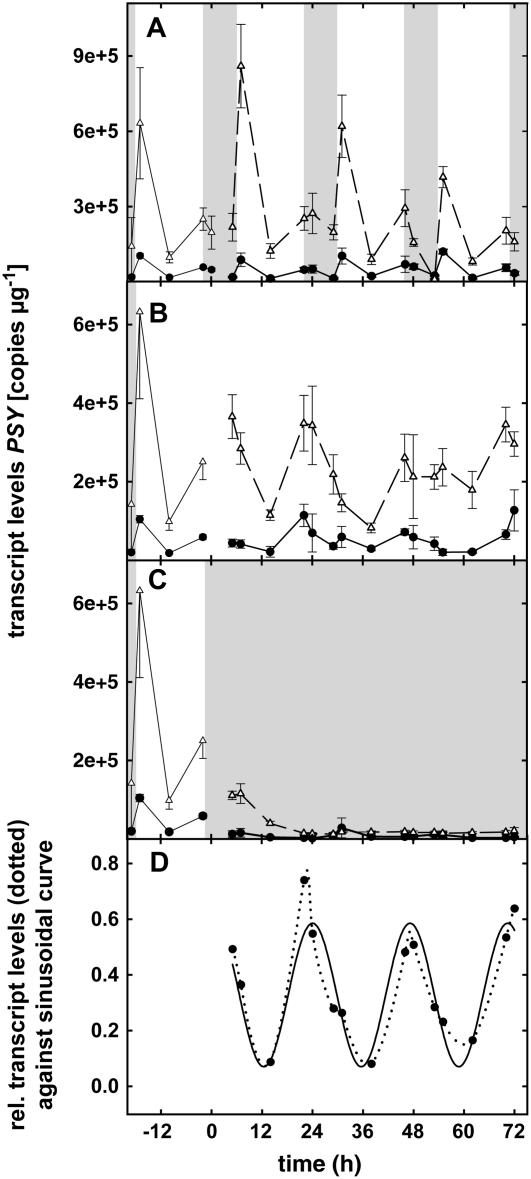
Figure 3.
PcPSY gene expression under LD (16-h light/8-h dark) conditions (A), under LL conditions (B), and under DD conditions (C). In each graph, the two curves represent two independent experiments (n = 3 ± se) and the curves shown in the first 24 h of each graph represent the mean of 3-d expression levels under LD conditions. D, Sinusoidal regression (y = y0 + a sin [2πx/b + c]; with a = 0.2574; b = 23.1706; c = 1.3461; y0 = 0.3279) on relative data under LL conditions (mean of both experiments; adjusted R2 = 0.9695, P < 0.001). Darkness is represented as shaded areas. Transcript levels are related to RNA quantity.
PcISPS transcript levels appeared to be high in the morning (Fig. 1A). PcDXR expression (Fig. 2A) seems to peak later in the afternoon. The expression levels of PcPSY (Fig. 3A) tend to peak in the morning, go down at midday, and exhibit a second, less intense, peak in the evening. These 3-d experiments also showed the repeatability of these diurnal variations, underlined by the stable conditions within our climate chambers.
Because all three studied genes showed diurnal variations in expression level, the hypothesis of a circadian element involved in their transcriptional regulation was tested by placing the poplar shoot cultures in either continuous light (LL) or continuous darkness (DD) and sampling over 3 d.
A striking feature was the dramatic decrease of the level of expression when the plants were placed in DD (Figs. 1C, 2C, and 3C). No significant fluctuation was observed under these conditions.
Under LL, two patterns of expression were revealed, namely, no significant variations in the level of transcription for PcDXR (Fig. 2B), but rhythmic fluctuations for both PcISPS (Fig. 1B) and PcPSY (Fig. 3B).
For PcISPS (Fig. 1B), mean transcript levels were clearly higher (about twice) under LL than under LD light periods (Fig. 1A) for both experiments, confirming light as an enhancer of this gene expression. For PcPSY (Fig. 2, A and B), however, the expression level was equivalent under LD and LL conditions. Therefore, if light is essential to trigger the expression, it may not be the only regulating factor for PcPSY. The most obvious feature of PcISPS and PcPSY expression levels is their rhythmic fluctuation. To identify possible circadian rhythms, the values of expression were made relative (minimal value brought to 0, maximal value brought to 1), the mean of the six values of the two experiments was calculated, and these fluctuations were tested against a sinusoidal curve. The results obtained are represented in Figures 1D and 3D for PcISPS and PcPSY, respectively. Separately for each experiment, the correlation between the observed fluctuations and the calculated curve is also high for both PcISPS (adjusted R2 = 0.9416, P < 0.005 for experiment 1 and adjusted R2 = 0.9652, P < 0.05 for experiment 2; data not shown) and PcPSY (adjusted R2 = 0.9076, P < 0.001 for experiment 1 and adjusted R2 = 0.977, P < 0.05 for experiment 2; data not shown). This indicates that a defined period exists within the observed fluctuations. Interestingly, this period is about 24 h and therefore defines circadian fluctuations. It could be observed that these fluctuations are shifted in phase when compared to LD conditions. Under LL, the maximal values of PcPSY are reached at times corresponding to subjective evening and maximal values of PcISPS are reached at times corresponding to subjective night.
