Abstract
Objective: This report evaluated the efficacy and tolerability of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder (ADHD), diagnosed using DSM-IV-TR criteria, who did or did not receive prior treatment with stimulants for ADHD by examining pooled data from 3 randomized, double-blind, placebo-controlled studies.
Method: Three patient populations were evaluated: (1) all patients (i.e., all-patient group), (2) patients who were treated previously with stimulants (i.e., prior-stimulant group), and (3) patients who either were treated previously with ADHD medications other than stimulants or were not treated with any medications for ADHD (i.e., medication- or stimulantnaive group). Tolerability was evaluated by monitoring adverse events reported by both patients and parents. The 3 studies were conducted between November 2003 and June 2004.
Results: Of 638 patients randomized, 633 received modafinil (N = 420) or placebo (N = 213), 303 had received prior stimulant treatment (modafinil, 194; placebo, 109), and 330 had no prior stimulant experience (modafinil, 226; placebo, 104). Modafinil improved symptoms of ADHD, as assessed by ADHD-RS-IV School Version total scores (mean change from baseline to final visit compared with placebo) in the all-patient group (−16.4 vs. −8.3) (p < .0001), the prior-stimulant group (−14.2 vs. −9.3) (p < .001), and the medication-or stimulant-naive group (−18.3 vs. −7.3) (p < .0001). Similar improvements were observed on the ADHD-RS-IV Home Version and for overall clinical condition. Insomnia, headache, and decreased appetite were the most commonly reported adverse events. Discontinuation because of adverse events was similar in the modafinil and placebo groups (5% vs. 3%).
Conclusions: This post hoc analysis extends previous findings that modafinil was well tolerated and improved the symptoms and behaviors of ADHD at school and at home as assessed by teachers, parents, and clinicians and improved patients' overall clinical condition. Improvements were shown regardless of history of stimulant use.
Attention-deficit/hyperactivity disorder (ADHD) is a neurobehavioral syndrome characterized by maladaptive and developmentally inappropriate levels of inattention, impulsivity, and hyperactivity. Pharmacotherapies used in the treatment of symptoms include the central nervous system (CNS) stimulants (i.e., methylphenidate and the amphetamines),1–4 the most commonly prescribed ADHD medications, and atomoxetine.5–8 While these are effective medications for children and adolescents with ADHD, it is estimated that approximately 30% of all patients do not respond adequately to stimulant medication or experience poor tolerability.9 For these reasons, new pharmacologic agents are needed to treat those unresponsive to standard ADHD medications.
One potential new treatment for ADHD is modafinil, an attention-promoting agent that appears to act on multiple areas of the ascending arousal and attention systems to increase frontal cortical activity. In a preclinical study, modafinil was shown to activate the prefrontal cortex selectively, without causing widespread activity throughout the CNS.10 Recently, a new, concentrated formulation of modafinil that was developed to administer targeted doses of 340 mg/day or 425 mg/day11 to children and adolescents with ADHD was evaluated in 3 double-blind, placebo-controlled studies.12–14 Modafinil film-coated tablets were shown to be well tolerated and to improve symptoms of ADHD and overall clinical condition.
In the double-blind, placebo-controlled studies of modafinil, a majority of patients had received prior therapy with stimulants. In guiding treatment, an understanding of how prior experience with stimulants may affect responses to subsequent pharmacologic intervention may be of help to physicians. Therefore, the main objective of this study was to evaluate the efficacy and tolerability of modafinil in children and adolescents with ADHD with or without prior experience with stimulants. To this end, data from the 3 double-blind, placebo-controlled studies were combined and evaluated in a post hoc analysis.
METHOD
Patients
Patients who participated in the 3 studies were all 6 to 17 years of age, with a diagnosis of ADHD based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).15 In addition, patients had total and/or subscale scores on the School version of the teacher/ investigator-rated Attention-Deficit/Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV) that were at least 1.5 standard deviations (SDs) above normal values for their age and gender16 and a Clinical Global Impressions-Severity of Illness (CGI-S) rating of 4 or higher (“moderately ill” or worse).17
Inclusion and exclusion criteria were identical for all 3 studies and are described in detail elsewhere.12–14 Briefly, patients were excluded if they had a history or current diagnosis of pervasive developmental disorder or schizophrenia or other psychotic disorders (DSM-IV Axis I), evidence of suicide risk, current psychiatric comorbidity that required pharmacotherapy, or other active clinically significant disease. Patients whose symptoms were well controlled and who were satisfied with current therapy for ADHD (with low levels of adverse events) were excluded, as were those who had failed to respond to 2 or more adequate courses (dose and duration of treatment) of stimulant therapy, with trials of a range of doses of immediate- and controlled-release formulations.
