Abstract
Lymphatic vessel plasticity and stability are of considerable importance when attempting to treat diseases associated with the lymphatic vasculature. Development of lymphatic vessels during embryogenesis is dependent on vascular endothelial growth factor (VEGF)-C but not VEGF-D. Using a recombinant adenovirus encoding a soluble form of their receptor VEGFR-3 (AdVEGFR-3-Ig), we studied lymphatic vessel dependency on VEGF-C and VEGF-D induced VEGFR-3 signaling in postnatal and adult mice. Transduction with AdVEGFR-3-Ig led to regression of lymphatic capillaries and medium-sized lymphatic vessels in mice under 2 weeks of age without affecting collecting lymphatic vessels or the blood vasculature. No effect was observed after this period. The lymphatic capillaries of neonatal mice also regressed partially in response to recombinant VEGFR-3-Ig or blocking antibodies against VEGFR-3, but not to adenovirus-encoded VEGFR-2-Ig. Despite sustained inhibitory VEGFR-3-Ig levels, lymphatic vessel regrowth was observed at 4 weeks of age. Interestingly, whereas transgenic expression of VEGF-C in the skin induced lymphatic hyperplasia even during embryogenesis, similar expression of VEGF-D resulted in lymphangiogenesis predominantly after birth. These results indicate considerable plasticity of lymphatic vessels during the early postnatal period but not thereafter, suggesting that anti-lymphangiogenic therapy can be safely applied in adults.
The lymphatic vasculature collects extravasated fluid, macromolecules, and cells of the immune system from the interstitium and after filtration through a series of lymph nodes returns them back to the blood circulation. The lymph vessels also absorb and transport dietary lipids from the intestine.1 In contrast to blood vessels, lymphatic capillaries start blind ended, have a discontinuous basement membrane, and are not covered by pericytes, whereas collecting lymphatic vessels are surrounded by a smooth muscle cell layer. The lymphatic endothelial cells lack tight interendothelial junctions and are attached to the surrounding extracellular matrix by anchoring filaments, while valves serve to prevent lymph backflow in the absence of a strong propulsive pressure.2 Defects of lymphatic vessel function can lead to lymphedema, a condition characterized by swelling of extremities due to fluid accumulation in tissues.3 Lymphatic vessels also represent the primary route of metastatic spread for many types of human cancers,4 and they are furthermore involved in the regulation of inflammatory responses in various pathological conditions.5,6
The mechanisms controlling development of the blood vasculature are relatively well characterized, but the molecular mechanisms regulating the growth and function of lymphatic vessels are only starting to be elucidated. Vascular endothelial growth factor receptor (VEGFR)-3 is initially expressed in all endothelial cells of mouse embryos.7VEGFR-3-deficient mice die at embryonic day 9.5 (E9.5) because of a failure in remodeling of the primary vascular plexus.8 Later in embryonic development, when the lymphatic vessels start to sprout at approximately E10.5, the expression of VEGFR-3 decreases in blood vessels and becomes restricted almost exclusively to the lymphatic endothelium.7 The two known ligands of VEGFR-3, vascular endothelial growth factor (VEGF)-C and VEGF-D, have been shown to induce primarily lymphangiogenesis.9,10 VEGF-C is necessary for the initial sprouting and migration of lymphatic endothelial cells from embryonic veins, and mice lacking VEGF-Cdie prenatally,11 whereas VEGF-D is dispensable for embryonic lymphatic development.12
We have shown that the ligand-binding domain of VEGFR-3 fused to the Fc-region of human immunoglobulin γ chain (VEGFR-3-Ig) is a potent inhibitor of VEGF-C-induced tumor lymphangiogenesis and lymphatic, but metastasis.13,14 To apply inhibition of ligand-induced VEGFR-3 signaling to prevent lymphatic metastasis in cancer patients, it would be important to know how this affects the normal lymphatic vasculature. Here we show that inhibition of the interaction of VEGFR-3 with its ligands, by adenovirus-encoded soluble VEGFR-3-Ig, recombinant VEGFR-3-Ig protein, or by inhibitory VEGFR-3 antibodies, causes systemic regression of normal lymphatic capillaries and medium-sized lymphatic vessels during the first 2 weeks of postnatal life. After that time, the lymphatic vasculature becomes independent of VEGFR-3 ligands, and the lymphatic vessels regenerate even in the presence of neutralizing concentrations of VEGFR-3-Ig. Furthermore, we demonstrate that whereas transgenic overexpression of VEGF-C induces lymphangiogenesis during embryogenesis, VEGF-D stimulates lymphatic vessel growth predominantly after birth, indicating additional changes related to postnatal lymphatic maturation.
Materials and Methods
Mice and Adenoviruses
The VEGFR-3+/LacZ, K14-VEGF-C, K14-VEGF-C156S, and K14-VEGF-D mice have been described previously.8–10 NMRI nu/nuand nu/+mice were from Harlan (Horst, The Netherlands). All experiments involving mice were approved by the Provincial State Office of Southern Finland and they were performed in accordance with institutional guidelines. Recombinant adenoviruses encoding VEGFR-3-Ig (AdVEGFR-3-Ig), VEGF-C (AdVEGF-C), β-galactosidase (AdLacZ), and VEGFR-2-Ig (AdVEGFR-2-Ig) were produced as described.13,15
In Vitro Testing of the Adenoviruses
HepG2 cells were transduced with 100 pfu/cell of AdVEGFR-3-Ig or AdLacZ and metabolically labeled with 100 μCi/ml [35S]methionine and [35S]cysteine (Redivue ProMix; Amersham Pharmacia Biotech, Uppsala, Sweden). The labeled fusion protein was precipitated with protein A-Sepharose (Amersham Pharmacia Biotech) and analyzed by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Alternatively, unlabeled conditioned medium from AdVEGFR-3-Ig-transduced HepG2 cells or, as a control, polyclonal rabbit antibodies against human VEGF-C (antiserum 882),16 were used to bind metabolically labeled VEGF-C from the conditioned media of HepG2 cells transduced with AdVEGF-C or AdLacZ adenoviruses. The complexes were precipitated, washed, and analyzed by 12.5% SDS-PAGE under reducing conditions.