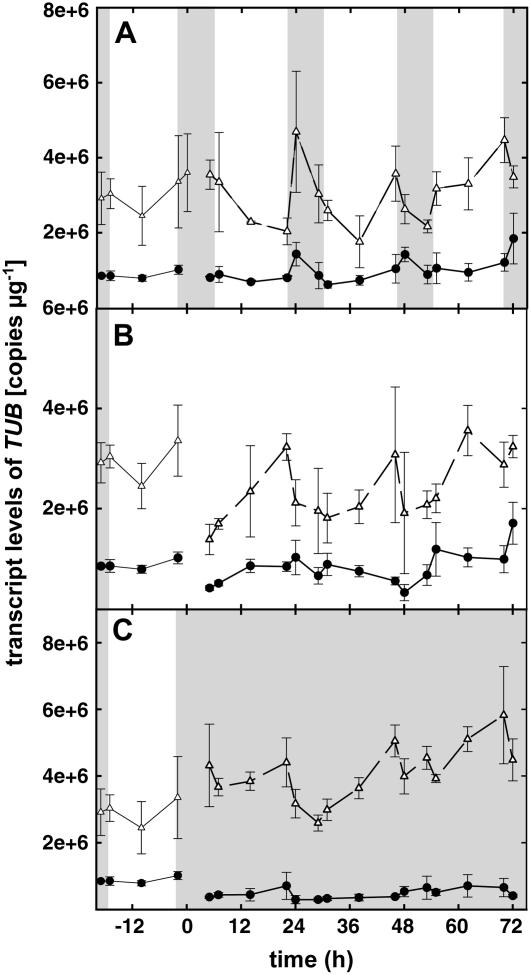
Transcript levels of β-tubulin (TUB) were also measured as a control (housekeeping gene), as shown in Figure 4. Fluctuations in gene expression of this gene were not as regular as for the three isoprenoid genes under LD conditions, the peaks up and down being observed at different times for different days, even if always in the evening. Under DD, the PcTUB expression level stays constant as well as expression under LL in the first experiment. In the second experiment, slight fluctuation of expression can be observed under LL. However, showing a different pattern than the genes of interest, PcTUB testifies for the specificity of the observed patterns of the isoprenoid biosynthesis-related genes.
Figure 4.
PcTUB gene expression under LD (16-h light/8-h dark) conditions (A), under LL (B) conditions, and under DD conditions (C). In each graph, the two curves represent two independent experiments (n = 3 ± se) and the curves shown in the first 24 h of each graph represent the mean of 3-d expression levels under LD conditions. Darkness is represented as shaded areas. Transcript levels are related to RNA quantity.
ISPS Protein Concentration and Activity and Isoprene Emission under Different Light Regimes
Because we observed circadian regulation of the level of transcript for PcISPS, we wanted to check whether these variations were also present at the level of PcISPS protein concentration, activity, and, finally, emission of isoprene, knowing that emission shows daily variations under day-night cycles (Mayrhofer et al., 2005).
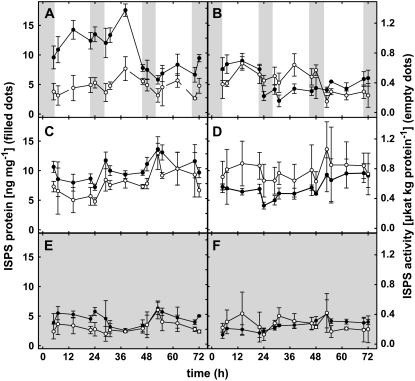
The results of protein concentration and enzyme activity measurements are presented in Figure 5 for the three light regimes tested (LD [A and B]; LL [C and D]; and DD [E and F]) for both experiments (A, C, and E and B, D, and F), respectively. It became evident that ISPS protein concentration as well as activity was lower under DD than LL, reaching a maximum of 6 ng mg−1 protein for the concentration and 0.45 μkat kg−1 protein for the activity in the dark, and being over 4 or 7 ng mg−1 protein and 0.6 or 0.4 μkat kg−1 protein in the light (values for both experiments, respectively). This difference between values under dark and light conditions was not as obvious under LD conditions. For one experiment (Fig. 5A), it seemed that during the late night both protein concentration and enzyme activity go down slightly. However, these variations are not significant and could not be observed in the second experiment (Fig. 5B). Globally, we observed a tendency for both activity and protein concentration to peak around midday, but no clear pattern could be statistically extracted. In addition, under LL conditions, enzyme activity and protein levels of ISPS seem to increase over time (Fig. 5, C and D).
Figure 5.
PcISPS protein quantity and enzyme activity under LD (16-h light/8-h dark) conditions in the first (A) and second (B) experiment, under LL conditions in the first (C) and second (D) experiment, and under DD conditions in the first (E) and second (F) experiment, respectively. PcISPS protein quantity (•) and enzyme activity (○; n = 3 ± sd). Darkness is represented as shaded areas.
Another interesting feature is the correlation between protein level and PcISPS activity when both experiments are analyzed separately, the activity-to-protein ratio indeed being lower in the second experiment (Fig. 5, B, D, and F) than in the first one (Fig. 5, A, C, and E). However, some exceptions to this correlation occur, particularly under LD conditions of the first experiment. Indeed, on the second day, protein levels stayed low (after a decrease during the night), whereas the level of activity appears as high as during the first day. At the third day, protein and activity were both at lower levels. These discrepancies between protein level and enzyme activity resulted in different turnover (Kcat) values ranging from 3.7 to 5.1 mmol isoprene mol−1 ISPS under both darkness and LD conditions and under LL, respectively.
