Abstract
Duchenne muscular dystrophy (DMD) is the most common and lethal genetic muscle disorder, caused by recessive mutations in the dystrophin gene. One of every 3,500 males suffers from DMD, yet no treatment is currently available. Genetic therapeutic approaches, using primarily myoblast transplantation and adenovirus-mediated gene transfer, have met with limited success. Adeno-associated virus (AAV) vectors, although proven superior for muscle gene transfer, are too small (5 kb) to package the 14-kb dystrophin cDNA. Here we have created a series of minidystrophin genes (<4.2 kb) under the control of a muscle-specific promoter that readily package into AAV vectors. When injected into the muscle of mdx mice (a DMD model), two of the minigenes resulted in efficient and stable expression in a majority of the myofibers, restoring the missing dystrophin and dystrophin-associated protein complexes onto the plasma membrane. More importantly, this AAV treatment ameliorated dystrophic pathology in mdx muscle and led to normal myofiber morphology, histology, and cell membrane integrity. Thus, we have defined minimal functional dystrophin units and demonstrated the effectiveness of using AAV to deliver the minigenes in vivo, offering a promising avenue for DMD gene therapy.
Duchenne muscular dystrophy (DMD) is an X-linked genetic muscle disease affecting 1 of every 3,500 males born (1–3). The progressive muscle degeneration and weakness usually confine the patients to wheelchairs by their early teens and lead to death by their early twenties. DMD is caused by recessive mutations in the dystrophin gene, the largest gene known to date, which spans nearly 3 million bp on the X-chromosome (2) with a high rate of de novo mutations. Dystrophin is an enormous rod-like protein (427 kDa) localized beneath the inner surface of myofiber plasma membrane in both skeletal and cardiac muscles (3). Dystrophin functions through four major structural domains. The N-terminal domain binds to the F-actin of cytoskeletal structures, whereas the C-terminal cysteine-rich (CR) domain along with the distal C terminus (CT), anchors to the plasma membrane via dystrophin-associated protein (DAP) complexes. The central rod domain contains 24 triple-helix rod repeats and four hinges (4). Thus, dystrophin crosslinks and stabilizes the muscle cell membrane and cytoskeleton. The absence of a functional dystrophin results in the loss of DAP complexes and causes instability of myofiber plasma membrane. These deficiencies in turn lead to chronic muscle damage and degenerative pathology.
Because of the lack of effective treatment for DMD, novel genetic approaches including cell therapy and gene therapy have been actively explored. However, clinical trials of myoblast transplantation have met with little success, owing to the poor survival of the transplanted cells (5). Gene therapy as an alternative strategy has been extensively studied in animal models. Somatic gene transfer using both nonviral DNA vectors carrying dystrophin cDNA (6) and RNA/DNA oligonucleotides (7) achieved transgene expression but with very limited efficiency. Adenovirus-based vectors have been successfully tested in dystrophic animal models (8, 9). Nonetheless, the immunogenicity and inefficiency of infecting mature muscle cells remain major hurdles to overcome before the vector can be safely used in humans.
Adeno-associated virus (AAV) vectors are the only viral vector system that is based on a nonpathogenic and replication defective virus (10). AAV vectors have been successfully used to establish efficient and long-term gene expression in vivo in a variety of tissues without significant immune response or toxicity (11–14). Unlike other viral and nonviral vectors, AAV readily bypasses extracellular barriers because of its small viral particle size (20 nm) that facilitates efficient transduction of muscle myofibers of various maturity (15). Currently, AAV vectors offer the best gene transfer efficiency and longevity among all viral and nonviral vectors tested in muscle tissues. The unparalleled efficiency and safety have led to an increasing interest in AAV-mediated gene therapy for genetic muscle disorders (16–18) as well as for metabolic diseases. However, until recently (19, 20) a major obstacle for AAV vectors is the limited packaging size that only allows for genes smaller than 4.5 kb (13, 17, 21, 22), therefore precluding such a large gene as dystrophin with a cDNA of 14 kb. Here we have created miniature versions of dystrophin genes ideal for AAV vector-mediated DMD gene therapy.
Materials and Methods
Construction of Minidystrophin Genes and AAV Vector Production.