Treatment of Mice with Adenovirus-Encoded Ligand Traps, Blocking Antibodies, or Recombinant Proteins
One-, four-, or seven-day-old mouse pups were injected intraperitoneally with 5 × 108 pfu/30 to 50 μl and mice 14 days or older with 1 × 109 pfu/60 to 100 μl of AdVEGFR-3-Ig, AdVEGFR-2-Ig, AdLacZ, or a corresponding volume of phosphate-buffered saline (PBS). Mice were injected intraperitoneally once a day or every second day with 30 mg/kg or 60 mg/kg of mF4-31C1, a rat monoclonal antibody against mouse VEGFR-3 that blocks ligand binding17; 25 mg/kg of recombinant VEGFR-3-Ig fusion protein18; 20 mg/kg AFL4, a rat monoclonal antibody against mouse VEGFR-3 that blocks ligand binding19; or control (nonblocking rat monoclonal antibodies against mouse VEGFR-2,19 recombinant VEGFR-1-Ig fusion protein18 or PBS) in a volume of 20 to 100 μl.
Visualization of Blood and Lymphatic Vessels
Fluorescent whole-mount immunostaining was performed as described previously20 with polyclonal rabbit antibodies against mouse LYVE-111 and monoclonal rat antibodies against mouse PECAM-1 (BD Pharmingen, San Diego, CA) using Alexa Fluor 594-conjugated goat anti-rabbit and Alexa Fluor 488-conjugated goat anti-rat antibodies (Molecular Probes, Eugene, OR) for detection. Ear tissues were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and analyzed with a LSM510 Meta confocal microscope (Carl Zeiss, Heidelberg, Germany). Other tissues were analyzed with a stereomicroscope (Leica, Wetzlar, Germany).
Paraffin sections from paraformaldehyde-fixed tissues were immunostained with monoclonal rat antibodies against VEGFR-319 or PECAM-1 (BD Pharmingen) or rabbit antibodies against LYVE-111 using tyramide signal amplification kit (NEN Life Sciences, Boston, MA). The lymphatic vessels of VEGFR-3+/LacZ mice were stained with X-gal (Sigma-Aldrich). For visualization of functional lymphatic vessels, fluorescein isothiocyanate-conjugated dextran (2000 kd; Sigma-Aldrich, St. Louis, MO) was injected intradermally into the ear or tail, and the uptake of the dye by lymphatic vessels was analyzed by fluorescence microscopy.
Detection of VEGFR-3-Ig in Serum
The concentration of VEGFR-3-Ig fusion protein in the serum was determined by specific enzyme-linked immunosorbent assay as described.18 Data are expressed as average ± SD. To test VEGFR-3-Ig binding properties, serum obtained from AdVEGFR-3-Ig-transduced mice was used to precipitate metabolically labeled VEGF-C from the conditioned medium of VEGF-C-transfected 293T cells.
Pharmacokinetics of mF4-31C1 and VEGFR-3-Ig
Female nu/numice were injected intraperitoneally with 20 mg/kg of mF4-31C1. Two mice per group were bled at the indicated times after treatment. Plasma concentration of mF4-31C1 was determined using an enzyme-linked immunosorbent assay. Briefly, 96-well Maxi-sorp microtiter plates (Nunc, Roskilde, Denmark) were coated with the extracellular domain of mouse VEGFR-3 fused with secreted human alkaline phosphatase (sR3-AP).17 Plasma samples, serially diluted in blocking buffer (PBS containing 5% freeze-dried milk), were added to the wells and incubated at room temperature for 1 hour. Bound mF4-31C1 was detected with donkey anti-rat IgG-horseradish peroxidase conjugate (Amersham Pharmacia Biotech) using TMB peroxidase substrate (KPL, Gaithersburg, MD). The change in optical density was measured using a microplate reader (Molecular Devices, Sunnyvale, CA). Pharmacokinetic parameters were calculated by noncompartmental analysis using the WinNonlin program (Pharsight Corporation, Mountain View, CA). To determine the systemic half-life of VEGFR-3-Ig in mice, 10 μg of recombinant VEGFR-3-Ig protein were injected intravenously into three female C57BL/6 mice in a volume of 200 μl, and blood samples were collected from the tail vein at the indicated time points. Serum VEGFR-3-Ig concentration was quantified by enzyme-linked immunosorbent assay.18 Data are expressed as average ± SD.
