Abstract
Clinical and experimental data indicate that anti-neutrophil cytoplasmic autoantibodies (ANCAs) cause glomerulonephritis and vasculitis. Here we report the first evidence that complement is an important mediator of ANCA disease. Transfer of anti-myeloperoxidase (MPO) IgG into wild-type mice or anti-MPO splenocytes into immune-deficient mice caused crescentic glomerulonephritis that could be completely blocked by complement depletion. The role of specific complement activation pathways was investigated using mice with knockout of the common pathway component C5, classic and lectin binding pathway component C4, and alternative pathway component factor B. After injection of anti-MPO IgG, C4−/− mice developed disease comparable with wild-type disease; however, C5−/− and factor B−/− mice developed no disease. To substantiate a role for complement in human ANCA disease, IgG was isolated from patients with myeloperoxidase ANCA (MPO-ANCA) or proteinase 3 ANCA (PR3-ANCA) and from controls. Incubation of MPO-ANCA or PR3-ANCA IgG with human neutrophils caused release of factors that activated complement. IgG from healthy controls did not produce this effect. The findings suggest that stimulation of neutrophils by ANCA causes release of factors that activate complement via the alternative pathway, thus initiating an inflammatory amplification loop that mediates the severe necrotizing inflammation of ANCA disease.
Anti-neutrophil cytoplasmic autoantibodies (ANCA) are specific for proteins in the cytoplasm of neutrophils and monocytes. The major target antigens in patients with vasculitis and glomerulonephritis are myeloperoxidase (MPO) and proteinase 3 (PR3). ANCAs occur in greater than 80% of patients with active untreated Wegener’s granulomatosis, microscopic polyangiitis, and pauci-immune crescentic glomerulonephritis.1 There is compelling clinical and experimental evidence that ANCA IgG causes ANCA-associated vasculitis and glomerulonephritis. The strongest clinical evidence for causation is the observation that a newborn child developed glomerulonephritis and pulmonary hemorrhage shortly after delivery from a mother with MPO-ANCA-associated microscopic polyangiitis, apparently caused by transplacental transfer of ANCA IgG.2,3 Two compelling animal models of ANCA vasculitis and glomerulonephritis have been described by two different research groups.4,5 Xiao and colleagues4 induced glomerulonephritis and systemic vasculitis by intravenous injection of either anti-MPO IgG or anti-MPO splenocytes derived from MPO knockout mice immunized with murine MPO. Induction of glomerulonephritis by anti-MPO IgG in this model is enhanced by cytokines6 and requires neutrophils.7 Little and colleagues5 immunized rats with human MPO, resulting in the production of antibodies that cross reacted with rat MPO and caused vasculitis and glomerulonephritis. The pathogenic effects of these anti-MPO antibodies were augmented by cytokines.
Numerous in vitro studies indicate that ANCA IgG can activate neutrophils and cause them to release proinflammatory factors. The expression of ANCA antigens (MPO and PR3) at the surface of neutrophils where they are accessible to interact with ANCA IgG is enhanced by proinflammatory cytokines, such as tumor necrosis factor (TNF)-α.8,9 Incubation of TNF-α-primed neutrophils with ANCA IgG induces full activation with release of lytic and toxic granule enzymes and reactive oxygen species.8,9 Interaction of ANCA IgG with ANCA antigens in the microenvironment of neutrophils causes activation through both Fc receptor engagement and Fab′2 binding.10–13 Activation of neutrophil by ANCA IgG in the presence of cultured endothelial cells results in neutrophil adherence,14 neutrophil transmigration,15 and endothelial cell death.16,17 Little and colleagues5 have documented this process in vivo using their rat model to show by intravital microscopy that leukocytes activated with MPO-ANCA IgG adhere to and injure the microvasculature.
Before the observations reported in this article, a role for complement in the pathogenesis of ANCA-induced inflammation has not been suspected. This is in part because there is less complement deposition in vessel walls in ANCA vasculitis and glomerulonephritis as compared with the substantial complement deposition that is observed with immune complex disease and anti-glomerular basement membrane disease.18,19 However, an important mediator of vascular inflammation does not have to be present in vessel walls at high concentrations. For example, there is a paucity of IgG in the vascular lesions of ANCA vasculitis and glomerulonephritis, yet, as reviewed earlier, there is compelling evidence that ANCA IgG is the primary pathogenic factor causing these inflammatory lesions. ANCA disease is not associated with hypocomplementemia; however, this is not a sensitive indicator of complement involvement because certain forms of glomerulonephritis and vasculitis that have substantial vascular deposits of complement do not have hypocomplementemia, such as Henoch-Schönlein purpura and anti-GBM disease. In addition, complement activation has been identified as a major mediator of injury and inflammation in a variety of diseases in which there is little or no complement localization at the site of injury, for example, complement activation, probably through the alternative pathway, is an important mediator in ischemia reperfusion injury.20 The complement system can be activated through three different pathways: classic, lectin, and alternative.21–23 Among the many factors that can activate complement are mediators released by activated neutrophils.24–27 Based on the observations reported herein, we hypothesize that ANCA-induced activation of neutrophils results in the release of factors that activate the alternative complement pathway amplification loop, which in turn augments recruitment and activation of more neutrophils, resulting in the severe necrotizing leukocytoclastic inflammation that is so characteristic of acute ANCA disease.
Materials and Methods
Mice
MPO knockout (Mpo−/−) mice initially generated by Aratani and colleagues28 were maintained by the University of North Carolina Division of Laboratory Animal Medicine. Mpo−/− mice (8 to 10 weeks old) were used for immunization and as donors of anti-MPO antibodies and splenocytes. Rag2 mice were purchased from Taconic Farms (Germantown, NY). Factor B−/− mice that were initially generated by Matsumoto and colleagues29 were kindly provided by Dr. Zhi Liu, Department of Dermatology, University of North Carolina, Chapel Hill, NC. Age- and sex-matched wild-type (WT) B6 controls, C4−/− mice, and C5−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). All complement-deficient mice had a C57BL/6 (B6) background. Rag2−/− mice, 10 to 12 weeks old, were used as recipients for adoptive splenocyte transfer experiments. Factor B−/−, C4−/−, C5−/−, and WT B6 mice, 9 to 10 weeks old, were used for IgG transfer experiments. All animal experiments were approved by the Animal Studies Committee of the University of North Carolina at Chapel Hill, School of Medicine, and were used in accordance with National Institutes of Health guidelines.