The institutional review board of each participating center reviewed and approved the study protocol. Written informed consent was obtained from the patient's parent or legal guardian before the study began, with consent obtained from the patient prior to enrollment; all patients were free to withdraw from the study at any time. Each study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation's Good Clinical Practice guidelines.
Study Design and Dosing
Two of the studies were identical in design: both were randomized, double-blind, placebo-controlled, flexible-dose studies conducted between November 2003 and June 2004 at a total of 42 sites in the United States.12,13 Patients who satisfied all entry criteria and discontinued prior ADHD medication over a 1- to 4-week washout period were randomly assigned 2:1 within each center to receive modafinil film-coated tablets or matching placebo tablets once daily in the morning for 9 weeks. The dose of modafinil was individually titrated based on tolerability and response using the following schedule: 85 mg on days 1 and 2, 170 mg on days 3 to 7, 255 mg on days 8 to 14, 340 mg on days 15 to 21, and 425 mg on day 22. Titration was stopped when any of the following conditions was met: poor tolerability, no further expected incremental improvement in efficacy, patient request, or achievement of a Clinical Global Impressions-Improvement (CGI-I)17 rating of 1. Those patients who received placebo increased the number of tablets to match the titration schedule of patients who received modafinil. The minimum and maximum daily doses allowed during the study were 170 mg and 425 mg, respectively.
The third study, conducted in 17 U.S. centers between November 2003 and June 2004, was a 7-week, double-blind, randomized, fixed-dose, placebo-controlled study followed by abrupt discontinuation and a 2-week blinded observational phase.14 Patients were stratified by weight for dosing (340 mg for patients < 30 kg and 425 mg for patients ≥ 30 kg) and randomly assigned (2:1) to receive once-daily modafinil or placebo every morning. Study drug was titrated during the first 7 to 9 days of the study according to the following schedule: 85 mg on day 1, with the dose increased by 85 mg every other day until the predetermined dose was reached. Each patient remained on the randomized dose through week 7. For the final 2 weeks (the observation period), patients in the modafinil group were randomly assigned to receive either modafinil or placebo, whereas patients in the placebo group continued to take placebo. Results from the observation period are presented elsewhere.14
Patients in all 3 studies who completed at least 4 weeks of study drug and did not discontinue the study because of an adverse event were eligible for inclusion in a 1-year, open-label extension study.
Efficacy Assessments
Efficacy was evaluated using the School and Home versions of the Attention-Deficit/Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV),16 a rating scale designed to assess the 18 symptoms that make up the DSM-IV-TR diagnostic criteria for ADHD.15 The primary efficacy measure was the change from baseline to the final visit in total score on the ADHD-RS-IV School Version. Investigators completed the ADHD-RS-IV School Version based on an interview with the patient's primary teacher before each visit. Investigators completed the ADHD-RS-IV Home Version at each visit on the basis of an interview with the patient's parent or guardian (and the child, when appropriate) to assess perceptions of behavior during the evening hours (between 6:00 p.m. and 8:00 p.m.) and on weekends. The School and Home versions of the ADHD-RS-IV were completed at baseline and weeks 1, 2, 3, 5, 7, and 9 in the flexible-dose studies and at baseline and weeks 1, 2, 3, 5, and 7 in the fixed-dose study.
The CGI-S17 was used to evaluate the severity of the overall clinical condition associated with ADHD at baseline. The CGI-I17 was used by the physician to evaluate overall clinical condition. The CGI-I was assessed at weeks 1, 2, 3, 5, 7, and 9 in the flexible-dose studies and weeks 1, 2, 3, 5, and 7 in the fixed-dose study.