Quantitation of Lymphatic Vessels
At E14.5, we quantified the number of X-gal-stained skin lymphatic vessels at 0.3, 0.6, and 0.9 mm distance from the dorsal midline as well as the skin area covered by X-gal-positive lymphatic vessels on the dorsal side of K14-VEGF-D × VEGFR-3+/LacZ, K14-VEGF-C × VEGFR-3+/LacZ, and VEGFR-3+/LacZ embryos. At P1, P7, and P14, the area covered by lymphatic vessels in the skin of K14-VEGF-C, K14-VEGF-D, and wild-type littermate mice was quantified from photomicrographs of LYVE-1-stained skin sections (six photomicrographs/mouse) using the Image-Pro Plus program (Media Cybernetics, Silver Spring, MD). Data are expressed as average ± SD.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
For the determination of the relative expression levels of VEGF-C and VEGF-D in the K14-VEGF-C, K14-VEGF-D, and wild-type littermate mice, RNA was extracted from whole embryos at E14.5 and from ventral skin at E17.5 using the RNeasy mini kit (Qiagen, Hilden, Germany). Reverse transcription was performed from 0.5 μg of total RNA with the QuantiTect reverse transcription kit (Qiagen), and 1/20 of the reverse transcription reaction was used as a template in the quantitative PCR reaction performed with the DyNAmo HS SYBR Green qPCR kit (Finnzymes, Espoo, Finland) using the ABI 7500 SDS real-time PCR instrument (Applied Biosystems, Foster City, CA). The oligonucleotide primers used were the following: 5′-GGCTGGCAACATAACAGAGA-3′ and 5′-GTGGCATGCATTGAGTCTTT-3′ for human VEGF-C, 5′-CTTGCTGGAACAGAAGACCA-3′ and 5′-TACAGACACACTCGCAACGA-3′ for human VEGF-D, and 5′-ACA-ACTTTGGCATTGTGGAA-3′ and 5′-GATGCAGGGATG-ATGTTCTG-3′ for mouse GAPDH. Relative expression of human VEGF-C and VEGF-D were normalized to mouse GAPDH expression.
Results
AdVEGFR-3-Ig Establishes Long-Term Inhibition of VEGF-C and -D in Vivo
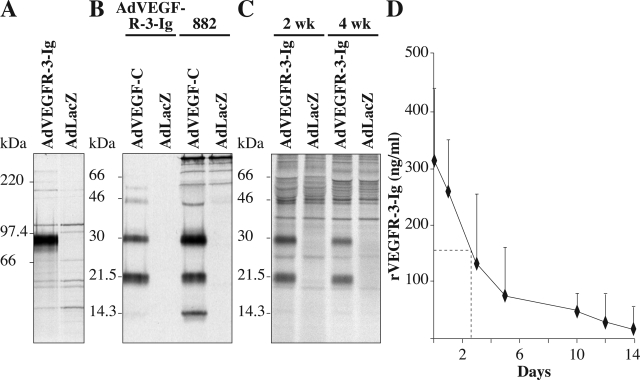
We used a recombinant adenovirus, AdVEGFR-3-Ig, to inhibit VEGFR-3 signaling in mice of different ages. AdVEGFR-3-Ig-transduced cells secreted a protein of the expected size (Figure 1A), which was able to bind VEGF-C (Figure 1B). Also, the VEGFR-3-Ig fusion protein in the serum of mice transduced intraperitoneally with AdVEGFR-3-Ig 1 week after birth, and analyzed at 2 or 4 weeks of age, was able to bind VEGF-C (Figure 1C). The systemic half-life of VEGFR-3-Ig in C57BL/6 mice was determined to be between 2 to 3 days (Figure 1D).
Figure 1-6926.
AdVEGFR-3-Ig establishes long-term inhibition of VEGF-C in vivo. A: AdVEGFR-3-Ig- or AdLacZ-transduced cells were metabolically labeled and the conditioned media were precipitated with protein A Sepharose. B and C: Metabolically labeled VEGF-C was precipitated with VEGFR-3-Ig produced by AdVEGFR-3-Ig-transduced cells or with the 882 polyclonal VEGF-C antiserum (B) or using serum from nude mice transduced intraperitoneally with AdVEGFR-3-Ig or AdLacZ 1 week after birth and analyzed at the age of 2 or 4 weeks (C). The bound proteins were analyzed by 7.5% (A) or 12% (B and C) SDS-PAGE under reducing conditions. D: Concentration of VEGFR-3-Ig in the circulation of C57BL/6 mice as a function of time after a single intravenous injection of 10 μg of the recombinant protein. Dashed line indicates half of the protein concentration obtained 5 minutes after injection.
AdVEGFR-3-Ig Induces Postnatal Lymphatic Vessel Regression
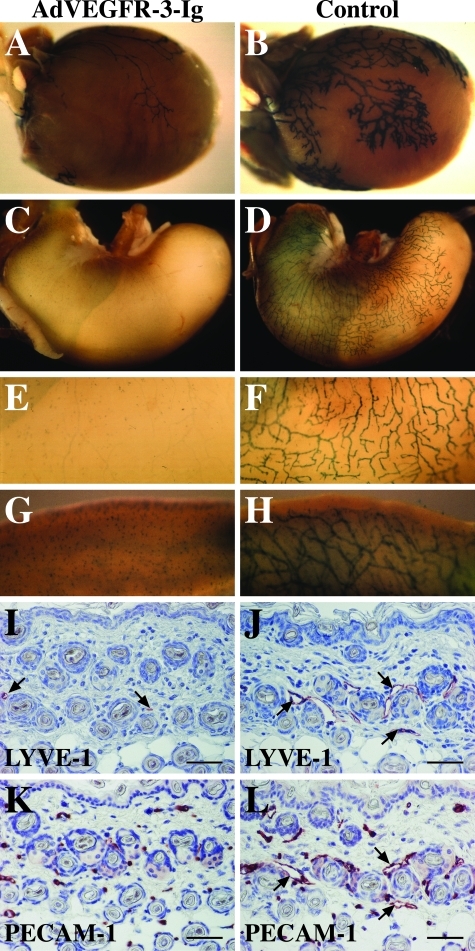
To determine whether the lymphatic vasculature of postnatal mice depends on ligand-induced VEGFR-3 signaling, VEGFR-3+/LacZ mice were transduced intraperitoneally with 5 × 108 pfu of AdVEGFR-3-Ig or AdLacZ or a corresponding volume of PBS at days 1, 7, or 14 after birth. An initial lymphatic vessel network is present on day 1 after birth (see Figure 6, I and L).18,21 The mice treated with AdVEGFR-3-Ig at the age of 1 or 7 days were almost completely devoid of lymphatic capillaries and medium-sized lymphatic vessels in the intestine, mesenterium, diaphragm, stomach, and heart, when analyzed 1 week after the injection. Only a few thin remnants of lymphatic vessels, likely without a lumen, and some isolated lymphatic endothelial cells remained in these tissues, as determined by whole-mount β-galactosidase staining (Figure 2, A–H; and data not shown). However, large collecting lymphatics, for example the thoracic duct, were not affected. This may reflect the expression of VEGFR-3 in lymphatic capillaries but not in collecting lymphatic vessels.22 Only occasional thin lymphatic vessels were detected in the skin sections of the AdVEGFR-3-Ig-treated mice by immunohistochemical analysis with antibodies against the lymphatic endothelial markers LYVE-1, VEGFR-3, or podoplanin (Figure 2I and data not shown). On the contrary, lymphatic vessels were abundant in the skin of AdLacZ- and PBS-treated control mice (Figure 2J). However, the blood vessels of the AdVEGFR-3-Ig-treated mice appeared normal in number and size, as analyzed by immunohistochemical staining with antibodies against the pan-endothelial marker platelet endothelial cell adhesion molecule 1 (PECAM-1) (Figure 2, K and L).