Kinetics of C3 Depletion by Cobra Venom Factor (CVF)
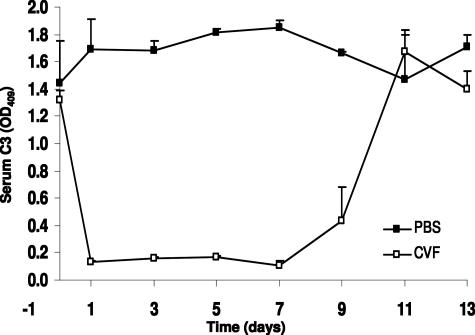
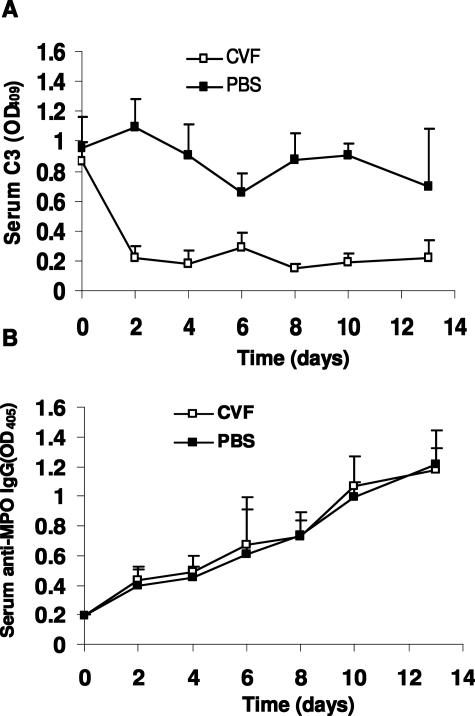
To determine the kinetics of C3 depletion by CVF, WT B6 mice (n = 6) were injected intraperitoneally with a single dose of 30 μg of CVF (Sigma-Aldrich, St. Louis, MO) in 0.3 ml of phosphate-buffered saline (PBS). Control mice (n = 6) received 0.3 ml of PBS. Serum C3 levels was measured on days 0, 1, 3, 5, 7, 9, 11, and 13 after injection of CVF by C3 antibody capture enzyme-linked immunosorbent assay (ELISA) using F(ab′)2 fragments of goat anti-mouse C3 as capture antibody and peroxidase-conjugated goat anti-mouse C3 as the second antibody (Cappel, Organon Teknika Corporate, West Chester, PA), according to the manufacturer’s protocol. C3 levels monitored by ELISA were markedly reduced with the lowest level within 24 hours after injection and remained at this low level for up to 7 days. Thereafter, C3 returned toward normal and reached the normal level at approximately day 11. Control mice injected with the same volume of PBS exhibited normal levels of circulating C3 (Figure 1).
Figure 1.
Profile of depletion of circulating complements by CVF. WT mice were given a single intraperitoneal injected dose of CVF (30 mg/0.3 ml PBS) or PBS (0.3 ml) and the kinetics of complement depletion were monitored by ELISA analysis of circulating C3 at indicated days after injection. Open squares represent data from CVF-treated mice (n = 6). Filled squares represent control mice that received PBS (n = 6). Bars indicate the SD.
Preparation of Nephritogenic Mouse Anti-Murine MPO Splenocytes and Antibodies
Purification of mouse MPO and the immunization of Mpo−/− mice were performed as previously described.4 In brief, mouse MPO was purified from WEHI-3 cells by Dounce homogenization, Concanavalin A affinity chromatography, ion exchange, and gel filtration chromatography. Mpo−/− mice were immunized intraperitoneally with 10 μg of purified murine MPO or bovine serum albumin in complete Freund’s adjuvant and subsequently boosted with antigen in incomplete Freund’s adjuvant. Development of antibodies was monitored by ELISA and anti-MPO antibody reactivity with neutrophils was confirmed by indirect immunofluorescence microscopy on murine neutrophils. The IgG fraction was isolated from serum by 50% ammonium sulfate precipitation and protein G affinity chromatography. Splenocytes were isolated from immunized and nonimmunized Mpo−/− mice by disrupting the spleens into cold RPMI 1640 medium and then washing twice with RPMI 1640. Red blood cells were removed with lysis buffer (Sigma-Aldrich) followed by washing with RPMI 1640 and final suspension in sterile PBS.
Induction of Glomerulonephritis with Anti-MPO IgG and Anti-MPO Splenocytes
Induction of experimental necrotizing and crescentic glomerulonephritis (NCGN) with anti-MPO IgG in WT mice or anti-MPO splenocytes in Rag2−/− immune-deficient mice was performed as previously described.4 Briefly, in antibody transfer experiments, mice were injected intravenously with 50 μg/g body weight (BW) of anti-MPO or control IgG in PBS and sacrificed on day 6. In splenocyte transfer experiments, Rag2−/− mice were injected intravenously with 5 × 107 anti-MPO or control splenocytes in 0.5 ml of PBS and sacrificed on day 13. At the point of sacrifice, serum and urine samples were collected to evaluate kidney disease, and serum samples also were collected for serological assays of antibody and complement activity.
In IgG transfer CVF complement depletion experiments, WT and control mice received intravenously 50 μg/g BW of anti-MPO IgG 4 hours after injection of one dose of CVF or PBS, respectively, and were sacrificed on day 6. The effect of CVF on circulating complement was determined by C3 antibody capture ELISA. The urine, serum, and kidney tissue samples were collected for further evaluation as below.
In splenocyte transfer CVF complement depletion experiments, Rag2−/− mice were injected intraperitoneally with 30 μg of CVF in 0.3 ml of PBS on days 0 and 6, and control mice received 0.3 ml of PBS on days 0 and 6. Both experimental and control mice received 5 × 107 anti-MPO splenocytes by intravenous injection 4 hours after receiving the first dose of CVF or PBS. The mice were sacrificed on day 13.
In IgG transfer experiments in complement knockout mice, C5−/−, C4−/−, fB−/−, or WT mice were injected intravenously with 50 μg/g BW of anti-MPO IgG and sacrificed on day 6. The urine, serum, and kidney tissues were collected for evaluation.
Functional Evaluation of Renal Injury
Mice were placed in metabolic cages 1 day before sacrifice and urine was collected for 12 hours. Urine was tested by dipstick (Roche Diagnostics Corp., Indianapolis, IN) for hematuria and leukocyturia. The extent of hematuria and leukocyturia was expressed as the mean on a scale of 0 (none) to 4 (severe). Albuminuria was determined by a mouse albumin ELISA quantitation kit (Bethyl Laboratories Inc., Montgomery, TX). Using the mean ± 2 SDs for the reference range established in 305 healthy B6 mice, abnormal hematuria was set at >0.9 and leukocyturia >0.2.7 Serum samples were collected at the time of sacrifice. Serum creatinine and blood urea nitrogen were measured using a Johnson & Johnson Ortho-Clinical Diagnostics VITROS 250 (Raritan, NJ).
Pathological Evaluation of Renal Injury
Samples of kidney tissue were collected at the time of sacrifice and fixed in 10% formalin, embedded in paraffin using routine protocols, sectioned at 4-μm, stained with hematoxylin and eosin and periodic acid Schiff, and evaluated by light microscopy. The extent of glomerular crescents and necrosis were expressed as the mean percentage of glomeruli with crescents and necrosis in each animal.