An additional efficacy measure was the Conners' Parent Rating Scale–Revised, Short Form (CPRS–R:S).18 Assessments were made within 24 hours of scheduled clinic visits, with items rated according to the patient's behavior since the last assessment. In the fixed-dose study, the CPRS–R:S was completed at baseline and weeks 3 and 7; in the flexible-dose studies, it was also completed at week 9.
Tolerability Assessments
Tolerability was evaluated by monitoring adverse events reported by both parents and patients at baseline, all study visits, and any time between visits. The severity of each adverse event was rated as mild, moderate, or severe. Vital signs and body weight were measured and hematology tests were performed at baseline and weeks 1, 2, 3, 5, 7, and 9 in the flexible-dose studies and at baseline and weeks 1, 2, 3, 5, and 7 in the fixed-dose study. Twelve-lead electrocardiograms (ECGs) and physical examinations were conducted at screening and week 9 in the flexible-dose studies and at screening and week 7 in the fixed-dose study. Serum chemistry and urinalysis were performed at baseline and week 9 in the flexible-dose studies and at baseline and week 7 in the fixed-dose study.
Statistical Analyses
Data from the 3 studies were pooled following statistical confirmation that there were no treatment-by-study interactions. Three patient populations were evaluated: (1) all patients (i.e., all-patient group), (2) patients who were treated previously with stimulants, including dexamphetamine, dexmethylphenidate, methylphenidate, or pemoline (i.e., prior-stimulant group), and (3) patients who either were treated previously with ADHD medications other than stimulants or were not treated with any medications for ADHD (i.e., medication- or stimulant-naive group). The efficacy analyses included data from patients who received at least 1 dose of study drug and had at least 1 postbaseline primary efficacy assessment. The safety analysis included data from patients who received at least 1 dose of study drug.
All statistical tests were 2-tailed, with a significance level of .05. Demographic and baseline characteristics were summarized using descriptive statistics. The effects of modafinil versus placebo on the efficacy assessments (except the CGI-I) were compared weekly and at the final visit (i.e., last postbaseline visit carried forward [week 9 or early termination in the flexible-dose studies and week 7 or early termination in the fixed-dose study]) using an analysis-of-covariance model, with treatment and center as factors and the corresponding baseline value as a covariate. CGI-I ratings were analyzed using the Cochran-Mantel-Haenszel test adjusted for center. CGI-I responders were defined as patients rated as “much improved” or “very much improved” on the CGI-I. To assess changes in body weight, individual weights were converted to standardized z scores, which represent the number of SDs above or below the mean for the age- and gender-specific general pediatric and adolescent populations.19,20 A clinically meaningful change was defined as a decrease or increase of 1 in z score (i.e., 1 SD) and an absolute value greater than 1.5 (i.e., below the 15th percentile for decrease and above the 85th percentile for increase).
RESULTS
Patients
Of the 638 patients enrolled in the 3 studies, 423 were randomly assigned to modafinil and 215 to placebo (Table 1). The proportion of patients who discontinued was greater in the placebo group (47%) than in the modafinil group (35%). A greater proportion of patients discontinued because of lack of efficacy in the placebo group (34%) than in the modafinil group (16%). Other reasons for discontinuation are listed in Table 1.
Table 1.
Disposition of Patients
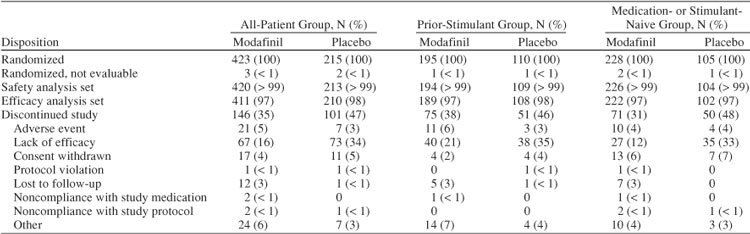
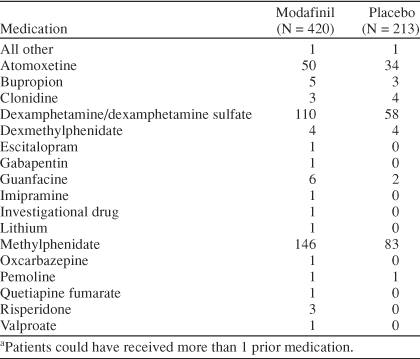
A total of 305 patients (48%) received prior stimulant therapy and 333 patients (52%) were medication- or stimulant-naive. The stimulant- or medication-naive group included more females, was slightly younger on average, had more patients who were moderately ill and fewer patients who were severely ill, had more patients diagnosed with the inattentive subtype and fewer patients diagnosed with the combined subtype, and on average weighed less than the group who received prior stimulant therapy (Table 2). Prior medications for ADHD are shown in Table 3.