Figure 6-6926.
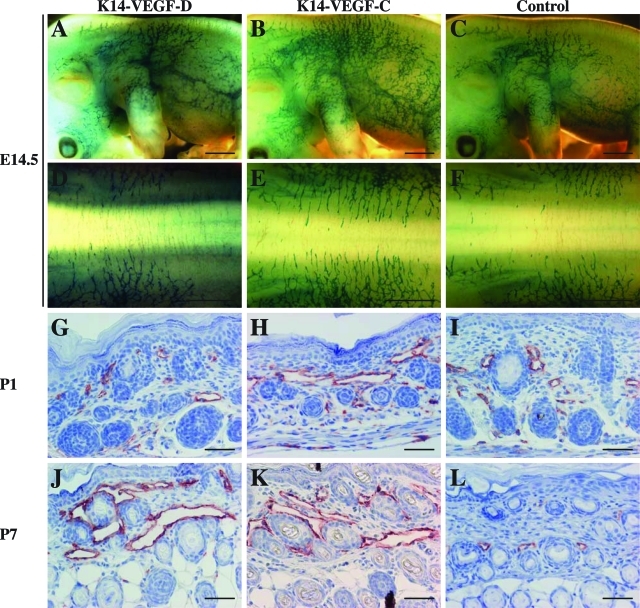
VEGF-D promotes lymphangiogenesis predominantly after birth. A–F: Whole-mount β-galactosidase staining of K14-VEGF-D × VEGFR-3+/LacZ, K14-VEGF-C × VEGFR-3+/LacZ, and control VEGFR-3+/LacZ mice at E14.5. G–L: Immunohistochemical staining for LYVE-1 (red) in the skin sections of K14-VEGF-D and K14-VEGF-C mice as well as in their wild-type littermates at days 1 (G–I) and 7 (J–L) after birth. Note the progression of lymphatic hyperplasia between P1 and P7 in the K14-VEGF-D skin (G and J). Scale bars: 1 mm (A–F); 50 μm (G–L).
Figure 2-6926.
Adenovirally encoded soluble VEGFR-3 causes lymphatic vessel regression in mice under 2 weeks of age but has no effect on the blood vasculature. VEGFR-3+/LacZmice were transduced intraperitoneally with 5 × 108 pfu of AdVEGFR-3-Ig or PBS on day 7 after birth and analyzed 1 week thereafter. The lymphatic vessels were visualized by β-galactosidase staining in the heart (A and B), stomach (C–F), and colon (G and H). The lymphatic and blood vessels in skin sections were visualized by immunohistochemistry using antibodies against the lymphatic-specific marker LYVE-1 (I and J, red) or the pan-endothelial marker PECAM-1 (K and L, red) of AdVEGFR-3-Ig-transduced and control mice, respectively. The vessels with wide lumens indicated with arrows in J and L are lymphatics, which are absent in I and K. Similar results were observed when the mice were injected at day 1 after birth and analyzed at the age of 7 days. Scale bars = 50 μm.
Despite the massive regression of the lymphatic vasculature, and in some cases almost complete lack of lymphatic capillaries and medium-sized lymphatic vessels, no signs of lymphedema, chylous fluid accumulation, or growth retardation were observed in the AdVEGFR-3-Ig-transduced mice (data not shown). The average VEGFR-3-Ig level in the sera of the mice that were treated with AdVEGFR-3-Ig at the age of 1 or 7 days was 572 ± 138 ng/ml (n = 18) 1 week after injection. Similar regression of the lymphatic vessels occurred in wild-type C57BL/6 littermates when analyzed by immunohistochemical staining of the skin, diaphragm, and intestine (data not shown), which indicates that the regression of lymphatic vessels in the VEGFR-3+/LacZ mice was not because of VEGFR-3haploinsufficiency. In wild-type NMRI mice slightly more lymphatic vessels persisted than in the C57BL/6 mice, indicating variability related to the genetic background (data not shown).
AdVEGFR-3-Ig also induced postnatal regression of the hyperplastic lymphatic vessels in transgenic mice expressing a VEGFR-3-specific mutant form of VEGF-C in the skin under control of the keratin 14 promoter (K14-VEGF-C156S) (Supplemental Figure 1, A–F; see http://ajp.amjpathol.org). This indicates that the abnormal, enlarged lymphatic vessels caused by an excessive amount of a VEGFR-3 ligand are dependent on continuous VEGFR-3 signaling during the postnatal period.