For immunofluorescence microscopy to detect glomerular localization of immune determinants, 4-μm frozen sections were stained with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Molecular Probes Invitrogen, Carlsbad, CA), IgM, IgA, and MPO (ICN/Cappel, Aurora, OH), respectively. Deposition of complement C3 was visualized with fluorescein isothiocyanate-conjugated goat anti-mouse C3 (ICN/Cappel). Immunofluorescence microscopy staining of glomeruli for IgG, IgM, IgA, C3, and MPO was expressed as the mean intensity of staining on a scale of 0 (none) to 4 (severe).
For immunohistochemistry to detect renal leukocytes infiltration, 4-μm frozen sections were stained with rat anti-mouse neutrophil Gr-1 (clone RB6-8C5; BD Pharmingen, Franklin Lakes, NJ) and rat anti-mouse monocytes/macrophages CD68 antibodies (clone FA11; Serotec, Raleigh, NC). Rat antibody binding was detected using peroxidase-conjugated secondary rabbit anti-rat IgG and tertiary goat anti-rabbit IgG antibodies (DAKO, Carpinteria, CA). Staining was visualized with 3-amino-9-ethylcarbazole and hydrogen peroxide. Sections were counterstained with hematoxylin. Leukocyte localization was expressed as the mean number of leukocytes per cross-section of glomeruli based on evaluating an average of 75 glomeruli per specimen (range, 60 to 90 glomeruli).
Measurement of Complement Activation by Factors Released by ANCA-Stimulated Human Neutrophils
IgG from healthy controls (n = 8), MPO-ANCA patients (n = 6), or PR3-ANCA patients (n = 8) was prepared from plasma by ammonium sulfate precipitation and protein G affinity column chromatography. IgG preparation were passed over AffinityPak endotoxin removal columns (Pierce, Rockford, IL) before use. The plasma was obtained during plasma exchange on ANCA-positive patients with active, biopsy-proven necrotizing and crescentic glomerulonephritis. The patients also were receiving immunosuppressive treatment. Neutrophils were isolated from healthy volunteers using Ficoll sedimentation. Neutrophils from a single donor were used for each experimental run that compared the effect of control IgG to various experimental preparations. Autologous serum was obtained from the same donors as the neutrophils. In preparation to determination of the ability of IgG to stimulate neutrophils to release complement-activating factors, neutrophils were primed for 15 minutes at 37°C with 2 ng/ml TNF-α. The primed neutrophils then were incubated with 200 μg/ml of either control IgG, MPO-ANCA IgG, or PR3-ANCA IgG for 15 minutes at 37°C. Additional controls included reaction mixtures that contained control IgG, MPO-ANCA IgG, or PR3-ANCA IgG but no neutrophils (to determine whether the IgG alone could activate complement) and reaction mixtures that contained TNF-primed neutrophils but no immunoglobulin (to determine whether primed neutrophils alone could activate complement). The reaction mixtures were centrifuged at 300 g for 5 minutes at 4°C. A 100-μl aliquot of supernatant was added to precooled tubes containing 100 μl of autologous serum and incubated for 1 hour at 37°C. The reaction was stopped by adding ethylenediaminetetraacetic acid to a final concentration of 10 mmol/L. The supernatants were stored frozen at −70°C until assayed for C3a. C3a, which is a sensitive marker of complement activation, was assayed in the supernatant using the Quidel C3a EIA kit (Quidel Corp., San Diego, CA) by the manufacturer’s instructions. All determinations were made in duplicate. Two or more control IgG samples were included with each assay, and results for individual control and experimental samples were expressed as a percentage of the result of the control IgG mean.
Results
Complement Is Required for Induction of Glomerulonephritis by Anti-MPO IgG
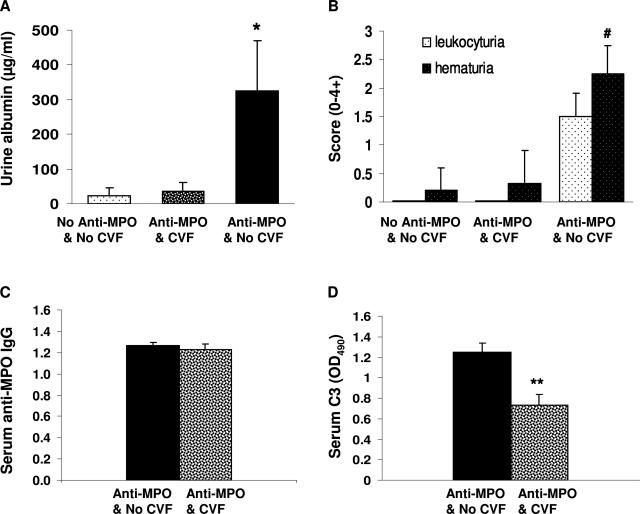
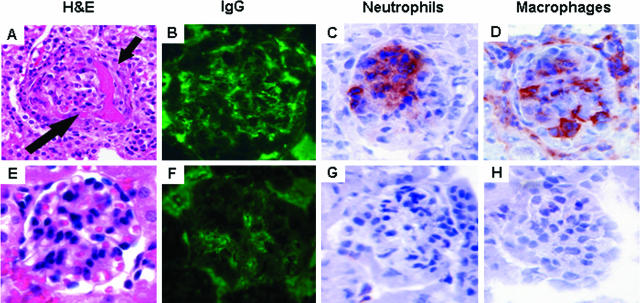
Pauci-immune NCGN that closely mimics human ANCA glomerulonephritis is induced in mice by a single injection of anti-MPO IgG derived from Mpo−/− mice that have been immunized with mouse MPO.4,6,7 By day 6, all mice that receive anti-MPO IgG develop segmental fibrinoid necrosis and crescents in ∼5 to 15% of glomeruli. Immunohistology demonstrates only mild staining for immunoglobulins and complement. The effect of complement depletion on the induction of NCGN in this model was evaluated. WT B6 mice were pretreated with (n = 7) or without (n = 7) a single intraperitoneal injection of CVF 4 hours before injection of anti-MPO IgG. Six days after injecting anti-MPO IgG, all mice that did not have complement depletion developed albuminuria, leukocyturia, and hematuria whereas the mice that received CVF stayed within the reference range (Figure 2, A and B). At the time of sacrifice, mice that received CVF had the same level of circulating anti-MPO IgG (Figure 2C) as the mice that did not receive CVF; however, as expected, they had decreased serum C3 levels (Figure 2D). All of the mice that received anti-MPO IgG without CVF developed NCGN with neutrophil and macrophage infiltration and low-level glomerular IgG and C3 deposition (Figure 3, A–C; and Tables 1 and 2). In contrast, none of the complement-depleted mice injected with the same dose of anti-MPO IgG developed glomerular necrosis or crescents (Figure 3, D–F; and Tables 1 and 2). The mice that did not receive CVF had a paucity of staining for C3 (0.5+) and mice that received CVF had no staining for C3 (Table 2).
Figure 2.