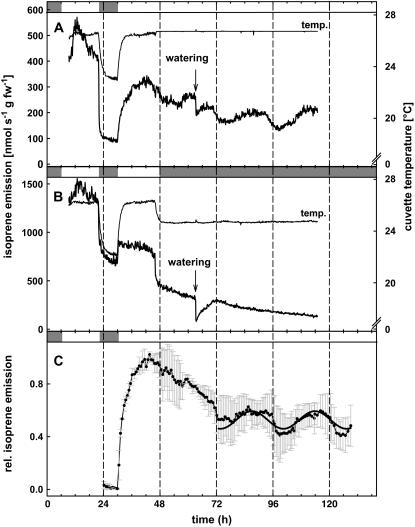
Emission of isoprene from the shoot cultures followed a clear diurnal pattern under LD conditions, as shown in Figure 6, A and B (first 48 h) for representative samples, being low overnight and high and stable over the course of the day. When shoots were placed in DD (Fig. 6B, after the first 48 h), isoprene emission dropped down very quickly after switching off the light and, subsequently, declined with a slower rate over the following 3 d. This is not surprising because gene expression, protein concentration, and enzyme activity are all switched down under such conditions. Remarkably, isoprene emission of shoot cultures does occur, even if at a low rate, under darkness. Under LL (Fig. 6A, after the first 48 h), we detected fluctuating isoprene emission rates with a 24-h period between two peaks, therefore defining circadian rhythm. Figure 6C shows the mean of relative values for three independent shoot cultures. Under LL conditions, isoprene emission displayed clear, daily changes on the third and fourth day after onset of LL. On these days, emission was at its strongest (at about 86 h), approximately 20% higher than emission at its lowest (at about 78 h) during the previous 24-h cycle and approximately 30% higher (at about 114 h) than the lowest (at about 100 h) emission in the last 24-h cycle. Relative values (minimal value brought to 0, maximal to 1) of fluctuation were tested against a sinusoidal curve, but, because of the decline in isoprene emission during the first 2 d, it was only possible to fit a curve on the data of the last 2 d. The fluctuation of isoprene emission during these days was highly significant (adjusted R2 = 0.9951, P < 0.0001; Fig. 6C), clearly testifying for circadian rhythm of isoprene emission. The highest rate of emission always occurred in the subjective afternoon, showing a switched circadian rhythm phase of isoprene emission under LL.
Figure 6.
Isoprene emission from cell-cultured poplars under LL conditions (A) and DD conditions (B). C, Related isoprene emission data under LL conditions (15 min means; n = 3 ± sd) with sinusoidal regression (y = y0 + a sin [2πx/b + c]; with a = 0.0670; b = 26.7854; c = 6.2800; y0 = 0.5269) on related data under LL conditions (mean of both experiments; adjusted R2 = 0.9951, P < 0.001) for the last 2 d. After two day-night cycles, plants were placed either under LL (A) or DD (B; 3-min means). During the third day, water was added to the cuvettes to maintain humidity. The following decrease in emission is indicated. A and B, One experiment out of three replicates. Dark and light periods are presented with gray and white boxes above the figures.
It is obvious that addition of water onto the agarose surface, even without touching the leaves and disturbing the gas flow, reduced the isoprene emission rate. Gas-exchange analysis of culture glasses after removal of the green tissue parts (stem and leaves) revealed that isoprene emission (background) was approximately 10% of the initial values.
Circadian Regulation of the PcISPS Gene Promoter
To get more insight into the circadian regulation of PcISPS, we isolated the promoter region of this gene by gene walking and primers designed for putative sequences available in the poplar genome databank and other databases (Fig. 7). The isolated sequence (accession no. AJ294819) of 1,612 bases (1,434 in front of the ATG codon) was aligned with the putative sequence from Populus trichocarpa (obtained from the draft genome) and with the published sequence from Populus tremuloides (accession no. AY341431). This alignment explains the failure of some forward primers designed according to the P. trichocarpa sequence and extending on variable regions. In general, different sequences show a high degree of similarity (on the common parts), ranging from 90% between Grey poplar and P. trichocarpa to 94% between Grey poplar and P. tremuloides, respectively. However, some small regions are diverging because of gaps and/or insertions.
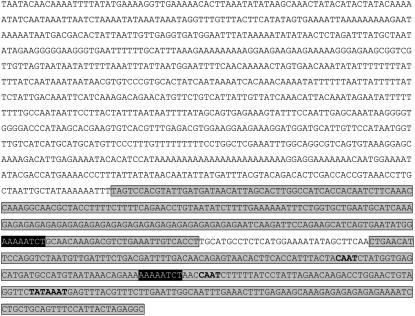
Figure 7.
PcISPS promoter sequence. The two circadian elements are shaded black. Core promoter sequences (TATA and CAAT boxes) are bold. Fragments 1 (top) and 2 (bottom) amplified for EMSA studies (see Fig. 8) are shaded light gray.
Regulatory elements were searched within the Grey poplar promoter sequence. Putative TATA and CAAT boxes are indications of the functionality of this sequence, TATA boxes being one type of core-promoter element essential for transcription initiation (Roeder, 1996). Using the databases plantCARE (Rombauts et al., 1999) and PLACE (Prestridge, 1991; Higo et al., 1999), many putative regulatory sequences were identified, particularly light, heat stress, and circadian-related ones. Moreover, two circadian elements (AAAAATCT) nearer to the start codon were found that are known to be recognized by late elongated hypocotyls (LHYs) and circadian clock-associated 1 (CCA1), two regulation factors enhancing morning expression of genes with such promoter elements (for review, see Devlin, 2002). In particular, this finding corresponds to the observation of the early expression of PcISPS in Figure 1A.
To prove the functionality of the cloned putative promoter, it was fused to the reporter genes coding for the enzyme β-glucuronidase and enhanced green fluorescent protein. This construct was then introduced into Arabidopsis and reporter genes were expressed in transgenic plants (data not shown), testifying that the PcISPS promoter is active in Arabidopsis plants.
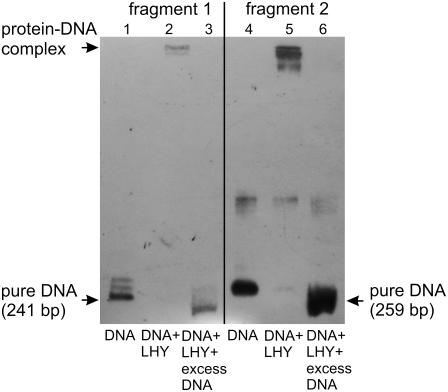
For analyzing whether LHY may have a role in transcription of the PcISPS gene, fragment 1 and fragment 2 (Fig. 7) were amplified by PCR, each fragment carrying one putative binding box for this circadian regulatory protein. Heterologously expressed AtLHY protein was used to perform an electrophoretic mobility shift assay (EMSA) to test whether this factor binds to the identified circadian elements on the PcISPS promoter. As presented in Figure 8, lines 2 and 5, AtLHY is indeed binding to the PcISPS promoter fragments. The 200-fold molar excess of unlabeled DNA used in the binding reactions loaded in lines 3 and 6 proved that the observed shift (due to the binding of AtLHY proteins onto PcISPS promoter fragments) is specific.