In addition to VEGFR-3, the fully processed, mature form of VEGF-C binds to and activates VEGFR-2, which is expressed predominantly in blood vessels and collecting lymphatic vessels.22 The exact role of VEGFR-2 in the lymphatic vasculature is unknown, although there is some indication of its involvement in lymphangiogenesis when stimulated either by VEGF or the mature form of VEGF-C.22–24 Treatment of postnatal mice with AdVEGFR-2-Ig did not have any apparent effect on blood or lymphatic vessels in the skin or diaphragm (Supplemental Figure 2, A and B; see http://ajp.amjpathol.org; and data not shown). The failure of VEGFR-2-Ig, which was produced and circulating in the blood at comparable levels to VEGFR-3-Ig, to affect the vessels of postnatal mice could in part depend on the lower binding affinity of VEGFR-2 for both VEGF-C (kd, 410 pmol/L)16 and for VEGF (75 to 250 pmol/L)25 as compared to that of VEGFR-3 for VEGF-C (135 pmol/L)16 or of VEGFR-1 for VEGF (25 pmol/L).25
Lymphatic Vessels Become Independent of the Ligand-Activated VEGFR-3 Pathway after the First Two Postnatal Weeks
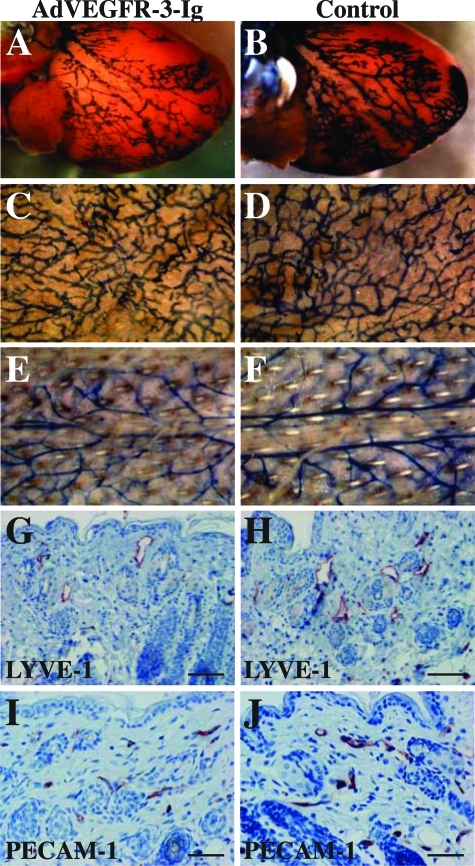
When the VEGFR-3+/LacZ mice were transduced with AdVEGFR-3-Ig at the age of 2 weeks or older and the lymphatic vessels were analyzed 1 week after the injection, no regression of the already formed lymphatic vessels or inhibition of further lymphangiogenesis was observed (Figure 3, A–H). The level of VEGFR-3-Ig in the sera of these mice was 562 ± 78 ng/ml (n = 5) at the time of analysis, which would neutralize ∼100 ng/ml of VEGF-C.18 The VEGFR-3-Ig in the sera of these mice was functional as demonstrated by its ability to precipitate VEGF-C, and no detectable amounts of potentially neutralizing antibodies against VEGFR-3-Ig were observed by Western blotting (data not shown).
Figure 3-6926.
Lymphatic vessels become resistant to inhibition of VEGFR-3 signaling after the first 2 postnatal weeks. β-Galactosidase staining of hearts (A and B), ears (C and D), and tail skin (E and F) and immunohistochemical staining of skin sections using antibodies against LYVE-1 (G and H, red) and PECAM-1 (I and J, red) of VEGFR-3+/LacZ mice transduced intraperitoneally with AdVEGFR-3-Ig or PBS, respectively, at the age of 2 weeks and analyzed 1 week thereafter. Scale bars = 50 μm.
Lymphatic Vessels Start to Regenerate during the Fourth Postnatal Week Despite Neutralizing Levels of VEGFR-3-Ig in the Serum
To avoid possible immune reactions against the adenovirus or the human protein and to allow longer observation times, the following experiments were done in nu/nu mice, which were transduced with AdVEGFR-3-Ig or AdLacZ 4 to 7 days after birth. At the age of 2 weeks, the functional lymphatic vessels in the ear, tail, and hind limb were visualized by fluorescent lymphangiography. In the ears of the AdVEGFR-3-Ig-treated mice, the tracer remained at the site of the injection or, in some cases, was taken up by a single thin lymphatic vessel, suggesting that the ears of the AdVEGFR-3-Ig-treated mice contained no or only few functional lymphatic vessels (Figure 4A). This was further confirmed by whole mount immunofluorescent staining for LYVE-1 (Figure 4E). In the tail of the AdVEGFR-3-Ig-treated mice, most of the small lymphatics were missing, but the fluorescent dextran was still drained through the main collecting lymphatic vessels, which appeared thinner than the ones in the control mice (data not shown). The para-aortic and axillary lymph nodes were smaller than those of the control mice, but the thoracic duct appeared unaffected (data not shown). In the diaphragm, almost complete regression of the lymphatic vessels was observed (data not shown).
Figure 4-6926.
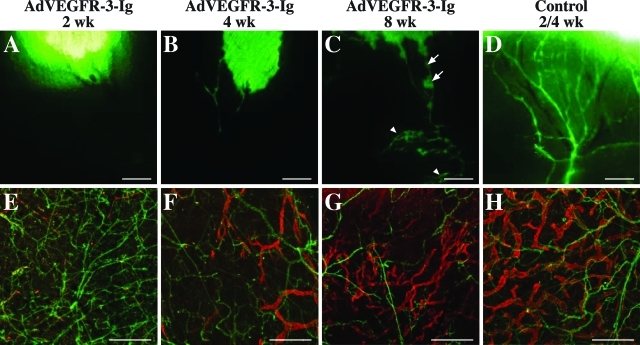
Lymphatic vessels start to regrow 4 weeks after birth despite neutralizing VEGFR-3-Ig levels in the serum. Fluorescent dextran lymphangiography in the ear skin (A–D) as well as whole-mount LYVE-1 (red) and PECAM-1 (green) staining of the ears (E–H) of nu/numice injected intraperitoneally with AdVEGFR-3-Ig (A–C and E–G) or AdLacZ (D and H) at the age of 1 week and analyzed at the indicated time points. Scale bars: 1 mm (A–D); 500 μm (E–H).