Mice depleted of complement are resistant to anti-MPO IgG-induced experimental glomerulonephritis. WT mice were pretreated with a single dose of CVF or PBS (n = 7 for each group). Four hours later, the mice received anti-MPO IgG (50 μg/g body weight). A and B: Urine analysis showed that untreated mice and complement-depleted mice that received anti-MPO IgG had no urine abnormalities, whereas noncomplement-depleted mice that received anti-MPO IgG had albuminuria, hematuria, and leukocyturia. C and D: ELISA analysis demonstrated the same circulating anti-MPO IgG titers in noncomplement-depleted and complement-depleted mice (C); and low circulating C3 in complement-depleted mice (D). Extent of hematuria and leukocyturia is expressed as the mean on a scale of 0 (none) to 4 (severe). *P < 0.05 and #P < 0.001 versus untreated WT. **P < 0.004 compared with noncomplement-depleted mice. Bars represent the mean ± SD.
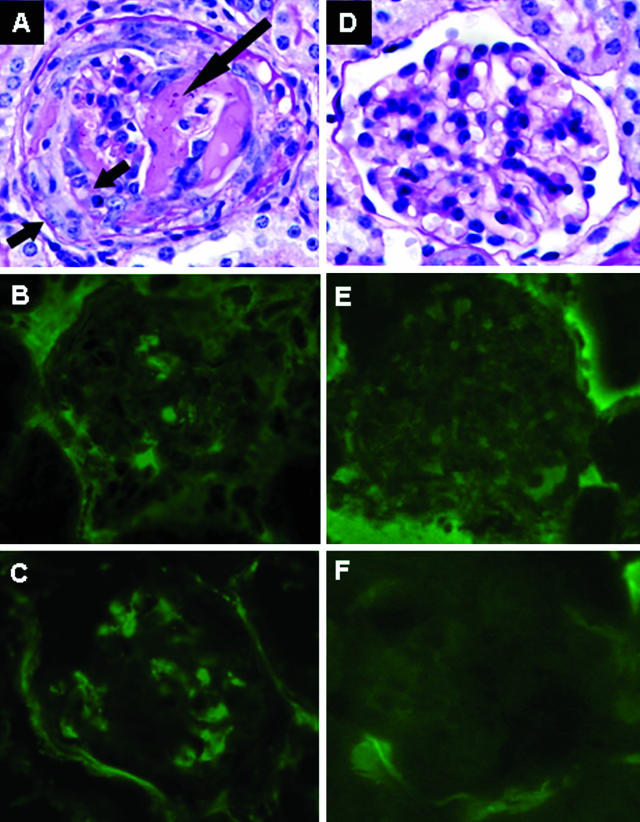
Figure 3.
Renal tissue was examined 6 days after mice received anti-MPO IgG. WT mice that received pathogenic IgG without complement depletion developed focal glomerular necrosis (long arrow) and crescents (short arrows) (A) and low-level glomerular IgG (B) and C3 (C) deposition. In contrast, the mice depleted of complement had no glomerular lesions after the same dose of anti-MPO IgG (D) and had no IgG (E) or C3 (F) in glomeruli. A and D are stained with PAS.
Table 1.
Pathology Finding in ANCA Mouse Model with and without CVF
| Groups | Mice | % Mice with crescents/necrosis | Crescents (%) | Necrosis (%) | PMN (Gr-1) | Macrophage (CD68) |
|---|---|---|---|---|---|---|
| Anti-MPO IgG | WT | 100 | 11.3 ± 1.2 | 5.3 ± 1.2 | 0.48 ± 0.17 | 0.82 ± 0.21 |
| Anti-MPO IgG + CVF | WT | 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.07 ± 0.03 | 0.10 ± 0.04 |
| Anti-MPO splenocytes | Rag2−/− | 100 | 36.5 ± 21.5 | 35.8 ± 15.4 | 2.39 ± 0.47 | 1.76 ± 0.19 |
| Anti-MPO splenocytes + CVF | Rag2−/− | 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.21 ± 0.07 | 0.11 ± 0.06 |
Table 2.
Immunofluorescence Microscopy Observations in ANCA Mouse Models with and without CVF Complement Depletion
| Group | IgG (0 to 4+) | IgM (0 to 4+) | IgA (0 to 4+) | C3 (0 to 4+) | MPO (0 to 4+) |
|---|---|---|---|---|---|
| Anti-MPO IgG | 0.5 ± 0.0 | 0.4 ± 0.3 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.0 ± 0.0 |
| Anti-MPO IgG + CVF | 0.5 ± 0.0 | 0.3 ± 0.3 | 0.7 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Anti-MPO splenocytes | 3.0 ± 0.0 | 1.0 ± 0.0 | 2.0 ± 0.0 | 2.5 ± 0.6 | 0.4 ± 0.3 |
| Anti-MPO splenocyte + CVF | 0.8 ± 0.3 | 0.3 ± 0.3 | 0.8 ± 0.3 | 0.2 ± 0.3 | 0.0 ± 0.0 |
Complement Is Required for Induction of NCGN by Anti-MPO Splenocytes
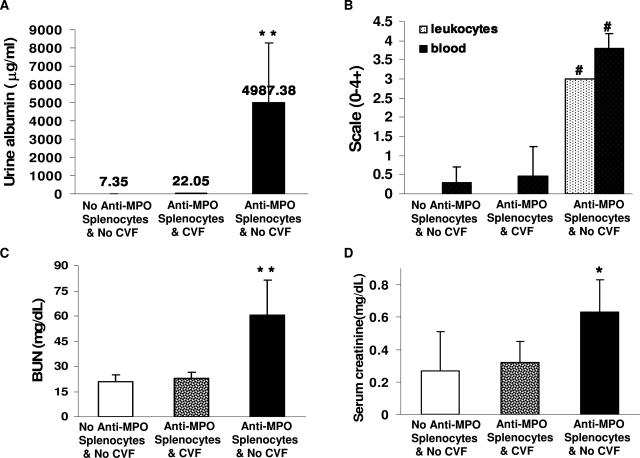
Intravenous administration of anti-MPO splenocytes causes more severe disease than injection of anti-MPO IgG, although it is complicated by a background of immune complex disease.4 In our previously reported experiments,4 all mice that received 1 × 108 or 5 × 107 anti-MPO splenocytes developed NCGN with crescents in ∼80% of glomeruli. None of the mice that received 1 × 107 anti-MPO splenocytes developed NCGN. Immunohistology demonstrates a moderate accumulation of immunoglobulins and complement in glomeruli of mice that receive either anti-MPO splenocytes or control splenocytes. The effect of complement depletion with CVF was evaluated in this more aggressive form of NCGN induced by anti-MPO splenocytes. Rag2−/− mice were treated intraperitoneally with two doses of 30 μg of CVF in 0.3 ml of PBS (n = 8) or PBS alone (n = 8) at day 0 and day 6. Four hours after injection of the first dose of either CVF (n = 8) or PBS (n = 8), mice received an intravenous injection of 5 × 107 splenocytes from Mpo−/− mice that had been immunized with mouse MPO. Control Rag2−/− mice received no CVF and no splenocytes. On day 13, all mice injected with anti-MPO splenocytes without complement depletion developed albuminuria, hematuria, leukocyturia, elevated serum creatinine, and elevated blood urea nitrogen (Figure 4, A–D). In contrast, mice with complement depletion injected with anti-MPO splenocytes had unremarkable urine findings, creatinine, and blood urea nitrogen that did not differ significantly frommice that received neither CVF nor anti-MPO splenocytes (Figure 4, A–D). Pathological evaluation of kidneys revealed that all noncomplement-depleted mice that received anti-MPO splenocytes developed severe NCGN with glomerular neutrophil and macrophage infiltration (Figure 5, A–D; and Table 1). In contrast, none of the complement-depleted