Minidystrophin constructs were made mainly by the PCR cloning method using Pfu polymerase (Stratagene) and human dystrophin cDNA (GenBank NM 004006) as the template. For consistency, the numbering of the nucleotide only includes the 11,058 bp dystrophin protein coding sequence. As depicted in Fig. 1, minigene Δ3849 contains nucleotides 1–1668 (N terminus, hinge 1, and rods 1 and 2), 8059–10227 (rods 22, 23, and 24, hinge 4, and CR domain), and 11047–11058 (the last 3 aa of dystrophin). Similarly, minigene Δ3990 contains nucleotides 1–1668, 7270–7410 (hinge 3), 8059–10227, and 11047–11058. Finally, minigene Δ4173 contains nucleotides 1–1992 (N terminus, hinge 1, and rods 1, 2, and 3), 8059–10227, and 11047–11058. The above constructs were made by blunt-end ligation of the Pfu-amplified PCR products of each individual segment, so that all of the protein coding sequences are precisely spliced together in-frame. The minidystrophin genes then were subcloned into an AAV vector plasmid containing a muscle-specific creatine kinase (MCK) promoter, a 595-bp HindIII/BstEII fragment from plasmid p(+enh206)358MCKCAT (23), and a 60-bp small poly(A) signal sequence (24), resulting in vector constructs AAV-MCK-Δ3849, AAV-MCK-Δ3990, and AAV-MCK-Δ4173. Similarly, the minigenes also were cloned into an AAV vector plasmid containing a cytomegalovirus (CMV) promoter (620 bp) and the small poly(A) signal sequence to generate vector constructs AAV-CMV-Δ3849 and AAV-CMV-Δ3990.
Figure 1.
Construction of highly truncated minidystrophin genes. Dystrophin has four major domains: the N-terminal domain (N), the CR domain, the CT domain, and the central rod domain, which contains 24 rod repeats (R) and four hinges (H). The minidystrophin genes were constructed by deleting a large portion of the central rods and hinges and nearly the entire CT domain (except the last 3 aa). The minidystrophin genes subsequently were cloned between an MCK promoter (or a CMV promoter) and a poly(A) sequence in AAV vectors.
The recombinant viral vector stocks were produced precisely according to the three-plasmid cotransfection method as described by Xiao et al. (25). The AAV viral vectors were subsequently purified twice through CsCl density gradient ultracentrifugation using the previously published protocols (26). The vector titers of viral particle number were determined by DNA dot blot method (26) and were approximately 5 × 1012 to 1 × 1013 viral particles per ml.
Mice and Vector Administration.
All experiments involving animals were approved by the University of Pittsburgh Animal Care and Use Committee. The healthy mice C57/B10 and dystrophic mice mdx were purchased from The Jackson Laboratory. The 10-day-old mdx pups or 50-day-old mdx adult mice were injected into the hindleg gastrocnemius muscle with 50 μl (5 × 1010 viral particles) of different AAV minidystrophin vectors. Muscle samples were collected for examination at various time points after vector injection.
Immunofluorescent (IF) Staining.
Muscle cryosections of 8 μm thickness were immunofluorescently stained with the Mouse-on-Mouse Kit from the Vector Laboratories according to the manufacturer's protocol, except that the cryosections were immediately treated with the blocking buffer without the fixation step (27). Monoclonal antibodies against dystrophin (NCL-Dys3 and NCL-Dys2) and against α-, β-, and γ-sarcoglycans (NCL-a-SARC, NCL-b-SARC, and NCL-g-SARC) were purchased from NovoCastra Laboratories (Burlingame, CA). Muscle cell nuclei were counterstained with 0.01% 4′,6-diamidino-2-phenylindole (DAPI) (Sigma) for 10 min. Photographs were taken with a Nikon TE-300 fluorescent microscope.
In Vivo Myofiber Plasma Membrane Integrity Test.
Evans Blue dye (10 mg/ml PBS) was injected into the tail vein of C57/B10 mice, mdx mice, and AAV vector-treated mdx mice at the dose of 0.1 mg/g of body weight (28). After dye injection, mice were allowed continuous swimming for 20 min. At 15 h after Evans Blue injection, muscles were collected and cryosectioned. Evans Blue dye-positive myofibers were observed under the fluorescent microscope with rhodamine filters.
Results
Construction of Minidystrophin Genes.
To explore the feasibility of using AAV vectors for DMD gene therapy, we have devised strategies to create minidystrophin genes, which are small enough to be packaged into AAV vectors, and yet retain the essential functions needed for protecting muscle from the pathological symptoms. Previous studies on patients with mild muscular dystrophy revealed that although they endured large in-frame deletions in the central rod domain of dystrophin, those patients suffered only slight symptoms (29–31). This phenomenon suggests that a major portion of the rod domain is dispensable. In addition, transgenic studies in mdx mice showed that two deletion mutants in the CT, one lacking exons 71–74 and the other lacking exons 75–78, displayed full functions in preventing dystrophic phenotypes (32). This result suggests that the CT domain also may be dispensable. In contrast, N-terminal deletions variably impair dystrophin functions (33). Based on the above observations, we have created by rational design several minigenes, in each deleting up to 75% of the central rod domain (19 of the 24 rods; 2 of the 4 hinges) as well as nearly the CT domain (exons 71–78) (Fig. 1).