Lymphatic vessels of nu/numice transduced with AdVEGFR-3-Ig at the age of 4 days had started to regenerate when the mice were 4 weeks of age despite the presence of 480 ± 202 ng/ml (n = 5) of functional VEGFR-3-Ig in their sera (Figure 4, B and F, and Figure 1C). In the ear fluorescent dextran was taken up by a few thin lymphatic vessels (Figure 4B). The regeneration of the lymphatic vessels was confirmed in whole-mount immunohistochemistry of the ears, mesenterium, and diaphragm as well as by staining of skin sections (Figure 4F and data not shown). In the ear skin and in the diaphragm, lymphatic regeneration seemed to occur by enlargement and sprouting of the remnants of the regressed lymphatic vessels (Figure 4F and data not shown).
At the age of 8 weeks, the lymphatic vasculature in the skin, diaphragm, and mesenterium had widely regenerated. However, both the function (Figure 4C) and the organization (Figure 4G) of the regenerated lymphatic vessels appeared abnormal when compared to skin lymphatic vasculature in control mice of the same age (data not shown; Figure 4H). In these mice, fluorescent dextran was taken up by fewer lymphatic vessels and more slowly than in the control mice, and the lymphatic vessels appeared leaky (Figure 4C, arrows). Furthermore, dextran backflow occurred from the precollecting lymphatic vessels to the smaller capillaries, suggesting defective valves in the regenerated lymphatic vasculature (Figure 4C, arrowheads). The blood vessels of the AdVEGFR-3-Ig-treated mice were not affected at any age (Figure 4, E–H).
Effects of VEGFR-3-Blocking Antibodies and Recombinant VEGFR-3-Ig
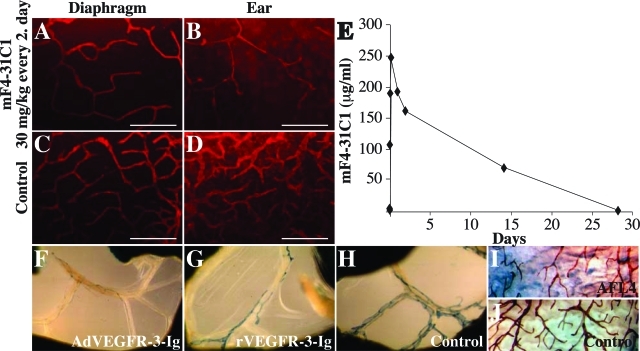
To exclude any contribution by responses against adenoviruses, we investigated if the lymphatic vessel regression could be reproduced by antibodies blocking VEGFR-3 signaling. The mF4-31C1 rat antibodies that block mouse VEGFR-317 or, as a control, nonblocking rat antibodies against mouse VEGFR-219 or PBS were injected intraperitoneally into nu/nu mice starting on day 4 after birth. Eight days thereafter the lymphatic vessels were analyzed by immunohistochemistry in diverse organs. Partial regression of lymphatic vessels was detected in the ear skin and diaphragm at a dose of 30 mg/kg injected every second day (Figure 5, A–D). Increasing the dose to 30 mg/kg or even to 60 mg/kg daily did not result in a more complete lymphatic vessel regression (data not shown), suggesting that 30 mg/kg every second day is a saturating dose. The systemic half-life of mF4-31C1 was found to be rather long, more than 5 days in nu/numice (Figure 5E). Partial regression of the lymphatics could also be observed in the mesenterium, stomach, and diaphragm of VEGFR-3+/LacZ mice injected intraperitoneally with a daily dose of 25 mg/kg of recombinant VEGFR-3-Ig protein (Figure 5G and data not shown) or of nu/nu mice injected daily with 20 mg/kg of AFL4 rat antibodies against mouse VEGFR-3 (Figure 5I and data not shown).
Figure 5-6926.
Intraperitoneally injected blocking antibodies against VEGFR-3 or recombinant VEGFR-3-Ig induce a partial regression of lymphatic vessels. A–D:nu/nu mice were injected intraperitoneally with mF4-31C1 blocking antibodies against VEGFR-3 at a dose of 30 mg/kg (A and B) or with PBS (C and D) every 2nd day starting at day 4 after birth. Eight days later the diaphragm (A and C) and the ears (B and D) were analyzed by fluorescent whole-mount staining with LYVE-1 antibodies (red). Similar results were obtained when a dose of 60 mg/kg every day was used. E: Pharmacokinetics of mF4-31C1 in nu/nu mice. F–H:VEGFR-3+/LacZ mice were either transduced with AdVEGFR-3-Ig at a single dose of 5 × 108 pfu (F) or injected intraperitoneally with recombinant VEGFR-3-Ig proteins (G) or control proteins (H) at a dose of 25 mg/kg once a day starting 1 day after birth and the mesenteric lymphatic vessels were stained with X-gal at the age of 7 days. I and J:nu/numice were injected intraperitoneally with AFL4 blocking antibodies against VEGFR-3 (I) or with control antibodies (J) at a dose of 20 mg/kg once a day starting 1 day after birth and the lymphatic vessels in the stomach were stained in whole mount with VEGFR-3 antibodies at the age of 7 days. Scale bars = 500 μm.