mice that received anti-MPO splenocytes developed glomerular necrosis or crescents or increased infiltration of inflammatory cells (Figure 5, F–H; and Table 1). There was a marked decrease in circulating C3 in mice given CVF that occurred within 2 days and persisted throughout the experiment (Figure 6A). There was no significant decrease in C3 levels in the mice that received splenocytes but no CVF. Both noncomplement-depleted and complement-depleted mice developed circulating anti-MPO antibodies within 2 days, and the titers steadily increased at similar levels until sacrifice at 13 days after receiving anti-MPO splenocytes (Figure 6B). Previous experiments have demonstrated that immune-deficient Rag2−/− mice that receive splenocytes from immune-competent mice develop an immune complex mediated mesangioproliferative glomerulonephritis without crescents.4 Severe crescent formation is induced only when the splenocytes are derived from Mpo−/− mice that have been immunized with MPO.4 Thus, the anti-MPO immune response and not the immune complex glomerulonephritis is the cause for the NCGN in this model. In the current experiments, the interpretation of the blockade of NCGN by CVF after anti-MPO splenocyte transfer is complicated by the marked reduction of immune complexes and complement deposition caused by CVF complement depletion (Table 2). The complement depletion could be blocking crescent formation by inhibiting a specific anti-MPO-mediated mechanism or by reducing the synergistic stimulation of concurrent immune complex disease or both.
Figure 4.
Complement depletion by CVF abolishes the development of urine abnormalities, and elevation of serum blood urea nitrogen and creatinine in Rag2−/− mice 13 days after they received 5 × 107 anti-MPO splenocytes (A–D). Bars represent the SD. *P < 0.05, **P < 0.01, #P < 0.001 compared with untreated Rag2−/− mice (ie, with no anti-MPO splenocytes and no CVF).
Figure 5.
Complement depletion prevents anti-MPO splenocyte-induced glomerular necrosis and crescents. Rag2−/− mice were treated with CVF or PBS (n = 8 per group), and then, 4 hours later, were injected with 5 × 107 anti-MPO splenocytes. Pathological examinations were performed at day 13 of receiving anti-MPO splenocytes. In noncomplement-depleted Rag2−/− mice, transfer of anti-MPO splenocytes induced glomerular necrosis (long arrow) and crescent formation (short arrow) (A) (H&E), moderate glomerular IgG deposition (B) (fluorescein isothiocyanate anti-IgG), and neutrophil and macrophage infiltration (C, D). Complement-depleted Rag2−/− mice that received anti-MPO splenocytes had no lesions by light microscopy (E) (H&E), low-level IgG deposition (F), and no significant increase in glomerular neutrophils or macrophages (G, H).
Figure 6.
Circulating C3 and anti-MPO IgG levels in Rag2−/− mice during induction of NCGN. Rag2−/− mice that received pretreatment of CVF or PBS (n = 8 for each group) were injected with 5 × 107 anti-MPO splenocytes. Circulating C3 and anti-MPO IgG were monitored by ELISA at different time points up to 13 days after receiving splenocytes. A: Circulating C3 levels in CVF-treated Rag2−/− mice (open squares) and control Rag2−/− mice (filled squares). B: Circulating anti-MPO IgG levels in CVF-treated Rag2−/− mice (open squares) and control Rag2−/− mice (filled squares). Bars represent the SD.
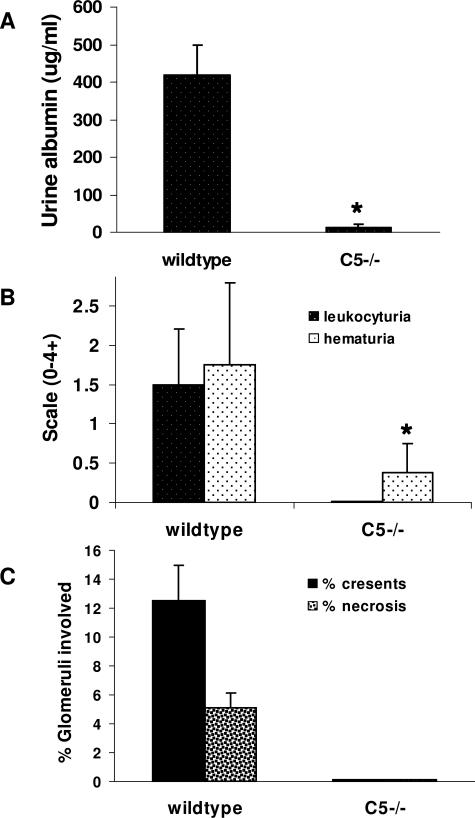
Mice Deficient in C5 Are Resistant to Anti-MPO ANCA-Induced NCGN
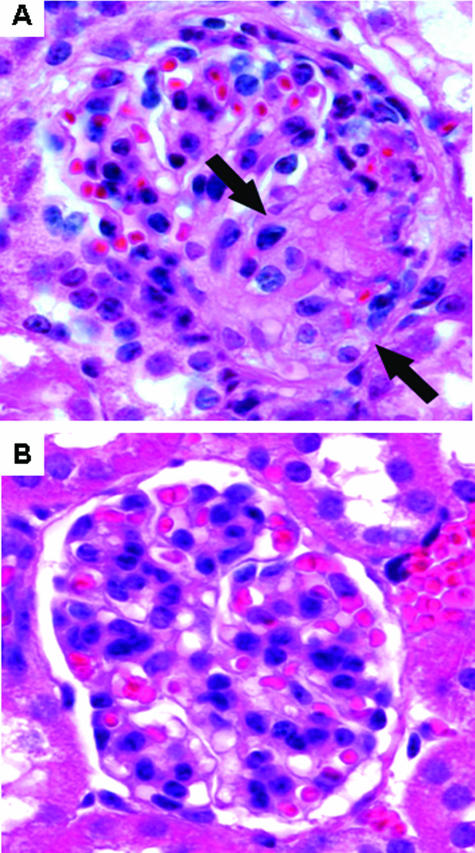
To confirm the observation that complement plays a role in anti-MPO-induced NCGN, C5-deficient mice (n = 7) were injected with anti-MPO IgG and compared with WT mice (n = 8) injected with the same dose of anti-MPO IgG. By day 6 after receiving anti-MPO antibodies, all WT mice developed albuminuria, leukocyturia, and hematuria, whereas none of the C5−/− mice that received anti-MPO developed significant albuminuria, leukocyturia, and hematuria (Figure 7, A and B). All eight WT mice sacrificed 6 days after injecting anti-MPO IgG developed glomerulonephritis with crescents (mean 13% of glomeruli with crescents) and necrosis (mean 5% of glomeruli with necrosis) (Figures 7C and 8A). In contrast, none of the C5−/− mice injected with the same dose of anti-MPO IgG had glomerular necrosis or crescents (Figures 7C and 8B). At day 6, immunofluorescence microscopy of glomeruli showed a paucity of glomerular IgG and C3 in WT mice and no IgG or C3 staining in C5−/− mice; and ELISA showed similarly high levels of circulating anti-MPO IgG in both WT and C5-deficient mice (data not shown).