These minigenes have enabled us to re-examine a previous hypothesis that a dystrophin could not be made smaller than one-half of its full length without causing muscular dystrophies (34). Our minidystrophin genes, as small as only one-third of the 11-kb full-length dystrophin coding sequence, are significantly smaller than the 6.3-kb Becker-form minidystrophin gene (29) that was widely used in transgenic and gene therapy studies in mdx mice (8). To ensure sufficient physical flexibility of the minidystrophin protein, all of our constructs such as Δ3849 still retain at least five rod repeats (R1, R2, R22, R23, and R24) and two hinges (H1 and H4) in the central rod domain (Fig. 1). Construct Δ3990 has an additional hinge (H3), whereas construct Δ4173 contains an additional rod (R3) (Fig. 1). The rationale of deleting central portion of the rod domain while preserving both distal rod repeats in our minigenes is based on the fact that those distal repeats were naturally retained in the mild Becker muscular dystrophy patients, who had large in-frame deletions in the rod domain (29–31). The above minidystrophin genes were packaged into AAV vectors under the control of a strong promoter CMV (CMV immediate early promoter) (Fig. 1). To ensure muscle-specific expression, the minigenes also were packaged into AAV vectors under the control of an MCK promoter (23) (Fig. 1).
Restoration of DAP Complexes.
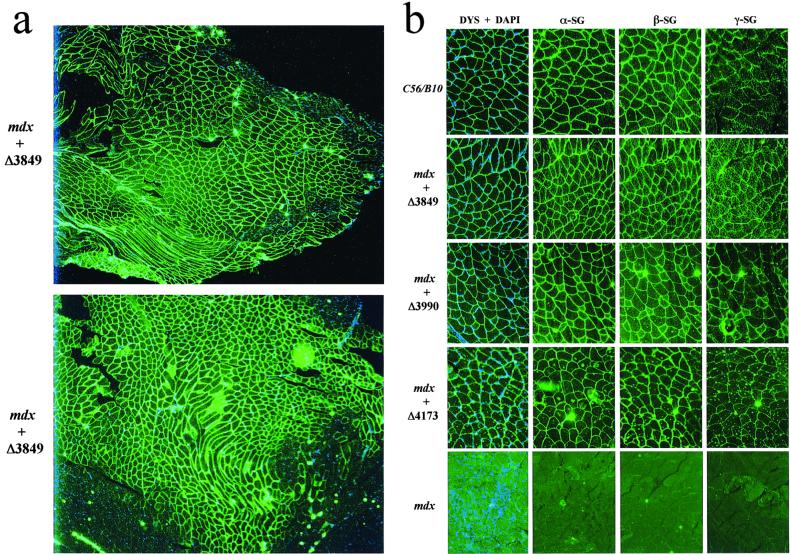
Because our minidystrophins lack nearly the entire distal CT domain, this prompted us to investigate whether those constructs still retain the major biochemical functionality including submembrane localization and interaction with DAP complexes. We initially injected the AAV MCK minidystrophin vectors into the hindleg muscle (gastrocnemius) of 10-day-old mdx mice. At 3 months and 6 months after vector injection, the muscles were collected for evaluation of minidystrophin expression and biochemical restoration of the DAP complexes, which were absent because of the primary deficiency of dystrophin. IF staining on thin sections of AAV-treated muscles, using an antibody (Dys3) specific to human dystrophin, revealed widespread vector transduction and correct submembrane location of the minidystrophins in a majority of the myofibers, especially in muscles treated with AAV vectors containing minigene Δ3849 or Δ3990 (Figs. 2 a and b and 3a). As expected, the equivalent muscle from the age-matched healthy C57/B10 mice showed an indistinguishable dystrophin staining pattern, when stained with an antibody (Dys2) that recognizes both mouse and human dystrophin CT region (Fig. 2b). As expected, this antibody (Dys2) failed to stain the AAV-treated mdx muscle because of deletion of the CT region in our minidystrophin genes (data not shown). This result further confirmed the identity of minidystrophins that were derived from the AAV vectors. Consistently, the untreated mdx control muscle showed no dystrophin staining (Fig. 2b) except the very few somatic revertant myofibers recognized by Dys2 antibody. Furthermore, injection of AAV minidystrophin vectors into the adult mdx muscle (gastrocnemius) showed similar results when examined for dystrophin expression at 2 and 4 months after injection of AAV MCK vectors (Fig. 3 c–f) or at 6 months after injection of AAV CMV vectors (Fig. 3 g and h). Importantly, there was no cytotoxic T lymphocyte destruction against the myofibers that persistently expressed minidystrophins of human origin from AAV vectors, either driven by a CMV promoter or by a muscle-specific MCK promoter.
Figure 2.