Figure 8.
EMSA. Identification of two regions in the PcISPS gene promoter that contain target sequences for the LHY protein. Line 1, Biotin-labeled fragment 1; line 2, biotin-labeled fragment 1 and LHY protein; line 3, biotin-labeled fragment 1, LHY protein, and 200-fold molar excess of unlabeled fragment 1; line 4, biotin-labeled fragment 2; line 5, biotin-labeled fragment 2 and LHY protein; line 6, biotin-labeled fragment 2, LHY protein, and 200-fold molar excess of unlabeled fragment 2.
DISCUSSION
Gene expression of PcISPS, PcDXR, and PcPSY in Grey poplar leaves follows diurnal variations under day-night conditions, being indeed enhanced by light and reduced during night and under DD. This indicates a primary role of light as a trigger of the expression of these isoprenoid biosynthesis-related genes. Formation of the carbon-rich compounds, such as isoprenoids, requires carbon pools. Because carbon fixation occurs during daylight, the observed diurnal variations in isoprenoid biosynthesis-related genes appear logical. It had even been shown that 75% of the carbon used to produce isoprene in poplar leaves come directly from photosynthesis (Schnitzler et al., 2004).
The role of isoprene is still controversial, but, being either a valve to evacuate carbon or energy overflow (Rosenstiel et al., 2004; Magel et al., 2006) or protection against high temperature or oxidative stress (Sharkey and Singsaas, 1995; Loreto and Velikova, 2001; Affek and Yakir, 2002), it is produced during the day when these functions can be exerted. To anticipate the necessity for isoprene, the PcISPS gene expression level seems to start increasing before dawn and peaks early after it. If light is necessary for the expression of the three studied genes, it is not the only regulating factor for PcPSY and PcISPS whose approximately 24-h period-length variations go on, even if shifted in time, under LL. This feature testifies to a circadian clock element of regulation because, in a recent review, McClung (2006) has characterized circadian rhythms as endogenously generated features persisting under constant environmental conditions, typically under LL and/or DD. In our data, mRNA undergoes rapid damping after plants were transferred to darkness. A similar feature was shown on mustard (Sinapsis alba) germin-like protein (SaGLP; Heintzen et al., 1994), in which mRNA could no longer be detected after 1 d in DD, even if its expression is endogenously clock controlled. Like SaGLP, PcISPS and PcPSY are under circadian control, but light positively influences their expression.
The presence of circadian regulatory elements and putative light elements in the promoter sequence of PcISPS could explain the observed regulation of this gene by the circadian clock and by light. The presence of two morning elements known to be recognized in Arabidopsis by the self clock-regulated factors LHY and CCA1 could be responsible for the early peak of expression of PcISPS. Recently, Wilkinson et al. (2006) showed that isoprene emission in oil palm (Elaeis guineensis) is under strong circadian control, but it was not determined at which levels circadian regulation influences isoprene emission, even if the authors seem to link it with the daily fluctuations of LHY and CCA1 transcript levels. LHY/CCA1 binding sites were observed previously to be conserved in light-harvesting complex gene promoters (Wang and Tobin, 1998) and also in several circadian-regulated gene promoters (McClung, 2000; Nozue and Maloof, 2006). Our experimental data, showing binding of AtLHY proteins to the two PcISPS promoter fragments containing a morning element, demonstrate that, in poplar, expression of PcISPS is probably controlled by the circadian clock at the transcriptional level.
TUB is a common housekeeping gene used as an internal control for data normalization in real-time PCR measurements. However, a recent analysis of common housekeeping genes in poplar, over the development, showed that TUB may not be the most appropriate housekeeping gene in this species under the tested conditions (Brunner et al., 2004). Concerning potential daily fluctuations of TUB transcript abundance, they had already been observed in developing tomato (Lycopsersicon esculentum) fruit (Piechulla and Gruissem, 1987), but were not characterized in poplar. In this study, TUB transcript levels in poplar displayed low, but obvious, diurnal fluctuations with the tendency to increase during the night. Although the span of transcript-level variation of PcTUB was approximately 25% of the span of isoprene biosynthesis-related genes and therefore does not much influence the last ones when used for normalization, TUB as a housekeeping gene may not be the most appropriate, in particular when different time points during the day or different light regimes are to be studied.
Absence of a correlation between PcISPS gene expression level and PcISPS protein/activity detected at the daily level was also observed at the seasonal level (Mayrhofer et al., 2005). The discrepancy between protein quantity and activity indicates that PcISPS concentration does not only determine overall PcISPS activity. As already proposed in Mayrhofer et al. (2005) at the level of seasonal variation, posttranslational regulation of the PcISPS protein may exist that would present at least two forms with different activities. However, the factors involved in this third level of regulation are still to be discovered in future experiments.
In field conditions, isoprene emission from poplars was known to present daily variations linked to temperature and light intensity (Mayrhofer et al., 2005). Our results on poplar shoot cultures grown under controlled conditions confirm this by showing that, during the night, isoprene emission of the shoots is indeed dramatically reduced, but not zero. Moreover, if we consider the emission observed under DD (wrapped culture glasses in an enlightened chamber), it appears to be low, but not as low as during a real night (whole chamber put in darkness). An explanation for this higher emission in the shoot cultures could be the higher temperature maintained in the wrapped pot in comparison to pots in dark chambers (2°C–3°C more). Even if the effect of temperature was not the scope of our study, it should not be forgotten as an important factor influencing isoprene emission. Besides, as in darkness, no new carbon is fixed; this continuous emission of isoprene testifies for a carbon source other than photosynthesis. Plants were grown on Murashige and Skoog medium, which contains sugar. These artificial growing conditions (compared to soil) could therefore explain this unusual nightly emission of isoprene, the saccharose being a potential carbon source. However, an experiment with 13C-labeled carbon would be necessary to confirm or refute this hypothesis. The low, but clear, variations of emission rate under LL present circadian periodicity and therefore testify to a clock element controlling isoprene emission. It is surprising that the first level of regulation of isoprene emission, namely, the expression rate of its synthesizing enzyme gene, and the emission itself present circadian rhythms, when neither the PcISPS protein level nor its activity display significant diurnal variations. Therefore, the observed fluctuations of emission do not seem to be due to PcISPS level variations. It is well documented that numerous genes and proteins involved in photosynthesis are clock regulated, as is fixed carbon allocation itself (Harmer et al., 2000). Because recently fixed carbon is the major pool used to produce isoprene in Grey poplar (Schnitzler et al., 2004), isoprene emission variations under LL may simply be the result of circadian fluxes of fixed carbon into the DOXP pathway. This would explain the lower amplitude of the fluctuations in comparison to LD cycles under which the instant PcISPS activity may vary.