Figure 7.
Lack of C5 blocked induction of NCGN by anti-MPO IgG. WT (n = 8) and C5−/− (n = 7) mice were injected intravenously with anti-MPO IgG. Six days after injection, urine and kidney samples were taken and examined. A and B: Urine albumin was determined by ELISA (A), and leukocyturia and hematuria were analyzed by dipsticks and expressed as the mean on a scale of 0 (none) to 4 (severe) (B). C: The extent of glomerular crescents and necrosis were expressed as the mean percentage of glomeruli with necrosis and crescents. Bars represent the SD. *P < 0.004.
Figure 8.
Six days after injection of anti-Mpo IgG, histological examination revealed glomerular necrosis and crescents (arrows) in all WT mice (A) but no glomerular lesion in C5−/− mice that received the same dose of anti-MPO IgG (B). H&E stain.
The Alternative Pathway but Not the Classic or Lectin-Binding Pathway Is Required for Induction of NCGN by Anti-MPO IgG
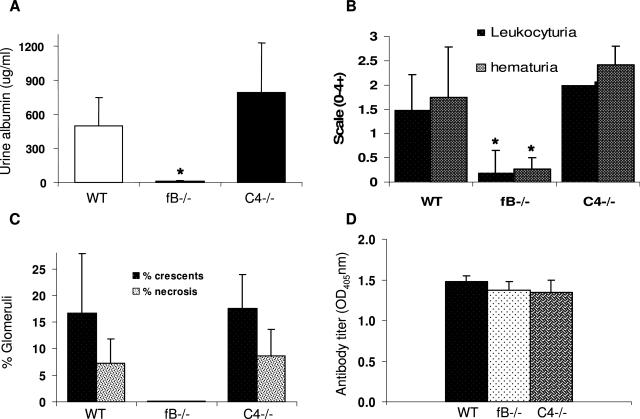
To gain insight into the relative roles of the three different pathways of complement activation in the mediation of anti-MPO induced NCGN, we evaluated the induction of NCGN by anti-MPO IgG in mice deficient in C4 or factor B (fB). C4 is required for activation through both the classic pathway and the lectin-binding pathway; whereas fB is required for activation through the alternative pathway. WT (n = 8), C4−/− (n = 6), and fB−/− (n = 8) were injected intravenously with the same nephritogenic dose of anti-MPO IgG. Six days after injection, all WT mice and C4−/− mice developed albuminuria, leukocyturia, and hematuria; however, none of the fB−/− mice developed urine abnormalities outside the reference range (Figure 9, A and B). Histological examination of glomeruli on day 6 after injection of anti-MPO IgG revealed that all WT and C4−/− mice had glomerular necrosis and crescents of similar severity (Figures 9C and 10). Crescents involved an average of 16.7% of glomeruli in WT mice and 17.6% in C4−/− mice. Necrosis involved an average of 7.3% of glomeruli in WT mice and 8.7% in C4−/− mice. In striking contrast, none of the fB−/− mice that received the same dose of anti-MPO IgG developed renal lesions (Figures 9C and 10). Anti-MPO IgG serum levels in WT, C4−/−, and fB−/− mice determined by ELISA were the same in all three groups of mice on day 6 (Figure 9D).
Figure 9.
Mice deficient in fB but not C4 were resistant to anti-MPO IgG-induced NCGN. WT (n = 8), fB−/− (n = 8), and C4−/− (n = 6) mice were injected intravenously with anti-MPO IgG and examined 6 days later. A and B: WT and C4−/− mice that received anti-MPO IgG showed albuminuria, leukocyturia, and hematuria, but fB−/− mice that received anti-MPO antibody had no urine abnormalities. Crescents and necrosis were observed in glomeruli of all WT and C4−/− mice that received anti-MPO IgG. No glomerular injury was seen in fB−/− mice injected with same amount of anti-MPO IgG. C: The extent of glomerular damage was expressed as the mean percentage of glomeruli with crescents and necrosis. D: Circulating anti-MPO antibody titers determined by ELISA were similar in WT, fB−/−, and C4−/− mice. Bars represent the SD. *P < 0.005.
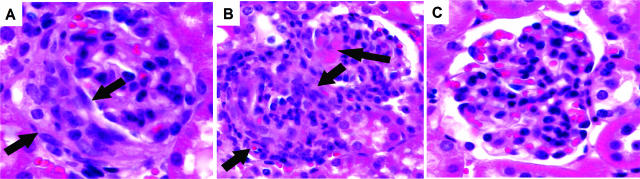
Figure 10.
A and B: Crescents and necrosis were observed in glomeruli of all WT mice (A) and all C4−/− mice (B) that received anti-MPO IgG (arrows). C: No glomerular injury was seen in fB−/− mice injected with same amount of anti-MPO IgG. H&E stain.
Stimulation of Human Neutrophils by ANCA IgG Causes Complement Activation
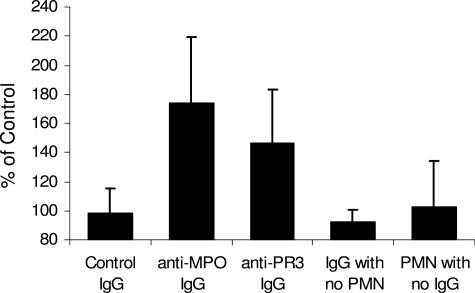
We hypothesized that activation of neutrophils by ANCA IgG results in the release of factors that cause complement activation, which in turn amplifies the recruitment and activation of more neutrophils. To test this hypothesis, we incubated TNF-α-primed normal human neutrophils with IgG isolated from patients with MPO-ANCA (anti-MPO) (n = 6) or PR3-ANCA (anti-PR3) (n = 8), or IgG from ANCA-negative healthy controls (n = 8). After 15 minutes, the suspensions were centrifuged and the supernatants from the reaction mixtures were added to normal human serum from the donor of the neutrophils. Complement activation was determined by ELISA measurement of C3a generation. Figure 11 demonstrates that anti-MPO IgG and anti-PR3 IgG but not control IgG induced neutrophils to release factors that caused complement activation with generation of C3a. An analysis of variance comparing the ranked values of the three groups gave a P value of 0.0016. Using a Duncan’s multiple range test both the MPO-ANCA and PR3-ANCA groups had statistically greater complement activation than the control group; however, the MPO-ANCA group was not different from the PR3-ANCA group. A nonparametric version of the analysis of variance (Kruskal-Wallis test) gave a P value of 0.0058, which further confirmed the differences between the groups. Anti-MPO IgG, anti-PR3 IgG, and control IgG in the absence of neutrophils did not cause C3a generation (Figure 11). TNF-α-primed neutrophils incubated under the same conditions in the absence of any IgG did not release factors that activated complement. Thus, ANCA-induced activation of human neutrophils results in the release of factors that cause complement activation.