IF analysis of the dystrophin and DAP complexes in gastrocnemius muscle. (a) Cryosections of mdx muscle, at 3 months after treatment with construct AAV-MCK-Δ3849 or AAV-MCK-Δ3990, were IF-stained with an antibody against dystrophin (green) and then counterstained for cell nuclei with DAPI (blue). Photos were taken with a ×4 microscope lens. Note the widespread minidystrophin expression and peripheral nucleation in a majority of the myofibers. Also note the extensive central nucleation in minidystrophin-negative areas. (b) Cryosections of muscles from 15-week-old normal C57/B10 mice, from mdx mice treated either with vector AAV-MCK-Δ3849, AAV-MCK-Δ3990, or AAV-MCK-Δ4173, or from untreated mdx mice were IF-stained with antibodies for dystrophin (green) and counterstained with DAPI (blue) for nuclei (DYS + DAPI). Note the lack of central myonuclei. The consecutive sections also were stained with antibodies for α-sarcoglycan (α-SG), β-sarcoglycan (β-SG), and γ-sarcoglycan (γ-SG). Photographs were taken with a ×20 lens.
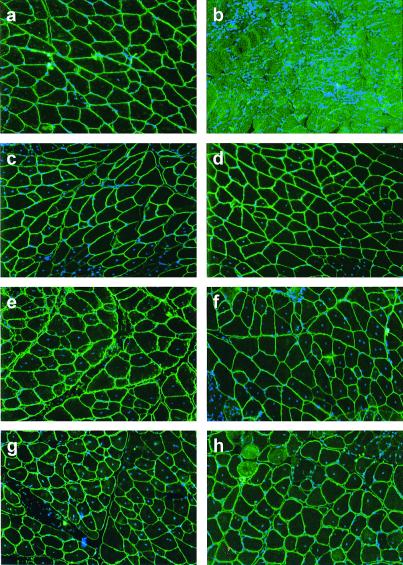
Figure 3.
Long-term minidystrophin expression in mdx mice treated at a young age (a) or as adults (c–h) with AAV vectors containing different minigenes under the control of different promoters. IF staining of minidystrophin (green) and myonuclei counterstaining with DAPI (blue) were performed on gastrocnemius muscles isolated from (a) MCK-Δ3849-treated 10-day-old mdx for 6 months, (b) untreated 6-month-old mdx, (c) MCK-Δ3849-treated adult mdx for 2 months, (d) MCK-Δ3990-treated adult mdx for 2 months, (e) MCK-Δ3849-treated adult mdx for 4 months, (f) MCK-Δ3990-treated adult mdx for 4 months, (g) CMV-Δ3849-treated adult mdx for 6 months, and (h) CMV-Δ3990-treated adult mdx for 6 months.
We next examined whether the minidystrophins were functional in restoring the missing DAP complexes on the myofiber plasma membrane, including the sarcoglycan complex, which is not found in untreated dystrophic muscle because of the primary deficiency of dystrophin. IF staining using three antibodies against α, β, and γ sarcoglycans, respectively, showed positive results in all of the consecutive thin sections adjacent to those stained with dystrophin antibodies (Fig. 2b). These results provided evidence of biochemical functionality of the minidystrophins, which lack the CT domain but are still capable of interacting with the DAP complexes.
Amelioration of Dystrophic Pathology.
To further investigate the functionality of our minidystrophins, it is essential to demonstrate that they can protect muscle from the pathological phenotypes. The onset of the pathology in mdx mice starts at around 3 weeks of age with massive waves of myofiber degeneration/regeneration. This process is characterized by the presence of central nuclei in myofibers, a primary pathological sign of muscular dystrophies. The absence or reduction of central nucleation after gene therapy would suggest that the therapy is successful. Therefore, we initially chose to test the AAV minidystrophin constructs in young mdx mice (10 days old) before the onset of central nucleation, to see whether muscle degeneration/regeneration can be prevented.
Histological examination of the mdx muscles at 3 and 6 months after AAV minidystrophin treatment, which was before the onset of central nucleation, showed nearly exclusive (≈98%) peripheral nucleation in the minidystrophin-positive myofibers, as revealed by dystrophin immunostaining and myonuclei counterstaining with DAPI [Figs. 2 a and b (first column) and 3a; Table 1]. The mutual exclusivity between minidystrophin expression and central nucleation in the vector-treated mdx muscle precisely mirrored that of the normal muscle (Fig. 2b and Table 1). In addition, the myofibers positive for minidystrophin expression also exhibited consistent myofiber sizes and polygonal shapes indistinguishable from those of the normal muscle (Fig. 2). By contrast, the untreated mdx muscle showed extensive (75.4%) central nucleation (Table 1), with additional signs of dystrophic pathology, including wide variation of myofiber sizes, round myofiber shapes, and fibrosis (Fig. 2b). Hence, AAV vector treatment prevented dystrophic pathology and led to normal histology in terms of peripheral nucleation, consistent myofiber size, and lack of fibrosis in the minidystrophin-positive areas. These results unequivocally demonstrated the absence of muscle degeneration because of the therapeutic effects of our minidystrophins in young mdx mice.
