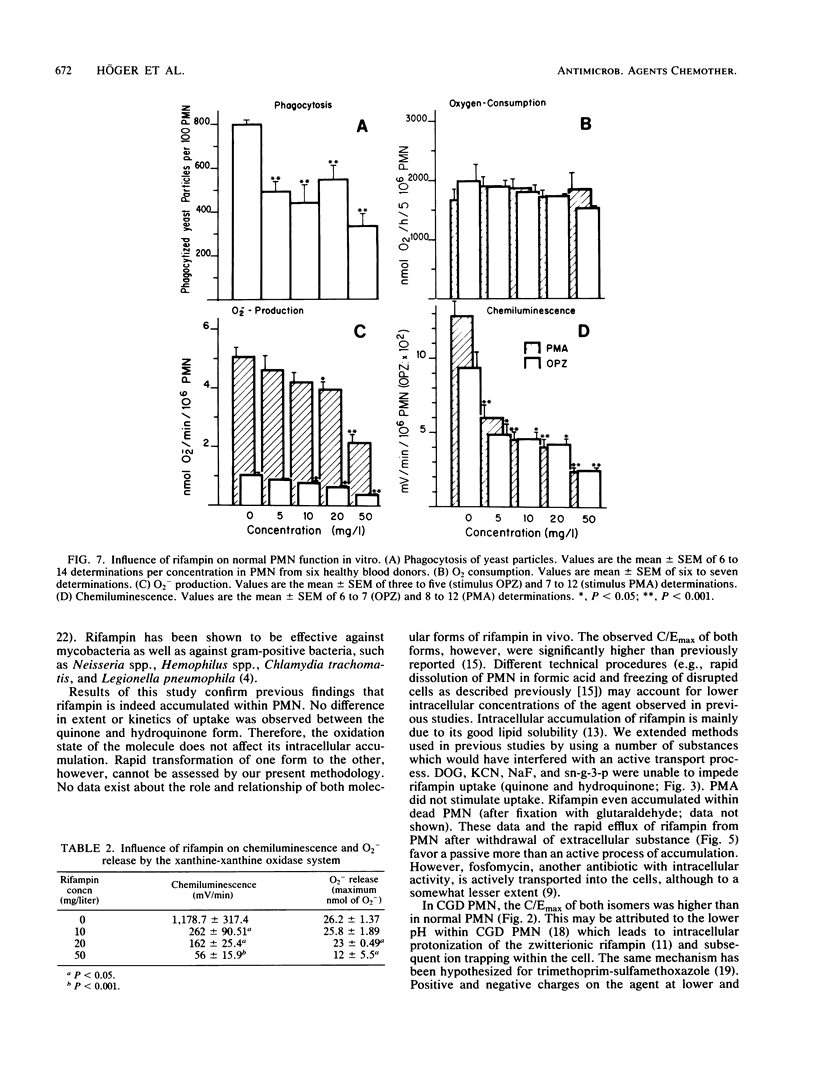
Abstract
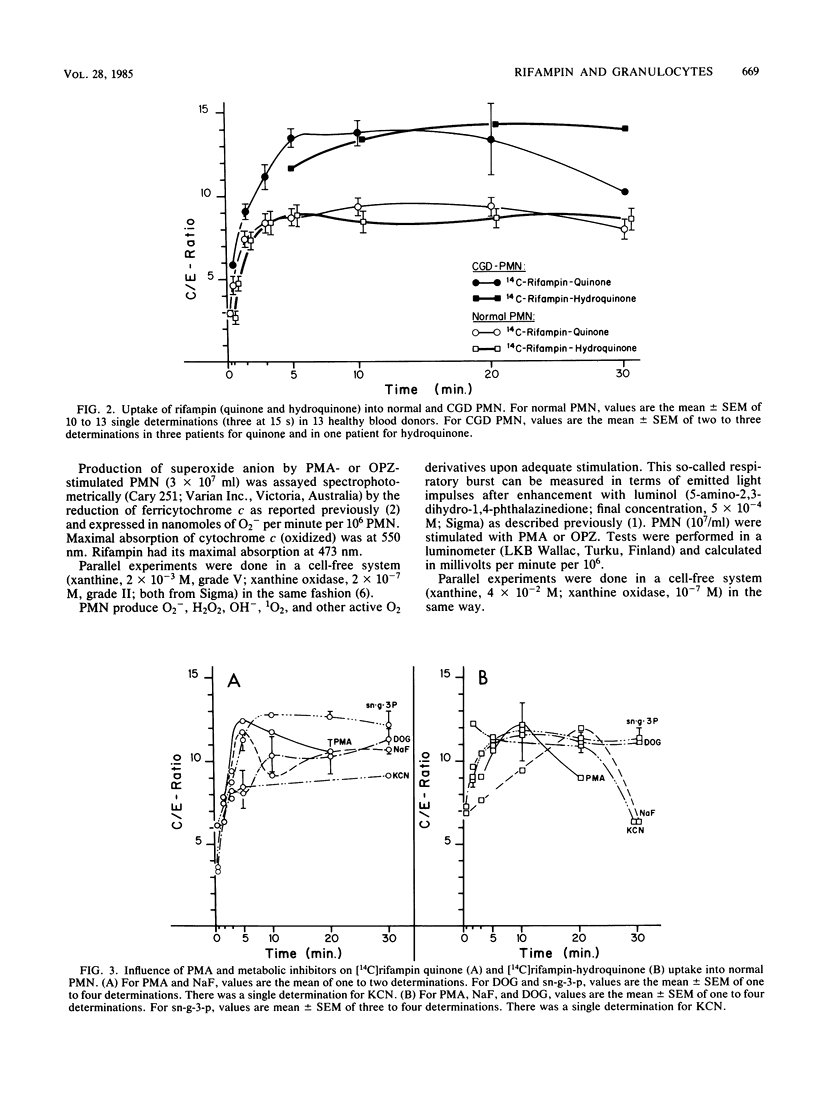
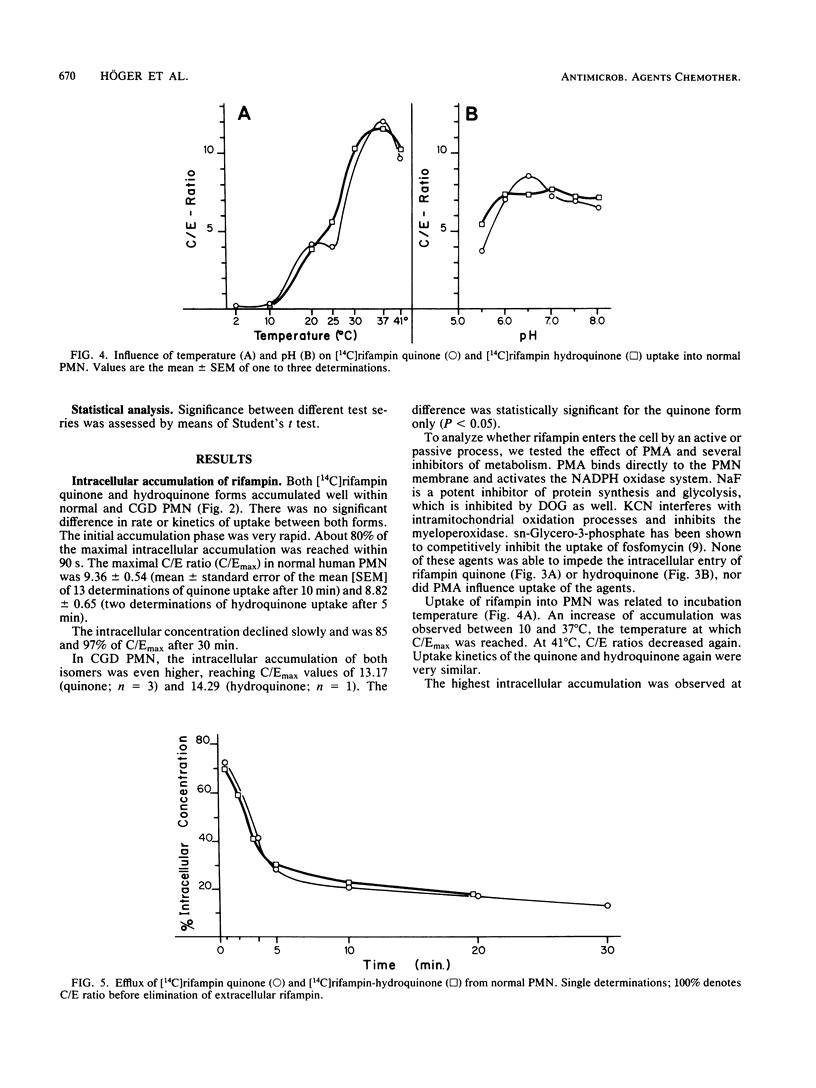
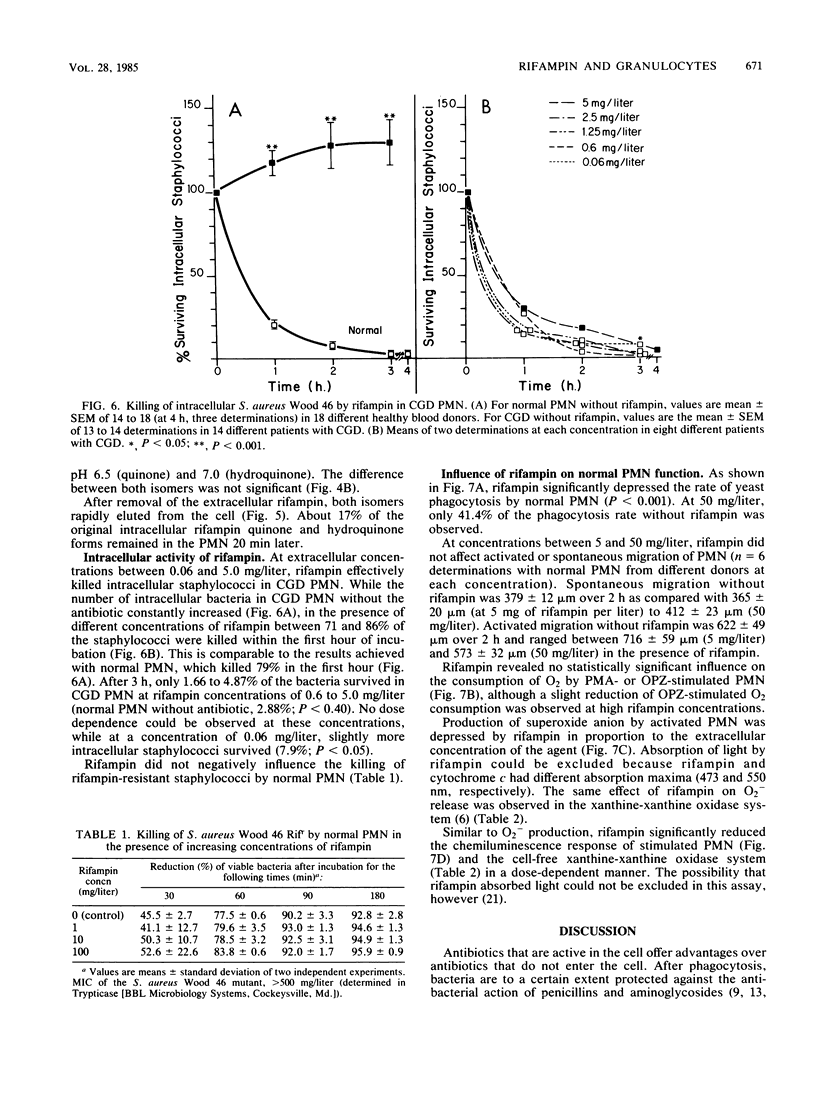
Quinone and hydroquinone forms of rifampin accumulated in normal polymorphonuclear leukocytes (PMN) (maximal cellular to extracellular concentration ratio [C/Emax] +/- standard error of the mean, 9.36 +/- 0.54 and 8.82 +/- 0.65, respectively, after 5 to 10 min) and chronic granulomatous disease PMN (C/Emax, 13.76 +/- 0.77 and 14.29, respectively). Uptake of rifampin was influenced by incubation temperature and extracellular pH but not by phorbol myristate acetate stimulation or metabolic inhibitors. At extracellular concentrations between 0.06 and 5.0 mg/liter, rifampin significantly reduced the number of staphylococci surviving inside chronic granulomatous disease PMN, thus compensating for the bactericidal defect inherent with this disease. Spontaneous migration and chemotaxis of normal PMN were unaffected by rifampin. However, phagocytosis of yeast particles and oxygen consumption of stimulated PMN were moderately depressed, and O2- production and chemiluminescence were significantly depressed in a dose-dependent manner. The bactericidal activity of normal PMN was not impaired. Inhibition of chemiluminescence and O2- release were also observed in a cell-free system. We conclude that rifampin possesses favorable characteristics for the effective elimination