DOXP pathway genes are regulated by development (Kuzma and Fall, 1993; Guevara-Garcia et al., 2005). DXR levels are shown to be at their highest values in young plants and during inflorescence development (Carretero-Paulet et al., 2002; Guevara-Garcia et al., 2005). In peppermint (Mentha × piperita), overexpression of DXR leads to higher accumulation of essential oil and cosuppression of this gene limits growth and leads to abnormal pigmentation (Mahmoud and Croteau, 2001), indicating a limiting and nonreplaceable role of DXR in the DOXP pathway for this species. Moreover, being the first committed step of the DOXP pathway, DXR is proposed to be one of the rate-limiting steps of isoprenoid biosynthesis (Mahmoud and Croteau, 2001; Carretero-Paulet et al., 2002). However, because PcDXR levels appear in our study to be neither synchronized with PcISPS nor with PcPSY fluctuations and, because they do not show circadian regulation, PcDXR may not be a key in daily regulation of the DOXP pathway and subsequent plastidic isoprenoid biosynthesis. Supporting our results, it is known that fluctuations of carotenoid biosynthesis in tomato do not require similar fluctuations of DXR (Rodríguez-Concepcíon et al., 2001). In addition, many studies suggest DXS as a regulating element of the DOXP pathway and carotenoid biosynthesis (Lois et al., 2000; Estévez et al., 2001; Guevara-Garcia et al., 2005). Therefore, it is possible that in Grey poplar no direct regulation of isoprene biosynthesis by PcDXR occurs. Knowing that DXR is not circadian regulated and that, in addition to its diurnal changes, its levels fluctuate strongly according to plant development (Carretero-Paulet et al., 2002), a possible significant role of DXR in the DOXP pathway more likely takes place in special stages of plant development.
PSY is the first dedicated and regulating enzyme of the carotenoid pathway (Von Lintig et al., 1997). Carotenoid functions are really diverse, ranging from primary metabolites involved in photosynthesis to secondary ones stored in chromoplasts (attracting pollinators and seed dispersers) or to vitamin and hormone precursors. Consistent with its role in synthesizing carotenoids for photosynthesis, this gene is highly expressed under light and repressed in the dark. Moreover, the main peak of PcPSY expression observed under LD early in the morning could be related to the need for the plant of these photosynthesis-related carotenoids. However, only further analysis of the downstream genes involved in this pathway could confirm this hypothesis. The circadian pattern observed under light conditions testifies to a role of the circadian clock in the regulation of PcPSY and may reflect the essential role of carotenoids in photosynthesis.
Gene expression of PcDXR and PcISPS is not synchronized, which raises the question of the pool of dimethylallyl diphosphate, a substrate of ISPS. Interestingly, dimethylallyl diphosphate pools have been shown to fluctuate diurnally in different species (Fisher et al., 2001; Brüggemann and Schnitzler, 2002), including poplar (Magel et al., 2006). Assuming that formation of photosynthates undergoes to a certain extent a circadian change, it might be hypothesized that the circadian change of isoprene emission is due to slightly enhanced metabolic flux within the DOXP pathway. To test this assumption, future experiments with stable 13CO2 feeding may help to clarify whether there might be changes in the labeling rate of isoprene as an indicator for fluctuations in carbon supply for isoprene biosynthesis under continuous conditions.
MATERIALS AND METHODS
Plant Material, Growth, and Experimental Designs
Six to seven wild-type Grey poplar (Populus × canescens) shoots were grown on one-half-strength concentrated Murashige and Skoog (1962) medium (approximately 200 mL), in 1-L glass containers, for 7 to 8 weeks under standard conditions (27°C day/24°C night), using a 16-h light/8-h dark photoperiod (from 6 am–10 pm) and 65 μmol m−2 s−1 photosynthetic photon flux density during the light period (for details of the cultivation procedure, see Leplé et al., 1992). DD was achieved by wrapping the jars in aluminum foil after which the temperature stayed at 25°C.
All experiments were repeated twice. Each sample consisted of all the leaves of one shoot. Nine containers (3/d over 3 d) were necessary per condition (LD, DD, and LL) for the circadian experiment. For all experiments, plants were placed in tested conditions at 10 pm the day before the first sampling.
For all experiments, samplings were done at 5 am (1 h before the end of the standard night), 7 am, 2 pm, 10 pm (just before start of the standard night), and midnight. For the darkness condition, containers were opened and samples were taken under red light. All samples, consisting of all leaves of one shoot, were immediately frozen in liquid nitrogen and stored at −80°C.
DNA Sequencing
Cycle-sequencing dideoxy chain termination reactions with Big Dye terminators (PE Applied Biosystems) were performed for both DNA strands of all DNA segments investigated, using universal forward and backward primers (Invitrogen) or sequence-specific oligonucleotides. Sequences were analyzed by using an ABI PRISM system 310 (PE Applied Biosystems).
To verify the specificity of primers on experimental cDNA, sequencing of purified products of real-time PCR was done by MWG Biotech AG using the same specific forward and backward primers as for quantitative reverse transcription (RT)-PCR.