Table 2.
Demographic and Clinical Characteristics at Baseline
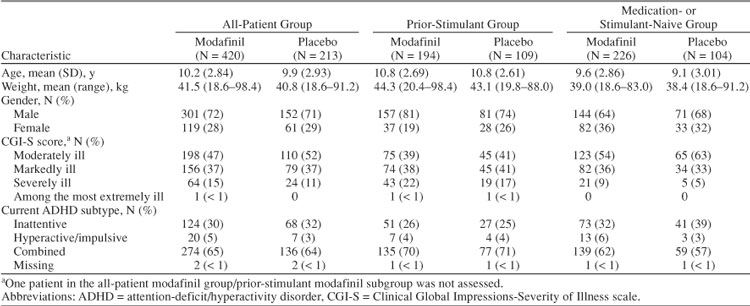
Table 3.
Prior Attention-Deficit/Hyperactivity Disorder Medicationa
Efficacy
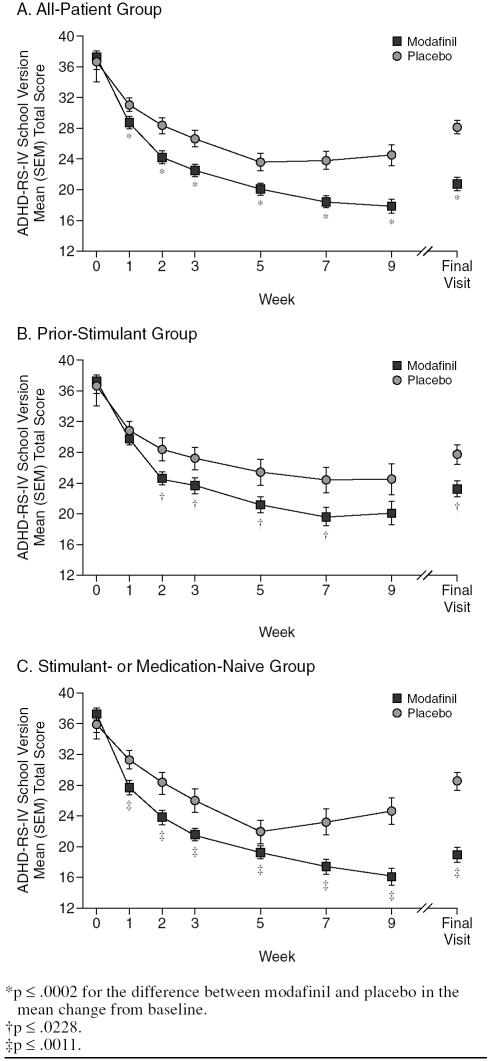
Modafinil improved symptoms of ADHD at all post-baseline visits and at the final visit. Significant improvements from baseline in the mean total score of the ADHD-RS-IV School Version were shown for modafinil compared with placebo in the all-patient group at week 1, with improvements increasing in magnitude throughout the study (all p ≤ .0002) (Figure 1A). At the final visit, the mean (SD) change from baseline in total score was −16.4 (12.5) for modafinil and −8.3 (10.1) for placebo (p < .0001; effect size 0.69) (Table 4). In addition, significant differences between modafinil and placebo in the all-patient group were shown for the change from baseline to the final visit in mean ADHD-RS-IV School Version sub-scale scores for inattention (mean [SD] change, −9.3 [7.3] vs. −5.1 [5.8]) and hyperactivity-impulsivity (−7.2 [6.9] vs. −3.3 [5.9]) (both p < .0001). Modafinil improved symptoms of ADHD on the ADHD-RS-IV School Version regardless of history of stimulant use. Greater reductions from baseline to postbaseline visits and the final visit in mean total scores were shown for modafinil compared with placebo in patients in the prior-stimulant group (all p ≤ .0228; effect size at final visit 0.41) (Figure 1B; Table 4) and in patients in the medication- or stimulant-naive group (all p ≤ .0011; effect size at final visit 0.97) (Figure 1C; Table 4).