Table 1.
AAV minidystrophin gene transfer in young and adult mdx mice
| Animals* and vectors | n | Age at vector injection | Months post injection | % Dystrophin-positive fibers | % Central nuclei† |
|---|---|---|---|---|---|
| mdx + Δ3849 | 4 | 10 days | 3 | 56 ∼ 88 | 1.02 (72/7,098) |
| mdx + Δ3990 | 4 | 10 days | 3 | 50 ∼ 80 | 0.99 (56/5,652) |
| mdx + Δ4173 | 4 | 10 days | 3 | 15 ∼ 25 | 0.93 (26/2,791) |
| mdx + Δ3849 | 4 | 10 days | 6 | 40 ∼ 60 | 2.80 (51/1,824) |
| mdx + Δ3990 | 2 | 10 days | 6 | 35 ∼ 45 | 2.30 (34/1,478) |
| mdx + Δ3849 | 2 | 50 days | 2 | 35 ∼ 50 | 34.76 (510/1,467) |
| mdx + Δ3990 | 2 | 50 days | 2 | 35 ∼ 40 | 34.18 (685/2,004) |
| mdx + Δ3849 | 4 | 50 days | 4 | 20 ∼ 25 | 44.24 (615/1,390) |
| mdx + Δ3990 | 4 | 50 days | 4 | 20 ∼ 30 | 46.18 (695/1,505) |
| C57/B10 | 4 | No injection | N/A | 100 | 1.45 (56/3,860) |
| mdx | 4 | No injection | N/A | <1 | 75.4 (238/3,160) |
N/A, Not applicable.
Untreated control mdx and C57/B10 mice were about 3 months old at the endpoints of experiments. AAV vectors were driven by MCK promoter.
All numbers were collected from dystrophin-positive myofibers that were photographed after IF staining and DAPI counterstaining, except in untreated mdx mice, which had extensive central nucleation and very few dystrophin-positive revertant myofibers.
Subsequently we also tested AAV minidystrophin vectors in treating adult mdx mice (45 days of age) after the onset of massive waves of degeneration/regeneration, to see whether the pathological process can be stopped or reversed. At the time of vector injection, a majority (≈75%) of the myofibers already underwent degeneration/regeneration process and displayed central nucleation. At 2 months, 4 months, and 6 months after AAV minidystrophin injection, widespread dystrophin expression was observed accompanied by normal myofiber morphology and lack of fibrosis in the dystrophin-positive areas (Fig. 3). By contrast, muscle of untreated mdx mice (Fig. 3b), or areas of treated muscle without successful vector gene transfer, manifested progressive degeneration and fibrosis. In addition, a reduction of central nucleation in minidystrophin-positive myofibers was observed (from approximately 75% before vector treatment to 35–50% after vector treatment; see Table 1). The partial reversal of central nucleation also was observed in healthy mouse muscle, where a majority of the myonuclei remained centrally located once experiencing a transient pathology such as myotoxin treatment (35). Persistence of central nucleation also was observed after treatment of adult mdx muscle with a gutless adenovirus vector containing the full-length dystrophin cDNA (P. Clemens, personal communication). Based on the above observations, our minidystrophin genes demonstrated therapeutic effects in ameliorating dystrophic pathology in both young and adult mdx muscles.
Protection of Myofiber Membrane Integrity.
Plasma membrane damage and leakage in dystrophic muscle is a major physiological defect and also a major pathological cause. To determine whether AAV minidystrophin treatment would be effective in protecting plasma membrane from mechanical damage, a myofiber membrane integrity test was performed by i.v. injection of Evans Blue dye. Evans Blue is a widely used vital red fluorescent dye that is excluded by the healthy myofibers, but is taken up by the dystrophic myofibers containing leaky cell membrane caused by contractile damages. A previous study of mdx mice revealed that the apoptotic myonuclei were found mostly in Evans Blue dye-positive myofibers, thus correlating plasma membrane leakage and muscle cell apoptosis (28).