Isolation of the PcISPS Promoter Sequence
Because the Grey poplar ISPS gene sequence (accession no. AJ294819) displays only 56 bases identified in front of the start codon, it was not possible to use it directly to find indications on the putative promoter sequence within the genome sequence of Populus trichocarpa (http://genome.jgi-psf.org/Poptr1/Poptr1.home.html). Therefore, the PcISPS sequence was BLASTed on the National Center for Biotechnology Information server to GenBank, revealing homologies with different terpene synthases, including two sequences of ISPS, one from Populus alba (accession no. AB198180) without a promoter part and one from Populus tremuloides (accession no. AY341431) displaying around 1 kb at the 5′ end of the start codon. This sequence (5′ end until the end of the first exon) was then used on the draft genome of P. trichocarpa. Sequences with the higher similarity were selected and aligned (using ClustalW; ClustalW WWW service at the European Bioinformatics Institute [http://www.ebi.ac.uk/clustalw]). The sequence with highest similarity to the P. tremuloides sequence was chosen and used for the second BLAST run on the genome. The same procedure was repeated three more times, until around 2.1 kb of the putative promoter region were identified. At different positions on the 5′ end of this sequence, 10 forward primers were randomly designed, each used combined to a reverse primer annealing in the exon 1 of the PcISPS gene (2325R primer designed in the first exon: CGTAATTGGCAGAGCGTCTG) with the following PCR conditions on a Biometra thermocycler, using polymerase platinum Taq (Invitrogen): 3-min denaturation at 94°C, 35 cycles at 94°C for 15 s, 50°C for 30 s, 72°C for 2 min, and 72°C for 4 min. The largest fragment (around 1.5 kb) was obtained with the primer 711F (CAAATAAACCTTAACATACAAATCATATTG). Two clones of this fragment were sequenced in both directions twice (nested sequencing, also repeated twice on both clones, had to be performed with internal primers because of the length of the sequence). Alignment of the obtained sequences leads to one sequence (published under accession no. AM084344) as the putative promoter region for the PcISPS gene, displaying 1,434 bp in front of the start codon.
RNA Isolation and cDNA Synthesis
Total RNA from frozen poplar leaves was isolated with the Qiagen RNeasy mini kit (Qiagen) following the Qiagen standard protocol. Amount and purity of isolated RNA were determined with NanoDrop ND-1000, a full-spectrum spectrophotometer having very high accuracy. The absorbance ratio 260/280 nm testified for very pure RNA, the mean of the samples ±sd being 2.116 ± 0.037.
For first-strand cDNA synthesis, 3 μg of total RNA were reverse transcribed using oligo(dT) primers and SuperScript II reverse transcriptase (Invitrogen) in a total volume of 20 μL according to the manufacturer's protocol. cDNA was stored at −20°C prior to analysis.
Quantitative RT-PCR
For quantitative PCR measurements of transcript levels, two to three primers pairs were designed for each gene of interest with PrimerExpress software (version 2.0.0; ABI-Prism). Their efficiency was tested by RT-PCR on the reference plasmid containing a sequenced fragment of the gene of interest and on experimental cDNA. The primer pairs used for further measurements were selected as being the most efficient ones, giving similar amplicons (checked on agarose gel) and dissociation curve patterns from plasmid and cDNA templates. Their sequences are as follows: for PcISPS, forward (5′-tttgcctactttgccgtggttcaaaac-3′) and reverse (5′-tcctcagaaatgccttttgtacgcatg-3′); for PcDXR, forward (5′-gcatatgtcttttccagcttctattgc-3′) and reverse (5′-ggaatagtaggttgcgcaggc-3′); for PcPSY, forward (5′-atgcatcacatatcacacccaaa-3′) and reverse (5′-ctcctagcatcttctccaacatctc-3′); for PcTUB (accession no. AY353093), forward (5′-gatttgtccctcgcgctgt-3′) and reverse (5′-tcggtataatgacccttggcc-3′). The resulting PCR segment lengths were 197 bp (PcISPS), 66 bp (PcDXR), 379 bp (PcPSY), and 151 bp (PcTUB), respectively. As a fluorescent marker for the increasing amount of double-stranded DNA, SYBR Green was used. The assays contained 12.5 μL 2× SYBR Green PCR master mix (Applied Biosystems), 300 nm of each primer, and 5 μL of total cDNA (diluted five times) in a final volume of 25 μL. After a hot start (10 min, 95°C), 45 PCR cycles were performed with a 15-s melting step at 95°C and a 1-min annealing/extension step at 60°C on a GeneAmp 5700 sequence detection system (Applied Biosystems). Transcript levels were related to the quantity of RNA used for RT. Transcript levels were calculated by the threshold cycle method with a standard curve under the GeneAmp 5700 sequence detection system software. To verify the amplification of the correct genes from poplar cDNA, all amplicons were purified from 0.8% agarose gels and sequenced.
Determination of PcISPS Activity
ISPS activity was assayed as previously described by Mayrhofer et al. (2005). A poplar-adapted plant extraction buffer (100 mm Tris-HCl, pH 8.0, 20 mm MgCl2, 100 mm CaCl2, 5% [v/v] glycerol, 0.1% [v/v] Tween 80, 20 mm dithiothreitol), with 250 mg polyvinylpolypyrrolidone was added prior to use and stirred for 15 min. Protein concentrations were determined by Bradford assay with bovine serum albumin as a standard.
Quantification of PcISPS Protein with ELISA
Quantification of ISPS protein was performed according to Schnitzler et al. (2005) using purified polyclonal anti-PcISPS IgG generated against N-terminal 6x-His-tagged PcISPS (Miller et al., 2001). For use as second antibody in ELISA, anti-PcISPS IgG was conjugated with horseradish peroxidase by BioGenes.
Heterologous Expression of LHY Protein from Arabidopsis and EMSA with PcISPS Promoter Fragments
Expression and purification of the recombinant Arabidopsis (Arabidopsis thaliana) LHY protein was performed using the AtLHY coding region fused to a C-terminal hexahistidine tag in pQE60 vector (Kim et al., 2003). Experiments were done according to QIAexpress protocols (Qiagen). Protocol 5 was used for the expression procedures, with the exception that Escherichia coli cells were disrupted by a French pressure cell press (SLM Instruments) two times at 1,000 psi and 0°C to 4°C. His-tagged AtLHY was purified under native conditions by affinity chromatography on a nickel-nitrilotriacetic acid column following QIAexpress protocol 11.