Figure 1.
Teacher/Investigator-Rated Attention-Deficit/ Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV) School Version: Mean Total Score by Visit
Table 4.
Changes From Baseline to the Final Visit in ADHD-RS-IV Total Scores
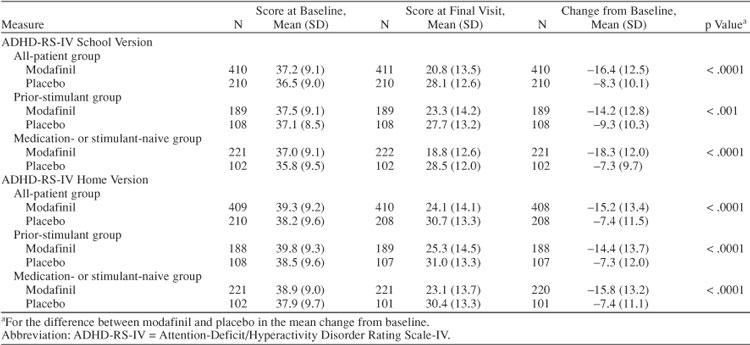
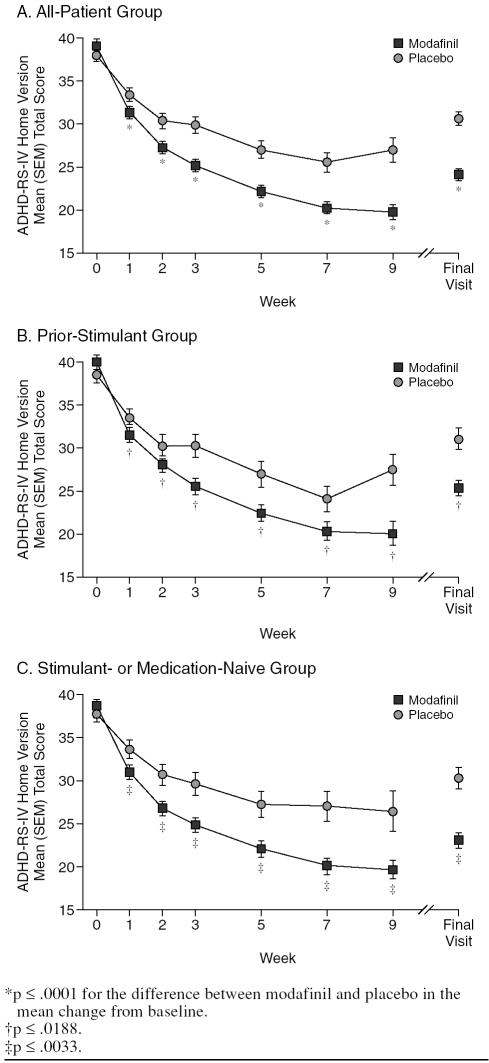
Improvements in mean total scores on the ADHD-RS-IV Home Version for those receiving modafinil in the all-patient group were consistent with improvements shown in total scores on the ADHD-RS-IV School Version (all p ≤ .0001) (Figure 2A; Table 4). Likewise, greater reductions for modafinil versus placebo in the all-patient group were shown in mean subscale scores for inattention (mean [SD] change from baseline to the final visit, modafinil −8.1 [7.29] vs. placebo −3.7 [6.33], respectively; p < .0001) and hyperactivity-impulsivity (−7.0 [7.20] vs. −3.7 [6.29], respectively; p < .0001). Modafinil improved total scores on the ADHD-RS-IV Home Version compared with placebo in the prior-stimulant group (all p ≤ .0188) (Figure 2B; Table 4) and in the medication-or stimulant-naive group (all p ≤ .0033) (Figure 2C; Table 4).
Figure 2.