VEGF-D Transgene-Induced Lymphatic Hyperplasia Occurs after Birth
We next analyzed the relative contributions of VEGF-C and VEGF-D to embryonic lymphangiogenesis. VEGF-C is necessary for the initial development of the lymphatic vasculature,11 whereas lymphatic development occurs normally in VEGF-D-deficient mice.12 In K14-VEGF-C and K14-VEGF-C156S mice, the first signs of lymphatic hyperplasia are detected at E13.5, and at E14.5 the cutaneous lymphatic vessels of these embryos are clearly hyperplastic.22 To investigate the ability of VEGF-D to stimulate lymphatic hyperplasia during embryogenesis, we crossed K14-VEGF-D, and as a control, K14-VEGF-C mice with VEGFR-3+/LacZ mice. Interestingly, no signs of lymphatic hyperplasia were observed in the K14-VEGF-D × VEGFR-3+/LacZ mice when compared to control VEGFR-3+/LacZ littermates at E14.5, the age when the cutaneous lymphatic vessels of K14-VEGF-C × VEGFR-3+/LacZ mice were already hyperplastic (Figure 6, A–F). At E14.5, the fold increase in the number of lymphatic vessels at 0.6 mm distance from the dorsal midline as compared to the VEGFR-3+/LacZ littermates (n = 3) was 0.79 ± 0.27 in K14-VEGF-D × VEGFR-3+/LacZ embryos (n = 7, P > 0.5), and 2.15 ± 0.67 in K14-VEGF-C × VEGFR-3+/LacZ embryos (n = 6, P < 0.01). The fold increase in the lymphatic vessel area on the dorsal side as compared to the VEGFR-3+/LacZ littermates (n = 7) was 1.33 ± 0.17 in K14-VEGF-D × VEGFR-3+/LacZ embryos (n = 7, P > 0.02), and 2.31 ± 0.17 in K14-VEGF-C × VEGFR-3+/LacZembryos (n = 2; P < 0.01). At this stage the K14-VEGF-D mice expressed the transgene at even higher levels than the K14-VEGF-C mice as determined by quantitative RT-PCR (data not shown), confirming that the lack of the lymphatic phenotype in the K14-VEGF-D embryos at E14.5 is not because of delay in transgene expression. The first signs of hyperplasia in the cutaneous lymphatic vessels of K14-VEGF-D mice were detected on day 1 after birth (Figure 6, G–I), and at the age of 7 days the lymphatic vessels of K14-VEGF-D mice were indistinguishable from those of K14-VEGF-C mice (Figure 6, J–L). At P7, the fold increase of the lymphatic area as compared to the one in wild-type littermates (n = 9) was 7.08 ± 0.17 in K14-VEGF-D mice (n = 6, P < 0.001) and 6.60 ± 0.59 in K14-VEGF-C mice (n = 4, P < 0.001). These results suggest that postnatal lymphatic maturation involves the acquisition of sensitivity to VEGF-D-induced growth signals.
Discussion
This study demonstrates that the lymphatic vasculature of postnatal, but not of adult mice is dependent on ligand-stimulated VEGFR-3 signals. Inhibition of this signaling results in regression of lymphatic capillaries and medium-sized lymphatic vessels during the first 2 weeks after birth. However, the lymphatic vasculature starts to regenerate independently of VEGF-C and VEGF-D by the age of 4 weeks. Surprisingly, we also found that the postnatal, but not embryonic, lymphatic vessels are sensitive to the induction of hyperplasia by human VEGF-D.
Mice heterozygous for VEGF-Cdeficiency,11 mice harboring an inactivating mutation in the VEGFR-3gene, the Chy mice,26 as well as mice expressing VEGFR-3-Ig under a keratin promoter in the skin18 are born essentially without lymphatic capillaries and medium-sized lymphatic vessels, whereas their large collecting lymphatic vessels appear normal. However, in these mice, lymphatic vascular regeneration occurs in the visceral organs but not in the skin starting at 2 weeks of age. Postnatal regeneration of lymphatic capillaries in most tissues, including the skin, has also been observed in mice deficient of neuropilin-2,27 which acts as a co-receptor for VEGF-C.26 It is as yet not known from where the lymphatic regeneration occurs in these various mice. The large lymphatic vessels in the neuropilin-2-deficient mice and the lymphatic remnants in the AdVEGFR-3-Ig-treated mice could serve as origins of lymphatic sprouting. In contrast, the lymphatic vessels are almost completely absent from the skin of the heterozygous VEGF-C gene targeted mice, Chy mice, and K14-VEGFR-3-Ig mice.
Our current results indicate that the VEGF-C/VEGFR-3 pathway is absolutely required for the maintenance of lymphatic vasculature during the first few weeks after birth. Later on the survival of lymphatic vessels becomes independent of VEGFR-3 ligands and their signals, indicating that a process of lymphatic capillary maturation occurs in postnatal mice at ∼2 weeks of age. After that, the regrowth of lymphatic vessels occurs without VEGFR-3 ligands, indicating that a VEGF-C- and VEGF-D-independent mechanism that induces lymphatic growth must exist in mice older than 2 to 3 weeks. Although the VEGF-C/VEGFR-3 pathway is not needed after this stage for the growth of the lymphatic vessels, it is apparently functional, because lymphangiogenesis can be stimulated by VEGF-C, when delivered for example via an adenovirus.15,22 Possible postnatal factors responsible for the VEGF-C/VEGF-D/VEGFR-3-independent pathway include VEGF,23,24 HGF,28 and the angiopoietins,20,29,30 which have all been shown to stimulate lymphangiogenesis.