The following PCR fragments of the PcISPS gene promoter region were used in the experiment: the 241-bp-long fragment 1 amplified using the forward primer (5′-TAGTCCACGTATTGATGATAACA-3′) and the reverse primer (5′-AGGTGACAATTTCAGACGTC-3′), and the 259-bp long fragment 2 amplified using the forward primer (5′-CTGAACATTCCAGGTCTAATG-3′) and the reverse primer (5′-CCTCTAGTAATGGAAACTGCA-3′). As determined by in silico analysis, each fragment contains one putative binding box for the LHY protein. Biotin end labeling of the DNA fragments was carried out using the biotin 3′-end DNA-labeling kit (Pierce). The LightShift Chemiluminescent EMSA kit (Pierce) was used to detect whether the AtLHY protein binds to the PcISPS promoter. Experimental procedures (binding reaction and revealing the biotin-labeled DNA on the membrane) were performed according to the manufacturer's instructions. In binding reactions, 10 ng of biotin-labeled PcISPS promoter fragment DNA (control) plus 2 ng of AtLHY protein (binding reaction), and, finally, about 2,000 ng of unlabeled PcISPS promoter fragment DNA (to check the binding specificity) were used. Samples were loaded on a 6% DNA retardation gel (Invitrogen). DNA and protein were then semidried and transferred after half an hour at 300 mA to a nylon membrane (Qiabrane nylon plus; Qiagen).
Analysis of Isoprene Emission with Proton Transfer Reaction-Mass Spectrometry
Measurements of isoprene emission from shoot culture containers were performed with an adapted head-space analysis system using online proton transfer reaction (PTR)-mass spectrometry (MS), a combination of a PTR reaction drift tube and quadrupole MS. The instrument allows fast detection of most VOCs in combination with low detection limits (10–100 pptv; for details, see Lindinger et al., 1998; Schnitzler et al., 2004; Tholl et al., 2006). Measurements were performed on two gas-tight culture containers in parallel, each containing six to seven cell-cultured shoots, aged 6 to 8 weeks, partially with a developed root system. Clean air adjusted to a dew point of 28°C was flushed at 500 mL min−1 into the containers and from the outlet air 100 mL min−1 was pumped into the PTR-MS to analyze VOCs (mass 69 for isoprene). Measurements were performed on each container alternatively (automatically switched each 3 min with 60-s stabilization time). To avoid drying of the plants used in a confined environment, water was added carefully on the surface of the agarose, neither touching the leaves nor interfering with gas exchange (low changes of the pressure in the containers) at the beginning (50 mL) of and once during (30 mL) the experiment. Temperature was measured continuously inside containers with thermocouples.
The first 24 to 48 h of the experiments were used to flush excess isoprene previously accumulated in the containers during the development of the plants and to let the plants adapt to the constant gas stream. After the isoprene level appears stable, isoprene emission was measured over a LD day-night cycle. At 10 pm of the following day, one container was placed in LL and the other in DD (covered with aluminum foil) and emissions were measured during three virtual day-night cycles.
For calibration of PTR-MS, a gas standard (Apel-Riemer) with a continuous flow (20 mL min−1) of a mixture of VOCs, including isoprene at 1.05 ppmv, was diluted into the gas stream of 500 mL min−1 and flushed through an empty container for one-half hour at the beginning and end of the experiments. Because the sensitivity of the PTR-MS went slightly down during the measuring period, the standard curve for isoprene through the experiment was corrected according to the declining signal of the primary ion (mass 21, deuterium isotope of H3O+). At the end of the experiments, isoprene emission from agar and roots (green material removed) was measured and these background values were subtracted from previously measured global isoprene emissions.
Statistical Analysis
Statistical and correlational analysis was performed with SPSS for Windows NT (release 8.0.0) and SigmaPlot for Windows (version 9.0), both programs from SPSS.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AM084344.
Acknowledgments
We gratefully acknowledge provision of a PcTUB fragment of poplar by Stanislav Kopriva (Norwich Research Park, UK) and the AtLHY-6x-His construct by Isabelle A. Carré (University of Warwick, UK).
This work was supported by the European Commission in the frame of the Marie-Curie Research Training Network ISONET (Ecological and physiological functions of biogenic isoprenoids and their impact on the environment) and the German Science Foundation within the German joint research group, Poplar—a model to address tree-specific questions (grant no. SCHN653/4).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sandrine Louis (sandrine.louis@imk.fzk.de).