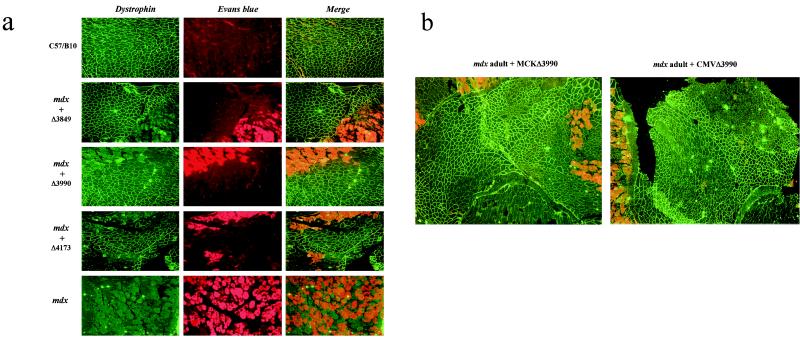
Initially, Evans Blue was administered into the tail vein of mdx mice that were treated at a young age (10 days old) with AAV vectors 3 months before. The age-matched untreated mdx mice and healthy C57/B10 mice were used as controls. To induce mechanical stress, the mice were allowed to exercise by continuous swimming for 20 min. Muscles then were collected and examined for dystrophin expression as well as for Evans Blue dye uptake. As expected, muscle from healthy mice revealed uniform dystrophin staining across the muscle sections and no uptake of the dye by the myofibers (Fig. 4a, top row). The AAV vector-treated mdx muscle showed results consistent with the healthy muscle, thus mutual exclusivity of dystrophin expression and dye uptake (Fig. 4a, three middle rows). Dye uptake (red fluorescence) was found only in myofibers that lacked minidystrophin expression in the areas not transduced by AAV vectors (Fig. 4a, three middle rows). By contrast, the untreated mdx muscle revealed absence of dystrophin and extensive dye uptake (Fig. 4a, bottom row). More importantly, AAV minidystrophin treatment of adult mdx muscle also achieved similar results in protecting myofibers from plasma membrane leakage when analyzed at 2 months and 6 months after vector injection (Fig. 4b). These results unequivocally demonstrated the physiological functionality of our minidystrophins in maintaining membrane integrity and protecting myofibers from mechanical damages in both young and adult mdx mice.
Figure 4.
Protection of muscle plasma membrane integrity by minidystrophin genes in mdx mice treated either at 10 days of age (a) or as adults (b). (a) Three months after AAV vector injection, either the treated mdx mice or the age-matched controls (normal C57/B10 and untreated mdx mice) were i.v. injected with Evans Blue dye. The gastrocnemius muscles then were collected and cryosectioned either from normal C57/B10 mice; from mdx mice treated at 10 days of age with AAV-MCK vectors Δ3849, Δ3990, or Δ4173; or from the untreated mdx mice. Normal dystrophin and minidystrophin expression was visualized by IF staining (Left, green). The leaky myofibers were visualized by the uptake of Evans Blue dye (Center, red fluorescence). Note the mutual exclusivity between dystrophin expression and Evans Blue dye uptake as shown by the merged images (Right). Photographs were taken with a ×10 lens. (b) Adult mdx gastrocnemius muscles were treated with AAV vectors containing Δ3990 minigene. Evans Blue dye tests were performed at 2 months after AAV-MCK-Δ3990 treatment (Left) or at 6 months after AAV-CMV-Δ3990 treatment (Right). Note the widespread minidystrophin expression (green) and the leaky myofibers (red), which were negative for minidystrophin staining.
Discussion
In summary, we have presented evidence that the dystrophin gene can be successfully reduced to one-third of its 11-kb full-length coding sequence, without compromising essential functions in protecting muscles from dystrophic phenotypes. Moreover, we have demonstrated that intramuscular injection of AAV vectors carrying human minigenes can achieve efficient and long-term therapeutic effects in a major muscle group of a DMD mouse model. The long-term correction of both biochemical and physiological defects in the dystrophic muscles was realized by the persistent minidystrophin expression from AAV vectors and the apparent lack of cytotoxic T lymphocyte immune response against myofibers expressing human dystrophin.
Previous attempts to generate minigenes that were shorter than one-half of the full-length dystrophin failed to preserve the essential protective functions. The minidystrophin genes tested in adenovirus vectors by Yuasa and colleagues (36, 37), although containing both intact N- and C-terminal domains and 1–3 central rod repeats, were functionally similar to a CT dystrophin construct (Dp71) (38), thus sufficient to restore DAP complexes but insufficient to restore myofiber morphology and prevent dystrophic pathology (unpublished results). By contrast, the minidystrophin genes reported here accommodated at least five rod repeats (R1, R2, R22, R23, and R24) and two hinges (H1 and H4). To retain as many repeat units in the rod domain without exceeding the packaging limit of AAV vectors, we have enabled the deletion of nearly the entire CT (819 bp) without sacrificing the primary functions of dystrophin, for example, submembrane anchoring and interaction with DAP complexes. Our results indicate that five rods and two hinges seem sufficient to provide both length and flexibility for the central domain. This conclusion is supported by the observation that minigenes Δ3849 and Δ3990 were equally functional in preventing the dystrophic phenotypes, although Δ3990 had an extra hinge (H3). Similarly, minigene Δ4173 had an extra rod (R3) but did not function better than minigenes Δ3849 or Δ3990 (Table 1). In fact, because the entire AAV-MCK-4173 vector cassette was nearly 5.2 kb in length, larger than the 5-kb packaging limit, its viral particle infectivity was impaired, which led to lower gene transfer efficiency (Fig. 4a and Table 1). Because a major role of dystrophin is to crosslink the myofiber cytoskeleton structure and plasma membrane, thus, to stabilize the myofiber during muscle contraction, we hypothesize that the length of the central rod domain is the critical factor in constructing functional minidystrophin genes. It is conceivable that if the minidystrophin is too short to span the sliding distance between the cytoskeleton and plasma membrane during muscle contraction, the crosslinks will be disrupted and the muscle membrane will become unstable and prone to mechanical damages.