Figure 11.
Incubation of TNF-α-primed normal human neutrophils with human anti-MPO IgG or anti-PR3 IgG caused release of factors that caused complement activation in normal serum as detected by generation of C3a. Normal TNF-α-primed neutrophils were first incubated with IgG and then the supernatant was reacted with normal serum and the activation of complement measured by C3a ELISA. C3a generation is expressed as a percentage of the mean of the results for control IgG. Anti-MPO IgG and anti-PR3 IgG caused C3a generation at 173.3 and 146.4%, respectively, compared with control IgG. The normal control replicate assays averaged 98.2%. The C3a generation by both anti-MPO and anti-PR3 ANCA was statistically significant compared with control (P = 0.0016). No C3a generation was caused by anti-MPO IgG, anti-PR3 IgG, or normal control IgG alone; or by TNF-α-primed neutrophils in the absence of IgG.
Discussion
Microscopic polyangiitis, Wegener’s granulomatosis, Churg-Strauss syndrome, and renal limited NCGN are manifestations of ANCA-associated small vessel vasculitis and glomerulonephritis.30 The acute pathological lesions in each of these expressions of ANCA disease are characterized by extensive neutrophil infiltration, leukocytoclasia, and necrosis.18,19,30 As demonstrated in the current and previous studies,4,6,7 vascular and glomerular inflammation and necrosis that is virtually identical to human ANCA disease is produced by administration of anti-MPO IgG to mice. Neutrophils are required for the induction of this injury.7 In this study, we have demonstrated that induction of glomerulonephritis with anti-MPO IgG or anti-MPO splenocytes requires the alternative complement pathway but not the classic pathway or the lectin pathway.
The complement system complements the mediation of inflammation through three pathways that are initiated by diverse stimuli and augment inflammation initiated both by the innate and adaptive immune responses.21 The classic pathway amplifies injury initiated by antibody binding to antigen, and thus interfaces with adaptive immune responses. The classic pathway also can be activated by innate immune responses through interaction with C-reactive protein.22 The lectin pathway is activated by pattern recognition of carbohydrate ligands, such as mannose, that are expressed by infectious pathogens. The classic and lectin pathways are unidirectional and thus are not self-sustaining if the stimulus is eliminated. In contrast, the alternative pathway is self-sustaining and thus acts as an amplification loop for inflammation that continues until it is down-regulated by specific control proteins, such as factor H and factor I.23 The alternative pathway begins with amplification of constitutive low-level C3 autoactivation, called “tickover,” which occurs normally and is held in check by control proteins unless an added impetus fully activates the amplification loop.
Activation of the classic, lectin, and alternative complement pathways all result in the conversion of C3 to C3b and C3a.21 The classic and lectin pathways generate the same C3 convertase (C4bC2b), whereas the alternative pathway generates a different C3 convertase (C3bBbP). Likewise, the classic and lectin pathways generate the same C5 convertase (C3bC4bC2b), whereas the alternative pathway generates a different C5 convertase (C3b2BbP). Thus, all three pathways converge at the activation of C5 to form the potent chemoattractant C5a and the membrane attack complex C5b-9. Thus C3 and C5 are required for activation of complement by each of the three pathways.
In both the anti-MPO IgG and anti-MPO splenocyte transfer mouse models of ANCA disease, pretreatment with Cobra venom factor, which causes extensive depletion of C3,31 completely blocked the induction of NCGN (Table 1). Likewise C5−/− mice were completely protected from the induction of NCGN by anti-MPO IgG (Figure 7). The absence of complement activation not only prevented the induction of glomerular necrosis and crescents, but also abrogated the marked influx of neutrophils and monocytes that is typical of this model (Table 1), probably because of blockade of generation of C5a, which is a potent chemoattractant and activator of neutrophils. Rather than total absence of complement-activating capacity, there may be a critical level of complement depletion below which lesions do not develop. Future studies will be needed to determine how much depression of complement activation is required to completely block disease induction.
Both the classic and lectin pathways require activation of C4 to produce C3 convertase (C4bC2b) and C5 convertase (C3bC4bC2b). C4−/− mice are unable to generate these convertases and thus cannot activate C3 or C5. Nevertheless, there was no protection of C4−/− mice from the induction of NCGN by anti-MPO IgG (Figure 9). This observation indicates that neither the classic nor lectin pathways of complement activation are required for the induction of this model of ANCA NCGN. This observation also substantiates the contention that ANCA disease is not a form of typical immune complex mediated inflammation, which involves activation of complement via the classic or alternative pathways.21,32 In marked contrast, the absence of the alternative pathway component factor B completely protected fB−/− mice from induction of NCGN by anti-MPO IgG (Figure 9). This requirement for factor B in the absence of a requirement for C4 indicates that the mediation of the inflammation involves the alternative pathway but not the classic or lectin pathways.
Based on the evidence reviewed in the introduction that ANCA IgG activates cytokine-primed neutrophils8–17 and reports in the literature that activated neutrophils release factors that can activate the alternative complement pathway,24–27 we hypothesized that activation of neutrophils by ANCA IgG causes the release of factors that activate complement. This hypothesis was substantiated by demonstrating that activation of human neutrophils by human MPO-ANCA or PR3-ANCA IgG releases factors that activate complement with the generation of C3a (Figure 11). These studies do not identify what factor or factors are responsible for the complement activation, but a number of candidates are suggested by the literature, including reactive oxygen radicals,24 MPO,25 proteases,26 and properdin.27 Properdin is synthesized by neutrophils and stored in secondary granules that are released on activation.33 C5a stimulates properdin release from neutrophils.32 Thus, ANCA activation of neutrophils could set in motion a cycle of properdin release with C5a generation that in turn causes more neutrophil recruitment, activation, and properdin release to perpetuate the cycle.
Once the alternative pathway is activated, it is self-sustaining and thus acts as an amplification loop for inflammation that continues until it is down-regulated by specific control proteins, such as factor H and factor I.23 In a microenvironment containing immune complexes, eg, ANCA antibodies bound to ANCA antigens, C3b can associate with the Fc region of immunoglobulins and be protected from regulatory proteins,34 which could further enhance the amplification of inflammatory injury at sites of ANCA disease.
