Abstract
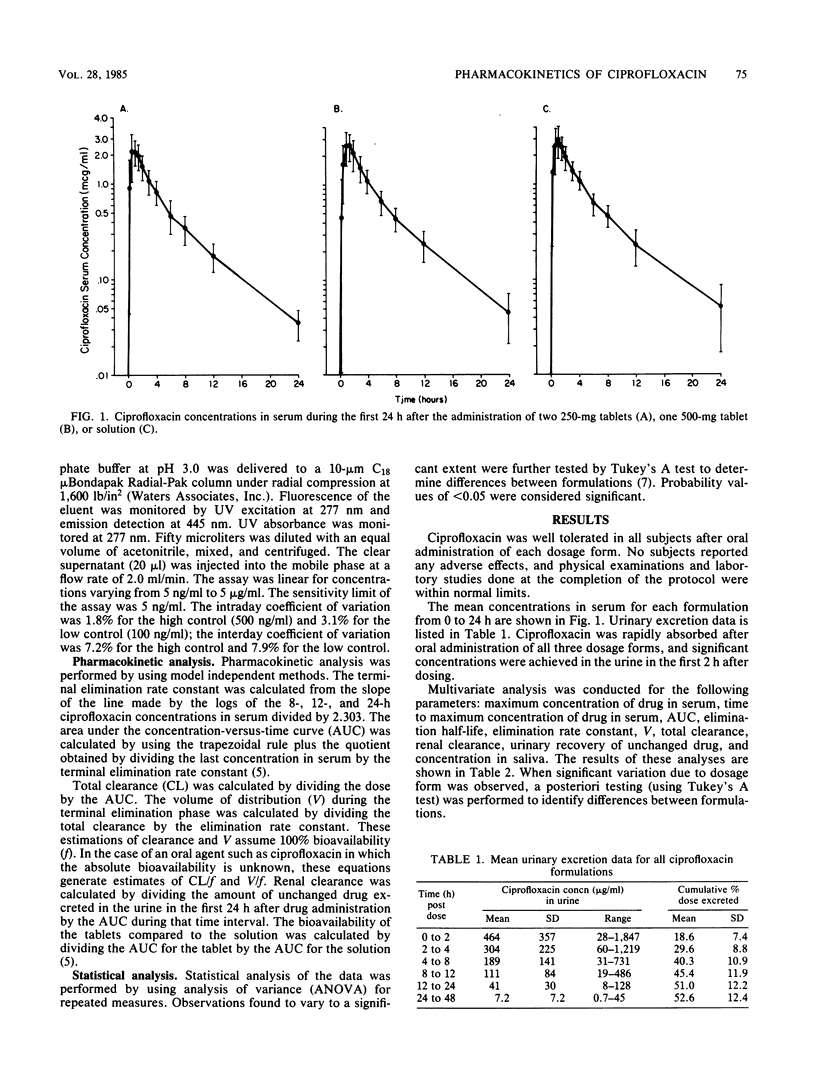
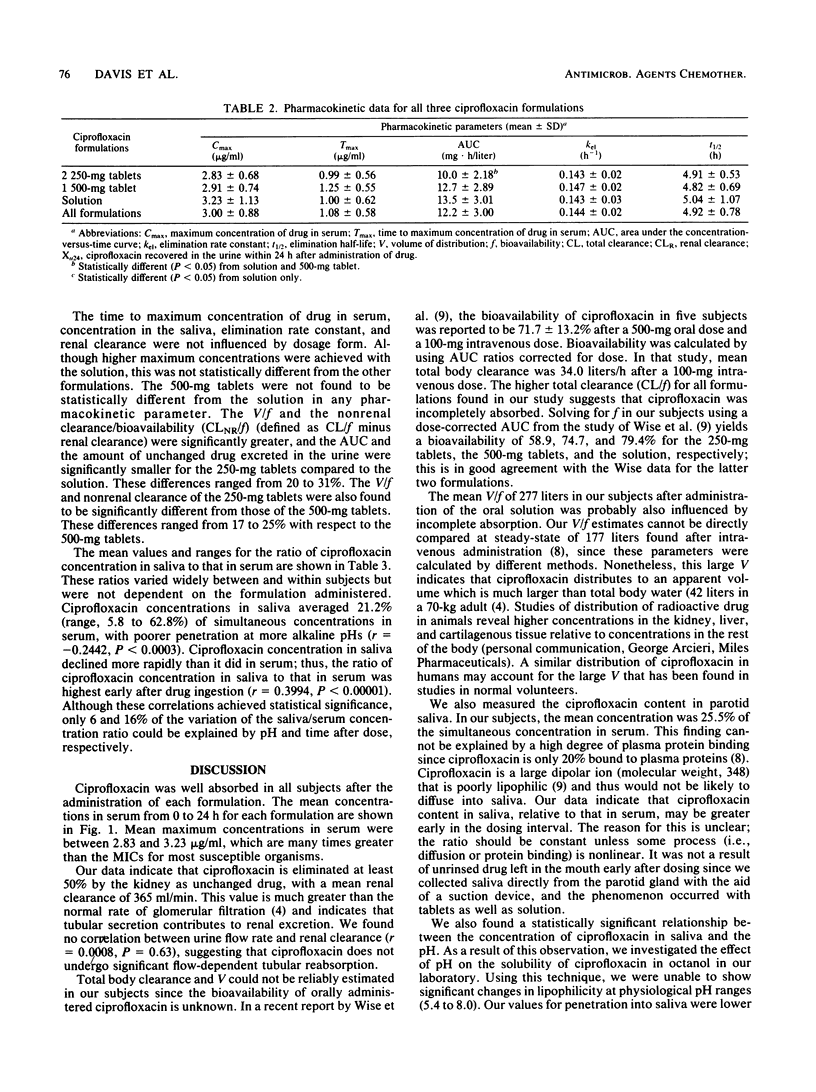
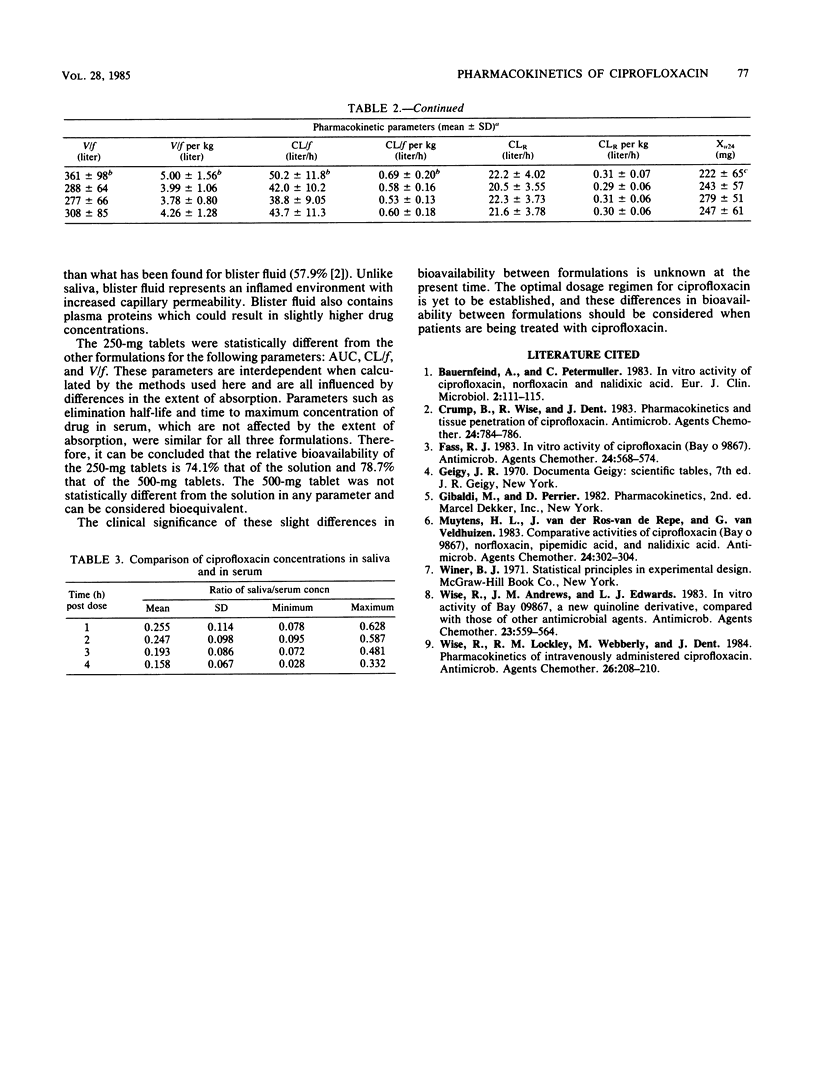
We compared the absorption of three formulations of ciprofloxacin after oral administration in 18 normal adult male volunteers. Each subject received 500 mg of ciprofloxacin as two 250-mg tablets, one 500-mg tablet, or a solution in a randomized crossover sequence. Pharmacokinetic parameters were determined by model independent methods. Because a solution is considered to be the ideal oral dosage form, the results determined for the tablets were compared to those for the solution. Mean values for the maximum concentration of drug in serum, the time to maximum concentration of drug in serum, and the elimination half-life were 3.23 micrograms/ml, 1.00 h, and 5.04 h, respectively, for the solution. The mean renal clearance of ciprofloxacin was 372 ml/min and accounted for at least 50% of the total clearance. We recovered 44.4, 48.6, and 55.8% of the administered ciprofloxacin from the urine as unchanged drug within 24 h after dosing with the 250-mg tablets, 500-mg tablets, or solution, respectively. The 500-mg tablets were found to be bioequivalent to the solution with regard to all pharmacokinetic parameters. The 250-mg tablet was not bioequivalent to either of the other formulations; the relative bioavailability values were 78.7 and 74.1%, respectively, for the 500-mg tablet and the solution. The clinical significance of this difference in bioavailability is yet to be determined.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauernfeind A., Petermüller C. In vitro activity of ciprofloxacin, norfloxacin and nalidixic acid. Eur J Clin Microbiol. 1983 Apr;2(2):111–115. doi: 10.1007/BF02001575. [DOI] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. In vitro activity of ciprofloxacin (Bay o 9867). Antimicrob Agents Chemother. 1983 Oct;24(4):568–574. doi: 10.1128/aac.24.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J., van Veldhuizen G. Comparative activities of ciprofloxacin (Bay o 9867), norfloxacin, pipemidic acid, and nalidixic acid. Antimicrob Agents Chemother. 1983 Aug;24(2):302–304. doi: 10.1128/aac.24.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]