References
- Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesenthal TA, Wu Q, Shepson PB, Wiebe HA, Anlauf KG, MacKay GI (1997) A study of relationships between isoprene, its oxidation products, and ozone, in the lower Fraser valley, BC. Atmos Environ 31 2049–2058 [Google Scholar]
- Brüggemann N, Schnitzler J-P (2002) Comparison of isoprene emission, intercellular isoprene concentration and photosynthetic performance in water-limited oak (Quercus pubescens wild and Quercus robur L) saplings. Plant Biol 4 456–463 [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH (2004) Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol 4 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero-Paulet L, Ahumada I, Cunillera N, Rodríguez-Concepcíon M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-d-erythritol 4-phosphate pathway. Plant Physiol 129 1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwent RG, Jenkin ME, Saunders SM, Pilling MJ (1998) Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos Environ 32 2429–2441 [Google Scholar]
- Devlin PF (2002) Signs of the time: environmental input to the circadian clock. J Exp Bot 53 1535–1550 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorestein N, Fäldt J, Miller B, Bohlmann J (2003) (E)-β-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15 1227–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway of terpenoids. Trends Plant Sci 6 78–84 [DOI] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Reindl A, Reichler S, Leon P (2001) 1-deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276 22901–22909 [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem 292 272–279 [DOI] [PubMed] [Google Scholar]
- Guevara-Garcia A, San Roman C, Arroyo A, Cortes ME, de la Luz Gutierrez-Nava M, Leon P (2005) Characterization of the Arabidopsis clb6 mutant illustrates the importance of posttranscriptional regulation of the methyl-d-erythritol 4-phosphate pathway. Plant Cell 17 628–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290 2110–2113 [DOI] [PubMed] [Google Scholar]
- Heintzen C, Fischer R, Melzer S, Kappeler S, Apel K, Staiger D (1994) Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol 106 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsper JPFG, Davies JA, Bouwmeester HJ, Krol AF, Van Kampen MH (1998) Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L cv Honesty. Planta 207 88–95 [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M-H, Goodman HM (2005) The Arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol 138 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH (2001) Endogenous timekeepers in photosynthetic organisms. Annu Rev Plant Physiol Plant Mol Biol 63 695–728 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Roeber J, Hastings JW (1984) Circadian changes in enzyme concentration account for rhythm of enzyme activity in Gonyaulax. Science 223 1428–1430 [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33 23–88 [Google Scholar]
- Kim JY, Song HR, Taylor BL, Carré IA (2003) Light-regulated translation mediates gated induction of the Arabidopsis clock protein LHY. EMBO J 22 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzma J, Fall R (1993) Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol 101 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L (1992) Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11 137–141 [DOI] [PubMed] [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998) Proton-transfer-reaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chem Soc Rev 27 347–354 [Google Scholar]
- Lois LM, Rodríguez-Concepcíon M, Gallego F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-d-xylulose 5-phosphate synthase. Plant J 22 503–513 [DOI] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Lu S, Xu R, Jia J-W, Pang J, Matsuda SPT, Chen X-Y (2002) Cloning and functional characterization of a β-pinene synthase from Artemisia annua that shows circadian regulation of expression. Plant Physiol 130 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magel E, Mayrhofer S, Müller A, Zimmer I, Hampp R, Schnitzler J-P (2006) Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmos Environ 40 S138–S151 [Google Scholar]
- Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 98 8915–8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer S, Teuber M, Zimmer I, Louis S, Fischbach RJ, Schnitzler J-P (2005) Diurnal and seasonal variation of isoprene biosynthesis-related genes in Grey poplar leaves. Plant Physiol 139 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR (2000) Circadian rhythms in plants: a millennial view. Physiol Plant 109 359–371 [Google Scholar]
- McClung CR (2006) Plant circadian rhythms. Plant Cell 18 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213 483–487 [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH, Adams WW, Driggers EM, Silver GM, Fall R (1992) Relationship among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98 1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15 473–497 [Google Scholar]
- Nozue K, Maloof JN (2006) Diurnal regulation of plant growth. Plant Cell Environ 29 396–408 [DOI] [PubMed] [Google Scholar]
- Piechulla B (1993) Circadian clock directs the expression of plant genes. Plant Mol Biol 22 533–542 [DOI] [PubMed] [Google Scholar]
- Piechulla B, Gruissem W (1987) Diurnal mRNA fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J 6 3593–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestridge DS (1991) Signal scan: a computer program that scans DNA sequences for eukaryotic transcriptional elements. CABIOS 7 203–206 [DOI] [PubMed] [Google Scholar]
- Roeder RG (1996) The role of general initiation factors in transcription by RNA polymerase II. Trends Plant Sci 21 327–335 [PubMed] [Google Scholar]
- Rodríguez-Concepcíon M, Ahumada I, Diez-Juez E, Sauret-Gueto S, Lois LM, Gallego F, Carretero-Paulet L, Campos N, Boronat A (2001) 1-Deoxy-d-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J 27 213–222 [DOI] [PubMed] [Google Scholar]
- Rombauts S, Déhais P, Van Montagu M, Rouzé P (1999) PlantCARE, a cis-acting regulatory element database. Nucleic Acids Res 27 295–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK (2004) Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biol 6 12–21 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Ohara K, Yazaki K (2005) Gene expression and characterization of isoprene synthase from Populus alba. FEBS Lett 579 2514–2518 [DOI] [PubMed] [Google Scholar]
- Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E (2001) Microarray analysis of diurnal and circadian regulated genes in Arabidopsis. Plant Cell 13 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler J-P, Graus M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A (2004) Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol 135 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler J-P, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach RJ (2005) Biochemical properties of isoprene synthase from poplar (Populus × canescens). Planta 222 777–786 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F (1993) Water stress, temperature and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 343 1–6 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374 769 [Google Scholar]
- Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52 407–436 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ (2004) Circadian regulation of the PhCCD1 carotenoids cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136 3504–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE (1999) The physiology and molecular bases of the plant circadian clock. Plant Physiol 121 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger GA, Schörken U, Wiegert T, Grolle S, De Graaf AA, Taylor SV, Begley TP, Bringer-Meyer S, Sahm H (1997) Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci USA 94 12857–12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Kuzyama T, Watanabe H, Seto H (1998) A 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA 95 9879–9884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Rose US, Schnitzler J-P (2006) Practical approaches to plant volatile analysis. Plant J 45 540–560 [DOI] [PubMed] [Google Scholar]
- Thompson AM (1992) The oxidizing capacity of the Earth's atmosphere: probable past and future changes. Science 256 1157–1165 [DOI] [PubMed] [Google Scholar]
- Von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, Kleinig H (1997) Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapsis alba and Arabidopsis thaliana seedlings. Plant J 12 625–634 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Tobin EM (1998) Constitutive expression of the circadian clock associated 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93 1207–1217 [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Owen SM, Possell M, Hartwell J, Gould P, Hall A, Vickers C, Hewitt CN (2006) Circadian control of isoprene emissions from oil palm (Elaeis guineensis). Plant J 47 960–968 [DOI] [PubMed] [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F, Yeh S, Weise E (2003) Biochemical regulation of isoprene emission. Plant Cell Environ 26 1357–1363 [Google Scholar]