of intracellular microorganisms. Further studies are needed to evaluate the in vivo significance of ion scavenging by rifampin, which could be hazardous to immunocompromised patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Farr B., Mandell G. L. Rifampin. Med Clin North Am. 1982 Jan;66(1):157–168. doi: 10.1016/s0025-7125(16)31449-3. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Gnarpe H. The effect of antibacterial agents on the association between bacteria and leukocytes. Scand J Infect Dis Suppl. 1982;33:115–120. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gray G. D., Smith C. W., Hollers J. C., Chenoweth D. E., Fiegel V. D., Nelson R. D. Rifampin affects polymorphonuclear leukocyte interactions with bacterial and synthetic chemotaxins but not interactions with serum-derived chemotaxins. Antimicrob Agents Chemother. 1983 Nov;24(5):777–783. doi: 10.1128/aac.24.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeprich P. D., Martin C. H. Effect of tetracycline, polymyxin B, and rifampin on phagocytosis. Clin Pharmacol Ther. 1970 May-Jun;11(3):418–422. doi: 10.1002/cpt1970113418. [DOI] [PubMed] [Google Scholar]
- Humber D. P., Nsanzumuhire H., Aluoch J. A., Webster A. D., Aber V. R., Mitchison D. A., Girling D. J., Nunn A. J. Controlled double-blind study of the effect of rifampin on humoral and cellular immune responses in patients with pulmonary tuberculosis and in tuberculosis contacts. Am Rev Respir Dis. 1980 Sep;122(3):425–436. doi: 10.1164/arrd.1980.122.3.425. [DOI] [PubMed] [Google Scholar]
