Abstract
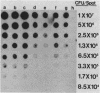
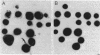
Two rapid DNA hybridization methods in which whole-cell lysates fixed to nitrocellulose were used were compared with Southern hybridization of purified plasmid or chromosomal DNA for the ability to identify the 2"-O-adenylyltransferase [ANT(2")] gene in 42 enzymatically defined isolates of gram-negative bacilli. A DNA restriction fragment isolated from an ANT(2") gene cloned into pBR322 and radiolabeled with 32P was used as the probe in all three procedures. Under conditions of high stringency, agreement was obtained between the Southern hybridization method and detection of the ANT(2") enzyme by the phosphocellulose paper binding assay or resistance phenotype in 39 of the 42 strains tested. By using these characterized strains, colony hybridization was shown to be unsatisfactory as a rapid technique for detecting the ANT(2") gene, due to the high number of false-positive and -negative signals obtained. Compared with Southern hybridization, however, spot hybridization (SPH) proved highly reliable for detecting the ANT(2") gene in both members of Enterobacteriaceae and Pseudomonas aeruginosa harboring R factors ranging in size from 23 to 150 kilobases. The relatively low copy number of the 150-kilobase plasmids decreased the sensitivity of SPH, necessitating a minimum cell density of 5 X 10(6) cells per spot. SPH proved to be a very useful method for rapidly screening large numbers of clinical isolates for this resistance determinant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M. A., Miller J. R., Summersgill J., Melo J., Raff M. J., Streips U. N. R-factor responsible for an outbreak of multiply antibiotic-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 1980 Dec;18(6):926–929. doi: 10.1128/aac.18.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Benveniste R. E. Enzymes that inactivate antibiotics in transit to their targets. Ann N Y Acad Sci. 1974 May 10;235(0):130–136. doi: 10.1111/j.1749-6632.1974.tb43262.x. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Kato T., Sato Y., Iyobe S., Mitsuhashi S. Plasmid-mediated gentamicin resistance of Pseudomonas aeruginosa and its lack of expression in Escherichia coli. Antimicrob Agents Chemother. 1982 Sep;22(3):358–363. doi: 10.1128/aac.22.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Ross D. G., Guzman M. A., Medeiros A. A., Hedges R. W., Botstein D. Dissemination of an antibiotic resistance plasmid in hospital patient flora. Antimicrob Agents Chemother. 1980 Apr;17(4):537–543. doi: 10.1128/aac.17.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., SMITH S. M. Transmission of colicinogeny between strains of Salmonella typhimurium grown together. J Gen Microbiol. 1962 Sep;28:671–687. doi: 10.1099/00221287-28-4-671. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. S., Jacoby G. A. Cloning the gentamicin resistance gene from a Pseudomonas aeruginosa plasmid in Escherichia coli enhances detection of aminoglycoside modification. Antimicrob Agents Chemother. 1982 Sep;22(3):525–526. doi: 10.1128/aac.22.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., McNeill W. F., Farrar W. E., Jr Transposable plasmid deoxyribonucleic acid sequence in Pseudomonas aeruginosa which mediates resistance to gentamicin and four other antimicrobial agents. J Bacteriol. 1979 Sep;139(3):877–882. doi: 10.1128/jb.139.3.877-882.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H. Gentamicin:adenine mononucleotide transferase: partial purification, characterization, and use in the clinical quantitation of gentamicin. J Infect Dis. 1974 Apr;129(4):391–401. doi: 10.1093/infdis/129.4.391. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taub F., Thompson E. B. An improved method for preparing large arrays of bacterial colonies containing plasmids for hybridization: in situ purification and stable binding of DNA on paper filters. Anal Biochem. 1982 Oct;126(1):222–230. doi: 10.1016/0003-2697(82)90133-6. [DOI] [PubMed] [Google Scholar]
- Tenover F. C., Gootz T. D., Gordon K. P., Tompkins L. S., Young S. A., Plorde J. J. Development of a DNA probe for the structural gene of the 2"-O-adenyltransferase aminoglycoside-modifying enzyme. J Infect Dis. 1984 Nov;150(5):678–687. doi: 10.1093/infdis/150.5.678. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Plorde J. J., Falkow S. Molecular analysis of R-factors from multiresistant nosocomial isolates. J Infect Dis. 1980 May;141(5):625–636. doi: 10.1093/infdis/141.5.625. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Holmes K. K., Handsfield H. H., Knapp J. S., Perine P. L., Falkow S. DNA hybridization technique for the detection of Neisseria gonorrhoeae in men with urethritis. J Infect Dis. 1983 Sep;148(3):462–471. doi: 10.1093/infdis/148.3.462. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]