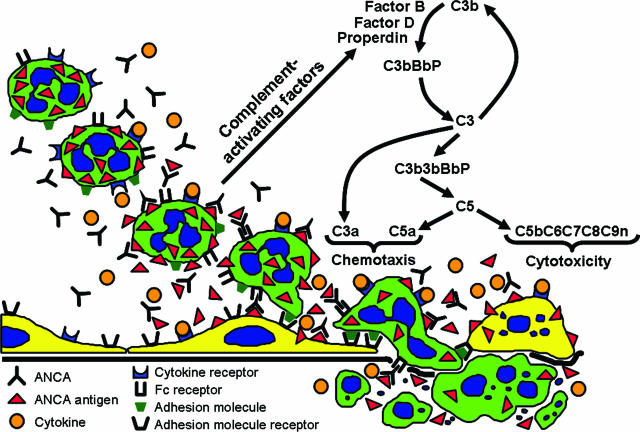
In conclusion, supported by the observations reported in this article, we propose that stimulation of neutrophils by ANCA IgG caused the release of factors that activate complement via the alternative pathway, which initiates an inflammatory amplification loop that mediates the severe necrotizing inflammation of ANCA disease (Figure 12).
Figure 12.
Diagram depicting a putative pathogenic mechanism for ANCA glomerulonephritis and vasculitis. Beginning in the top left, neutrophils are primed by cytokines to express more ANCA antigens (MPO and PR3) at the surface where they can interact with ANCA antibodies. This results in neutrophil activation both by Fc receptor engagement and Fab′2 binding. ANCA-activated neutrophils release factors (eg, properdin, proteases, oxygen radicals, and MPO) that activate the alternative complement pathway with the generation of the powerful neutrophil chemoattractant C5a and the membrane attack complex C5b-9. This complement activation amplifies neutrophil influx, neutrophil activation, and vessel damage, resulting in the aggressive necrotizing inflammation of ANCA disease.
Acknowledgments
We thank Dr. Susan Hogan for statistical analysis, Dr. Jiajin Yang for ANCA IgG, and Dr. Bei Zhang and Ms. Jue Yao for expert technical assistance.
Footnotes
Address reprint requests to J. Charles Jennette, M.D., Brinkhous Distinguished Professor and Chair, Department of Pathology and Laboratory Medicine, 303 Brinkhous-Bullitt Building, University of North Carolina, Chapel Hill, NC 27599-7525. E-mail: jcj@med.unc.edu.
Supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases grant 2 P01 DK58335-06). A.S. was supported by a fellowship grant from the Deutsche Forschungsgemeinschaft (SCHR 771/1-1).
References
- Jennette JC, Xiao H, Falk RJ. The pathogenesis of vascular inflammation by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235–1242. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunology. 2004;93:398–401. doi: 10.1016/S1081-1206(10)61400-7. [DOI] [PubMed] [Google Scholar]
- Schlieben DJ, Korbet SM, Kimura RE, Schwartz MM, Lewis EJ. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45:758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD. Anti-neutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106:2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol. 2005;167:47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk RJ, Terrell R, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leuk Biol. 1991;50:539–546. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994;153:1271–1280. [PubMed] [Google Scholar]
- Mulder AH, Heeringa P, Brouwer E, Limburg PC, Kallenberg CG. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994;98:270–278. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettritz R, Jennette JC, Falk RJ. Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol. 1997;8:386–394. doi: 10.1681/ASN.V83386. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ben-Smith A, Hewins P, Dove SK, Hughes P, McEwan R, Wakelam MJ, Savage CO. Activation of the G(i) heterotrimeric G protein by ANCA IgG F(ab′)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol. 2003;14:661–669. doi: 10.1097/01.asn.0000050223.34749.f4. [DOI] [PubMed] [Google Scholar]
- Radford DJ, Savage CO, Nash GB. Treatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum. 2000;43:1337–1345. doi: 10.1002/1529-0131(200006)43:6<1337::AID-ANR16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Radford DJ, Luu NT, Hewins P, Nash GB, Savage CO. Antineutrophil cytoplasmic antibodies stabilize adhesion and promote migration of flowing neutrophils on endothelial cells. Arthritis Rheum. 2001;44:2851–2861. doi: 10.1002/1529-0131(200112)44:12<2851::aid-art473>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- Savage CO, Pottinger BE, Gaskin G, Pusey CD, Pearson JD. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992;141:335–342. [PMC free article] [PubMed] [Google Scholar]
- Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135:921–930. [PMC free article] [PubMed] [Google Scholar]
- Jennette JC. Implications for pathogenesis of patterns of injury in small and medium-sized vessel vasculitis. Cleveland Clinic J Med. 2002;69:SII33–SII38. doi: 10.3949/ccjm.69.suppl_2.sii33. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Ward PA. Complement in ischemia reperfusion injury. Am J Pathol. 2003;162:363–367. doi: 10.1016/S0002-9440(10)63830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelen MA, Roos A, Daha MR. Role of complement in innate and autoimmunity. J Nephrol. 2005;18:642–653. [PubMed] [Google Scholar]
- Agrawal A. CRP after 2004. Mol Immunol. 2005;42:927–930. doi: 10.1016/j.molimm.2004.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- Shingu M, Nonaka S, Nishimukai H, Nobunaga M, Kitamura H, Tomo-Oka K. Activation of complement in normal serum by hydrogen peroxide and hydrogen peroxide-related oxygen radicals produced by activated neutrophils. Clin Exp Immunol. 1992;90:72–78. doi: 10.1111/j.1365-2249.1992.tb05834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt W. Complement activation by myeloperoxidase products released from stimulated human polymorphonuclear leukocytes. Immunobiology. 1996;195:334–346. doi: 10.1016/S0171-2985(96)80050-7. [DOI] [PubMed] [Google Scholar]
- Venge P, Olsson I. Cationic proteins of human granulocytes. VI. Effects on the complement system and mediation of chemotactic activity. J Immunol. 1975;115:1505–1508. [PubMed] [Google Scholar]
- Wirthmueller U, Dewald B, Thelen M, Schafer MK, Stover C, Whaley K, North J, Eggleton P, Reid KB, Schwaeble WJ. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158:4444–4451. [PubMed] [Google Scholar]
- Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci USA. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ. Small vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- Cochrane CG, Muller-Eberhard HJ, Aikin BS. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970;105:55–69. [PubMed] [Google Scholar]
- Hisano S, Matsushita M, Fujita T, Iwasaki H. Activation of the lectin complement pathway in Henoch-Schonlein purpura nephritis. Am J Kidney Dis. 2005;45:295–302. doi: 10.1053/j.ajkd.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Schwaeble WJ, Reid KB. Does properdin crosslink the cellular and the humoral immune response? Immunol Today. 1999;20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- Lutz HU, Jelezarova E. Complement amplification revisited. Mol Immunol. 2006;43:2–12. doi: 10.1016/j.molimm.2005.06.020. [DOI] [PubMed] [Google Scholar]