Parent/Investigator-Rated Attention-Deficit/ Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV) Home Version: Mean Total Score by Visit
Patients receiving modafinil showed greater improvement in overall clinical condition than those receiving placebo. For the all-patient group, the proportion of patients who were rated as CGI-I responders was greater for those receiving modafinil than for those receiving placebo at all study visits (all p ≤ .0001) and at the final visit (46% vs. 18%). For patients in the prior-stimulant group, 39% of patients receiving modafinil and 17% of those receiving placebo were classified as responders at the final visit. For patients in the medication- or stimulant-naive group, 51% of patients who received modafinil were rated as responders compared with 19% of those who received placebo.
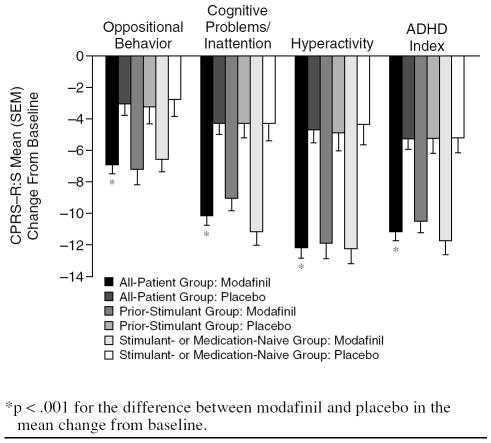
Greater improvements from baseline to the final visit in mean scores on the CPRS–R:S were shown for modafinil compared with placebo in the domains of oppositional behavior, cognitive problems/inattention, hyperactivity, and ADHD index scores (Figure 3) (all-patient group, all p < .001).
Figure 3.
Conners' Parent Rating Scale–Revised, Short Form (CPRS–R:S): Mean Change From Baseline to the Final Visit
Tolerability
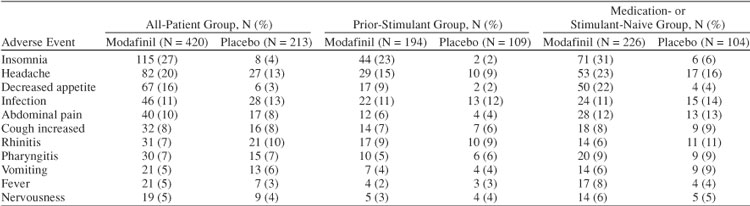
Overall discontinuation because of adverse events was similar in the modafinil and placebo groups (5% vs. 3%; Table 1). Discontinuations because of adverse events for those receiving modafinil (6%) were greater than for those receiving placebo (3%) in the prior-stimulant group, but similar in the medication- or stimulant-naive group (modafinil 4%, placebo 4%). For all patients, the most common adverse events were insomnia (modafinil 27% vs. placebo 4%), headache (20% vs. 13%), and decreased appetite (16% vs. 3%) (Table 5). A total of 8 serious adverse events (influenza syndrome, duodenitis, peptic ulcer, dehydration, hypertonia, asthma, erythema multiforme, and 1 case of possible Stevens-Johnson syndrome) were reported for 4 patients (< 1%) of 420 patients who received modafinil. No serious adverse events were reported for patients receiving placebo.
Table 5.
Adverse Events Reported in at Least 5% of All Patients
For all patients, no clinically meaningful changes in mean heart rate, blood pressure, or 12-lead ECG results were observed. Weight changes were small, with a mean increase of 1.0 kg in the placebo group compared with a mean decrease of 0.7 kg in the modafinil group. The mean z score for height was unchanged from baseline to final visit, and the mean z score for weight decreased by 0.2. Mean height and weight percentile scores decreased by 0.3 and 5.4, respectively, in modafinil patients. The mean change in absolute neutrophil count was −0.4 × 109/L for the modafinil treatment group and −0.1 × 109/L for the placebo group. There were no clinically meaningful changes from baseline in other mean laboratory evaluations.