Despite the fact that our minidystrophin genes demonstrated functionality in protecting myofiber membrane integrity even after exercises, it remains to be seen whether the protective effects withstand extreme conditions such as eccentric contractions. Further investigation also will be instructive to see whether these minigenes can restore the muscle contractile force deficit, although in mdx mice such deficit is not apparent unless the force output is normalized by muscle cross-sectional area. The minigenes created here are shorter than the 6.3-kb truncated dystrophin gene, which was isolated from a 61-year-old ambulant Becker muscular dystrophy patient who had very mild symptoms (29). It is plausible not to expect that the minigenes are as functional as the 6.3-kb Becker-form dystrophin gene. However, further in vivo functional comparisons between these genes as well as to the wild-type dystrophin gene by transgenic mouse studies will provide insightful information not only for muscle biology but also for DMD gene therapy.
Of particular importance in this study is the use of the AAV vector, which appears to be the best vector system currently available for muscle-directed gene therapy. Compared with other viral and nonviral vectors previously explored for DMD, AAV has the combined advantages of high-efficiency gene transfer, persistent transgene expression and low immunogenicity (12, 13, 17, 18, 21). The success of truncating large dystrophin gene into functional miniature versions enabled us to explore the utility of AAV vector in treating the most common and devastating genetic muscle disorder. Recent progress in stem-cell transplantation has offered a new hope for cell therapy of DMD (39). The functional dystrophin genes reported here also should find their utilities in stem-cell therapeutics after ex vivo gene transfer. Nevertheless, the primary advantage of AAV vectors is the direct in vivo gene delivery such as intramuscular injections. New developments in systemic vector delivery through the blood circulation (16) and tissue targeting of AAV vectors should render more widespread gene transfer in large groups of muscles. Finally, using AAV vectors rather than the traditional transgenic mouse technology, we have provided a more convenient and less time-consuming method to further discern the dystrophin functional domains in vivo and to optimize the minidystrophin genes for future clinical applications in DMD gene therapy.
Acknowledgments
We thank Drs. S. D. Hauschka and T. R. Flotte for the MCK promoter and poly(A) sequence; Dr. P. Clemens for the Animal Core; Dr. S. Takeda for sharing unpublished information; and Drs. M. Ontell, X. D. Fu, and M. Xiao for critical reading of the manuscript. We also thank Drs. L. W. Sun and S. J. Qiu for their assistance. This work is supported in part by National Institutes of Health Grants AR45967 and AR 45925 and an equipment grant from Parent Project USA. B.W. is a recipient of the Duchenne Muscular Dystrophy Research Center Postdoctoral Fellowship.
Abbreviations
- AAV
adeno-associated virus
- DMD
Duchenne muscular dystrophy
- CR
cysteine-rich
- CT
C terminus
- DAP
dystrophin-associated protein
- MCK
muscle-specific creatine kinase
- CMV
cytomegalovirus
- DAPI
4′,6-diamidino-2-phenylindole
- IF
immunofluorescent
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 13464.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240335297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240335297
References
- 1.Kunkel L M. Nature (London) 1986;322:73–77. doi: 10.1038/322073a0. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Hoffman E P, Bertelson C J, Monaco A P, Feener C, Kunkel L M. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman E P, Brown R H, Jr, Kunkel L M. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 4.Koenig M, Kunkel L M. J Biol Chem. 1990;265:4560–4566. [PubMed] [Google Scholar]
- 5.Gussoni E, Blau H M, Kunkel L M. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 6.Acsadi G, Dickson G, Love D R, Jani A, Walsh F S, Gurusinghe A, Wolff J A, Davies K E. Nature (London) 1991;352:815–818. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- 7.Rando T A, Disatnik M H, Zhou L Z. Proc Natl Acad Sci USA. 2000;97:5363–5368. doi: 10.1073/pnas.97.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan J C, Perricaudet M, et al. Nature (London) 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 9.Howell J M, Lochmuller H, O'Hara A, Fletcher S, Kakulas B A, Massie B, Nalbantoglu J, Karpati G. Hum Gene Ther. 1998;9:629–634. doi: 10.1089/hum.1998.9.5-629. [DOI] [PubMed] [Google Scholar]