- Höger P. H., Seger R. A., Schaad U. B., Hitzig W. H. Chronic granulomatous disease: uptake and intracellular activity of fosfomycin in granulocytes. Pediatr Res. 1985 Jan;19(1):38–44. doi: 10.1203/00006450-198501000-00011. [DOI] [PubMed] [Google Scholar]
- Kenny M. T., Strates B. Metabolism and pharmacokinetics of the antibiotic rifampin. Drug Metab Rev. 1981;12(1):159–218. doi: 10.3109/03602538109011084. [DOI] [PubMed] [Google Scholar]
- Kono Y. Oxygen Enhancement of bactericidal activity of rifamycin SV on Escherichia coli and aerobic oxidation of rifamycin SV to rifamycin S catalyzed by manganous ions: the role of superoxide. J Biochem. 1982 Jan;91(1):381–395. doi: 10.1093/oxfordjournals.jbchem.a133698. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. The antimicrobial activity of rifampin: emphasis on the relation to phagocytes. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S463–S467. doi: 10.1093/clinids/5.supplement_3.s463. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W., Saito S. Influence of agents with immunomodulating activity on phagocytosis and bactericidal function of human polymorphonuclear cells. J Rheumatol. 1983 Oct;10(5):688–693. [PubMed] [Google Scholar]