DISCUSSION
This post hoc analysis extends previous findings by showing that modafinil improved ADHD symptoms in children and adolescents regardless of their prior history of stimulant use. The findings are consistent with those reported in the 3 independent clinical trials,12–14 documenting that modafinil film-coated tablets improved core symptoms of ADHD at school and at home, as rated by teachers, parents, and investigators, compared with placebo. In this analysis, the mean improvement shown with modafinil in the ADHD-RS-IV School Version total score, the primary efficacy measure, was comparable to improvements shown in the individual studies, in which mean changes from baseline to the final visit ranged from −15.0 to −17.5 for modafinil compared with −7.3 to −9.7 for placebo (all p < .0001) (range of effect sizes, 0.63 to 0.76; effect size for combined group 0.69).12–14
Patients from both the medication- or stimulant-naive group and the prior-stimulant group had significantly greater improvement on the ADHD-RS-IV School Version compared with patients from the respective placebo groups (p ≤ .228) with a large effect size for the medication- or stimulant-naive group (0.97) and a moderate effect size for the prior-stimulant group (0.41). Overall, the magnitude of improvements on the various ADHD rating scales and subscales used was slightly greater for patients in the medication- or stimulant-naive group than for patients in the prior-stimulant group, with the exception of the oppositional behavior subscale of the CPRS–R:S, for which the reverse was true. With regard to overall clinical condition, a greater proportion of patients receiving modafinil in the medication- or stimulant-naive group was rated as responders (i.e., much improved or very much improved) than patients in the prior-stimulant group (51% vs. 39%), with proportions of responders in both groups being greater than those in their respective placebo groups (19% and 17%).
Regardless of prior stimulant experience, modafinil was reasonably well tolerated, with insomnia and decreased appetite being reported more commonly for modafinil than for placebo. Although patients in the stimulant- or medication-naive group appeared to report more adverse events than patients in the prior-stimulant group, a very small minority of patients in both groups (5% and 4%, respectively) discontinued because of an adverse event, indicating that most adverse events were tolerable and not a cause for discontinuation.
Clinical Implications
Few studies have reported how prior experience with stimulants may affect responses to subsequent pharmacologic intervention. In the Multimodal Study of Children with Attention-Deficit/Hyperactivity Disorder,21 prior medication status was shown to moderate outcomes on a parent-completed rating scale of social skills. Medication management was superior to community care in patients with prior treatment with medication, while in previously unmedicated patients, there was no significant difference between treatment groups. Although there are no large-scale studies to support the general recommendation9 that patients who do not adequately respond to or cannot tolerate stimulant therapy be switched to a different class of medication, there are reports that patients who switch from stimulants may still achieve a good response.22,23 The finding that modafinil-associated improvement in ADHD symptoms is not affected by prior stimulant history suggests that modafinil may be a therapeutic option for the treatment of children and adolescents with ADHD who do not respond to or cannot tolerate 1 trial of stimulant therapy. In addition, medication- or stimulant-naive patients would be expected to respond to modafinil. Although the effect sizes suggest that the response to modafinil was more robust in patients who were stimulant or medication naive than in patients who had received prior stimulant therapy, the study was not designed to compare these groups. Thus, we cannot definitively state that the response to modafinil would be greater for medication- or stimulant-naive patients than for patients who had received prior stimulant therapy.
Limitations
The results of this analysis of the combined data set should be viewed with an understanding of the limitations of this methodological approach. Because the study was a post hoc analysis, the findings need to be viewed as preliminary until confirmed in future studies. In addition, the post hoc analyses were not adequately powered to detect differences between the 2 groups of patients evaluated; despite this, significant differences were observed on the School and Home versions of the ADHD-RS-IV. Patients were excluded from the original studies combined for this analysis if they had failed to respond to 2 or more adequate courses of prior stimulant therapy. Therefore, the findings may not apply to patients who failed multiple treatments; however, they do apply to those who failed 1 stimulant trial. Future studies designed to assess a priori the efficacy and tolerability of modafinil in patients with multiple unsuccessful prior stimulant trials are needed to address this issue.
CONCLUSIONS
This post hoc analysis of data from 3 double-blind, placebo-controlled studies showed that modafinil film-coated tablets improved symptoms of ADHD compared with placebo, irrespective of prior exposure to stimulant therapy. Modafinil may represent a viable pharmacologic option for children and adolescents with ADHD, including those who have tried stimulant therapy without success.
Drug names: atomoxetine (Strattera), bupropion (Wellbutrin and others), clonidine (Catapres and others), dexmethylphenidate (Focalin), escitalopram (Lexapro and others), gabapentin (Neurontin and others), guanfacine (Tenex and others), imipramine (Tofranil and others), lithium (Eskalith, Lithobid, and others), methylphenidate (Ritalin and others), modafinil (Provigil), oxcarbazepine (Trileptal), quetiapine (Seroquel), risperidone (Risperdal).
