Abstract
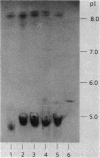
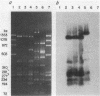
The most common cause of ampicillin resistance in Haemophilus influenzae type b is production of TEM-1 beta-lactamase; however, a novel enzyme with a similar substrate profile but a quite different isoelectric point has also been described. This beta-lactamase, designated ROB-1, has not been found previously in any other organism. In a survey of 46 ampicillin-resistant H. influenzae type b isolates, we found a second human isolate that produces ROB-1 and discovered that ampicillin-resistant isolates of the porcine pathogen Haemophilus pleuropneumoniae also produced ROB-1. In both Haemophilus species ROB-1 production was determined by plasmids that had considerable DNA sequence homology. However, the ROB-1 and TEM-1 beta-lactamase genes were not related. Our findings suggest that this form of ampicillin resistance has an animal reservoir and that conditions fostering its prevalence in animal strains may play a role in the spread of resistance to human pathogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Medeiros A. A., Cohenford M., Jacoby G. A. Genetic and biochemical properties of AER-1, a novel carbenicillin-hydrolyzing beta-lactamase from Aeromonas hydrophila. Antimicrob Agents Chemother. 1985 Apr;27(4):479–484. doi: 10.1128/aac.27.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D. C., Martin L. D., Libal M. C. Plasmid-mediated antimicrobial resistance in Haemophilus pleuropneumoniae. Am J Vet Res. 1982 Feb;43(2):269–272. [PubMed] [Google Scholar]
- Levy S. B. Playing antibiotic pool: to tally the score. N Engl J Med. 1984 Sep 6;311(10):663–665. doi: 10.1056/NEJM198409063111009. [DOI] [PubMed] [Google Scholar]
- Libal M. C., Gates C. E. Antimicrobial sensitivity patterns of Haemophilus pleuropneumoniae isolates from pigs with pneumonia. J Am Vet Med Assoc. 1982 Feb 15;180(4):399–399. [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Hedges R. W., Jacoby G. A. Spread of a "Pseudomonas-specific" beta-lactamase to plasmids of enterobacteria. J Bacteriol. 1982 Feb;149(2):700–707. doi: 10.1128/jb.149.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A., O'Brien T. F. Ampicillin-resistant Haemophilus influenzae type B possessing a TEM-type beta-lactamase but little permeability barrier to ampicillin. Lancet. 1975 Mar 29;1(7909):716–719. doi: 10.1016/s0140-6736(75)91630-x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. G., Medeiros A. A., Yolken R. H., Moxon E. R. Ampicillin treatment failure of apparently beta-lactamase-negative Haemophilus influenzae type b meningitis due to novel beta-lactamase. Lancet. 1981 Nov 7;2(8254):1008–1010. doi: 10.1016/s0140-6736(81)91214-9. [DOI] [PubMed] [Google Scholar]
- SHOPE R. E. PORCINE CONTAGIOUS PLEUROPNEUMONIA. I. EXPERIMENTAL TRANSMISSION, ETIOLOGY, AND PATHOLOGY. J Exp Med. 1964 Mar 1;119:357–368. doi: 10.1084/jem.119.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]