Previous studies have indicated that isolated lymphatic endothelial cells can survive and proliferate to some extent without VEGFR-3 ligand stimulation on fibronectin but not on uncoated tissue culture plates.31 This suggests that the extracellular matrix provides important growth-promoting signals for lymphatic vessels. Furthermore, it was shown that integrin β1 can directly interact with VEGFR-3 and modulate its phosphorylation, thus affecting lymphatic endothelial cell migration.32,33 Integrin α9β1-deficient mice die 6 to 12 days after birth because of chylothorax caused by a failure of lymphatic vessels, which indicates a crucial function for integrin α9β1 in lymphatic development.34 Indeed, integrin α9β1 was shown to directly bind VEGF-C and VEGF-D and to promote endothelial cell adhesion and migration.35 The molecular mechanism of postnatal lymphatic maturation and of the VEGFR-3 ligand-independent form of lymphangiogenesis could involve such stabilizing signaling by the extracellular matrix, possibly mediated by integrins. However, it remains to be elucidated why the matrix signals would become activated only a few weeks after birth and why they cannot compensate for the lack of ligand-stimulated VEGFR-3 signaling during embryogenesis and the immediate postnatal period. The fact that VEGF-D is not able to induce lymphangiogenesis during embryogenesis suggests that VEGF-D-induced VEGFR-3 activation might depend on such additional maturation signals for lymphatic endothelial cells.
VEGFgene deletion or daily administration of VEGFR-1-Ig in postnatal mice has been shown to result in endothelial cell apoptosis, leading to severely reduced numbers of blood vessels and increased postnatal lethality.36 The susceptibility of the blood vascular endothelial cells to VEGF depletion is lost, or at least greatly decreased, after the 4th postnatal week, suggesting a process of blood vessel maturation that results in VEGF-independent survival of blood vascular endothelial cells.36,37 In the adult, VEGF seems to be required mainly for active angiogenic processes, such as corpus luteum development and wound healing, and to be responsible for angiogenesis in several pathological conditions, such as tumor growth and arthritis.25 VEGF has also been implicated for the survival of fenestrated blood capillaries.38 Coverage of the blood vessels by pericytes starts early, during late embryonic development, indicating that it cannot be the only mechanism of vessel maturation. Because the ablation of the VEGFgene during the first postnatal week leads to death in 38% of the pups,36 it would be interesting to know if the blood vessels of the surviving mice can regenerate independently of VEGF in a similar manner to the regeneration of lymphatic vessels without VEGF-C and VEGF-D.
Spread through lymphatic vessels into regional lymph nodes is commonly the first step in the dissemination of human cancer. A correlation between VEGF-C expression by the primary tumor and metastasis to the sentinel lymph nodes has been reported for several types of human cancers.39 Overexpression of VEGF-C and VEGF-D in experimental tumors has been shown to induce tumor lymphangiogenesis and to promote tumor metastasis.13,40–42 Furthermore, VEGF-C/D-induced tumor lymphangiogenesis and lymphatic metastasis can be inhibited by blocking the interaction of VEGFR-3 with its ligands.13,14,41 It is not known if active lymphangiogenesis occurs in human tumors or if overexpression of VEGF-C by the tumor cells promotes lymphatic metastasis by some more subtle means, for example by facilitating interaction of the tumor cells with the lymphatic endothelium or by priming the lymph nodes for metastatic tumor cells. In any case, our study suggests that the blocking of VEGF-C and VEGF-D should be a safe method to inhibit tumor metastasis because normal lymphatic vessels are not affected by such treatment in adults.
Supplementary Material
Acknowledgments
We thank Drs. Caroline Heckman and Tatiana V. Petrova for critical comments on the manuscript; Dr. Hajime Kubo for the AFL4 antibodies; Drs. Eric Corcoran, Jim Tonra, and Kris Persaud for help with the pharmacokinetics of mF4-31C1; and Tapio Tainola, Sanna Lampi, Mari Helanterä, Paula Hyvärinen, Kaisa Makkonen, and Riikka Kivirikko-Ekman for excellent technical assistance.
Footnotes
Address reprint requests to Kari Alitalo, M.D., Ph.D., Molecular/Cancer Biology Laboratory, Biomedicum Helsinki, P.O.B. 63 (Haartmaninkatu 8), FI-00014 University of Helsinki, Finland. E-mail: kari.alitalo@helsinki.fi.
Related Commentary on page 347
Supported by the European Union (Lymphangiogenomics LSHG-CT-2004-503573), the National Institutes of Health (grant 5 R01 HL075183-02), the Academy of Finland (grants 202852 and 204312), the Novo Nordisk Foundation, the Finnish Cancer Organizations, the Science Foundation of Instrumentarium, the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Paulo Foundation, the K.A. Johanssons Foundation, the Biomedicum Helsinki Foundation, Pfizer (senior research fellowship to S.A.S.), and the Australian National Health and Medical Research Council (to S.A.S. and M.G.A.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of T.M.: Department of Molecular Neurobiology, Max-Planck-Institute of Neurobiology, Martinsried, Germany; current address of T.V.: Roche Oy, Espoo, Finland.
References
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Tammela T, Alitalo K, Wilting J. Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res. 2003;314:69–84. doi: 10.1007/s00441-003-0777-2. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VWM, Fang G-H, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25:2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Jackson DG, Ylä-Herttuala S, Jäättelä M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- Enholm B, Karpanen T, Jeltsch M, Kubo H, Stenback F, Prevo R, Jackson DG, Yla-Herttuala S, Alitalo K. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623–629. doi: 10.1161/01.res.88.6.623. [DOI] [PubMed] [Google Scholar]
- Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 2000;96:546–553. [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, Alitalo K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med. 2002;196:719–730. doi: 10.1084/jem.20020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Ylä-Herttuala S, Finegold DN, Ferrell RE, Alitalo K. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JF, Zhang X-F, Groopman JE. Stimulation of b1 integrin induces tyrosine phosphorylation of VEGF receptor-3 and modulates cell migration. J Biol Chem. 2001;276:41950–41957. doi: 10.1074/jbc.M101370200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202:205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin a9b1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H-P, Hillan KJ, Ryan AM, Kowalski J, Keller G-A, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.