Financial disclosure: Dr. Wigal has been a consultant for and has been on the speakers/advisory boards of Cephalon, McNeil, Shire, and Celltech and has received grant/research support from Cephalon, Eli Lilly, Shire, New River Pharmaceuticals, and the National Institute of Mental Health. Dr. Biederman receives research support from Shire, Eli Lilly, Pfizer, McNeil, Abbott, Bristol-Myers Squibb, New River Pharmaceuticals, Cephalon, Janssen, Neurosearch, Stanley Medical Research Foundation, Lilly Foundation, Prechter Foundation, National Institute of Mental Health, National Institute of Child Health and Human Development, and National Institute on Drug Abuse; is on the speakers bureaus of Shire, Eli Lilly, McNeil, Cephalon, and UCB Pharma; and is on the advisory boards of Eli Lilly, Shire, McNeil, Janssen, Novartis, and Cephalon. Dr. Swanson has been a consultant for, received grant/research support from, and been on the speakers/ advisory boards of McNeil, Shire, Cephalon, Novartis, UBC, and Eli Lilly. Dr. Yang is an employee of Cephalon. Dr. Greenhill has been a consultant for Eli Lilly, McNeil, Novartis, Pfizer, Janssen, and Cephalon; has received grant/research support from Eli Lilly, Novartis, McNeil, and Forest Labs; has received honoraria from Pfizer and Novartis; and has been on the speakers/advisory boards of Eli Lilly, Forest, and Novartis.
Footnotes
This study was sponsored by Cephalon, Inc., Frazer, Pa.
Financial disclosure is listed at the end of the article.
The authors thank John Jiang, Ph.D., of Cephalon, Inc., for providing the statistical analyses.
REFERENCES
- Brown RT, Amler RW, and Freeman WS. et al. Treatment of attention-deficit/ hyperactivity disorder: overview of the evidence. Pediatrics. 2005 115:e749–e757. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, and Swanson JM. et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. J Dev Behav Pediatr. 2001 22:60–73. [DOI] [PubMed] [Google Scholar]
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-ups: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113:754–761. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- Kelsey DK, Sumner CR, and Casat CD. et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004 114:e1–e8. [DOI] [PubMed] [Google Scholar]
- Michelson D, Faries D, and Wernicke J. et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics. 2001 108:E83. [DOI] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, and Busner J. et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002 159:1896–1901. [DOI] [PubMed] [Google Scholar]
- Weiss M, Tannock R, and Kratochvil C. et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005 44:647–655. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2004;7:77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Grasela T, and Robertson P Jr. et al. Population pharmacokinetics of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder [poster]. Presented at the annual meeting of the American Association of Pharmaceutical Scientists; Nov 6–10. 2005 Nashville, Tenn. [Google Scholar]
- Biederman J, Swanson JM, and Wigal SB. et al. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005 116:e777–e784. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Biederman J, and Boellner SW. et al. A randomized, double-blind, placebo-controlled study of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006 45:503–511. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Greenhill LL, and Lopez FA. et al. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J Clin Psychiatry. 2006 67:137–147. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association. 2000 [Google Scholar]
- DuPaul GJ, Power TJ, and Anastopoulos AD. et al. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press. 1998 [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health. 1976 [Google Scholar]
- Conners CK. Conners' Parent Rating Scales-Revised: Short Form (CPRS-R:S). Tonawanda, NY: Multi-Health Systems, Inc. 1997 [Google Scholar]
- Kuczmarski RJ, Ogden CL, and Guo SS. et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002 1–190. [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, and Flegal KM. et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002 109:45–60. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the Multimodal Treatment Study of Children with Attention-Deficit/ Hyperactivity Disorder. Arch Gen Psychiatry. 1999;56:1088–1096. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- Swanson J, Gupta S, and Guinta D. et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999 66:295–305. [DOI] [PubMed] [Google Scholar]
- Arnold LE. Methylphenidate versus amphetamine: a comparative review. In: Greenhill LL, Osman BB, eds. Ritalin: Theory and Practice. 2nd ed. Larchmont, NY: Mary Ann Liebert, Inc. 2000 127–140. [Google Scholar]