- 10.Muzyczka N. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 11.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O'Malley K L, During M J. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 12.Xiao X, Li J, Samulski R J. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao W, Berta S C, Lu M M, Moscioni A D, Tazelaar J, Wilson J M. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruchnic R, Cao B, Peterson Z Q, Xiao X, Li J, Samulski R J, Epperly M, Huard J. Hum Gene Ther. 2000;11:521–536. doi: 10.1089/10430340050015716. [DOI] [PubMed] [Google Scholar]
- 16.Greelish J P, Su L T, Lankford E B, Burkman J M, Chen H, Konig S K, Mercier I M, Desjardins P R, Mitchell M A, Zheng X G, et al. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- 17.Xiao X, Li J, Tsao T-Y, Dressman D, Hoffman E P, Watchko J F. J Virol. 2000;74:1436–1442. doi: 10.1128/jvi.74.3.1436-1442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordier L, Hack A A, Scott M O, Barton-Davis E R, Gao G-P, Wilson J M, McNally E M, Sweeney H L. Mol Ther. 2000;1:119–129. doi: 10.1006/mthe.1999.0019. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Li J, Xiao X. Nat Med. 2000;6:599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- 20.Duan D, Yue Y, Yan Z, Engelhardt J F. Nat Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 21.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne B J, Atkinson M, Flotte T R. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay M A, Manno C S, Ragni M V, Larson P J, Couto L B, McClelland A, Glader B, Chew A J, Tsai S J, Herzog R W, et al. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 23.Shield M A, Haugen H S, Clegg C H, Hauschka S D. Mol Cell Biol. 1996;16:5058–5068. doi: 10.1128/mcb.16.9.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flotte T R, Afione S A, Solow R, Drumm M L, Markakis D, Guggino W B, Zeitlin P L, Carter B J. J Biol Chem. 1993;268:3781–3790. [PubMed] [Google Scholar]
- 25.Xiao X, Li J, Samulski R J. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder R, Xiao X, Samulski R J. In: Current Protocols in Human Genetics. Dracopoli N, Haines J, Krof B, Moir D, Seidman C, Seidman J, Smith D, editors. New York: Wiley; 1996. pp. 12.1.1–12.2.23. [Google Scholar]
- 27.Li J, Dressman D, Tsao Y P, Sakamoto A, Hoffman E P, Xiao X. Gene Ther. 1999;6:74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda R, Nishikawa A, Tanaka H. J Biochem (Tokyo) 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 29.England S B, Nicholson L V, Johnson M A, Forrest S M, Love D R, Zubrzycka-Gaarn E E, Bulman D E, Harris J B, Davies K E. Nature (London) 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 30.Passos-Bueno M R, Vainzof M, Marie S K, Zatz M. Hum Mol Genet. 1994;3:919–922. doi: 10.1093/hmg/3.6.919. [DOI] [PubMed] [Google Scholar]
- 31.Mirabella M, Galluzzi G, Manfredi G, Bertini E, Ricci E, De Leo R, Tonali P, Servidei S. Neurology. 1998;51:592–595. doi: 10.1212/wnl.51.2.592. [DOI] [PubMed] [Google Scholar]
- 32.Rafael J A, Cox G A, Corrado K, Jung D, Campbell K P, Chamberlain J S. J Cell Biol. 1996;134:93–102. doi: 10.1083/jcb.134.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corrado K, Rafael J A, Mills P L, Cole N M, Faulkner J A, Wang K, Chamberlain J S. J Cell Biol. 1996;134:873–884. doi: 10.1083/jcb.134.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanin M, Freda M P, Vitiello L, Danieli G A, Pegoraro E, Angelini C. Muscle Nerve. 1996;19:1154–1160. doi: 10.1002/mus.880190902. [DOI] [PubMed] [Google Scholar]
- 35.Martin H, Ontell M. Muscle Nerve. 1988;11:588–596. doi: 10.1002/mus.880110611. [DOI] [PubMed] [Google Scholar]
- 36.Yuasa K, Miyagoe Y, Yamamoto K, Nabeshima Y, Dickson G, Takeda S. FEBS Lett. 1998;425:329–336. doi: 10.1016/s0014-5793(98)00251-8. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto K, Yuasa K, Miyagoe Y, Hosaka Y, Tsukita K, Yamamoto H, Nabeshima Y I, Takeda S. Hum Gene Ther. 2000;11:669–680. doi: 10.1089/10430340050015572. [DOI] [PubMed] [Google Scholar]
- 38.Cox G A, Sunada Y, Campbell K P, Chamberlain J S. Nat Genet. 1994;8:333–339. doi: 10.1038/ng1294-333. [DOI] [PubMed] [Google Scholar]
- 39.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]