- Segal A. W., Coade S. B. Kinetics of oxygen consumption by phagocytosing human neutrophils. Biochem Biophys Res Commun. 1978 Oct 16;84(3):611–617. doi: 10.1016/0006-291x(78)90749-0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Seger R. A., Baumgartner S., Tiefenauer L. X., Gmünder F. K. Chronic granulomatous disease: effect of sulfamethoxazole/trimethoprim on neutrophil microbicidal function. Helv Paediatr Acta. 1981;36(6):579–588. [PubMed] [Google Scholar]
- Serrou B. Letter: Rifampicin and immunosuppression. Lancet. 1974 Jul 20;2(7873):172–172. doi: 10.1016/s0140-6736(74)91611-0. [DOI] [PubMed] [Google Scholar]
- Siegel J. P., Remington J. S. Effect of antimicrobial agents on chemiluminescence of human polymorphonuclear leukocytes in response to phagocytosis. J Antimicrob Chemother. 1982 Dec;10(6):505–515. doi: 10.1093/jac/10.6.505. [DOI] [PubMed] [Google Scholar]
- Solberg C. O., Hellum K. B. Protection of phagocytosed bacteria against antimicrobial agents. Scand J Infect Dis Suppl. 1978;(14):246–250. [PubMed] [Google Scholar]
- Spisani S., Dovigo L., Corazza G., Carletti R., Traniello S. The effect of rifamycin SV on neutrophil functions in patients with rheumatoid arthritis. Scand J Rheumatol. 1982;11(2):65–69. doi: 10.3109/03009748209098164. [DOI] [PubMed] [Google Scholar]
- Vosbeck K., James P. R., Zimmermann W. Antibiotic action on phagocytosed bacteria measured by a new method for determining viable bacteria. Antimicrob Agents Chemother. 1984 Jun;25(6):735–741. doi: 10.1128/aac.25.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]


