Abstract
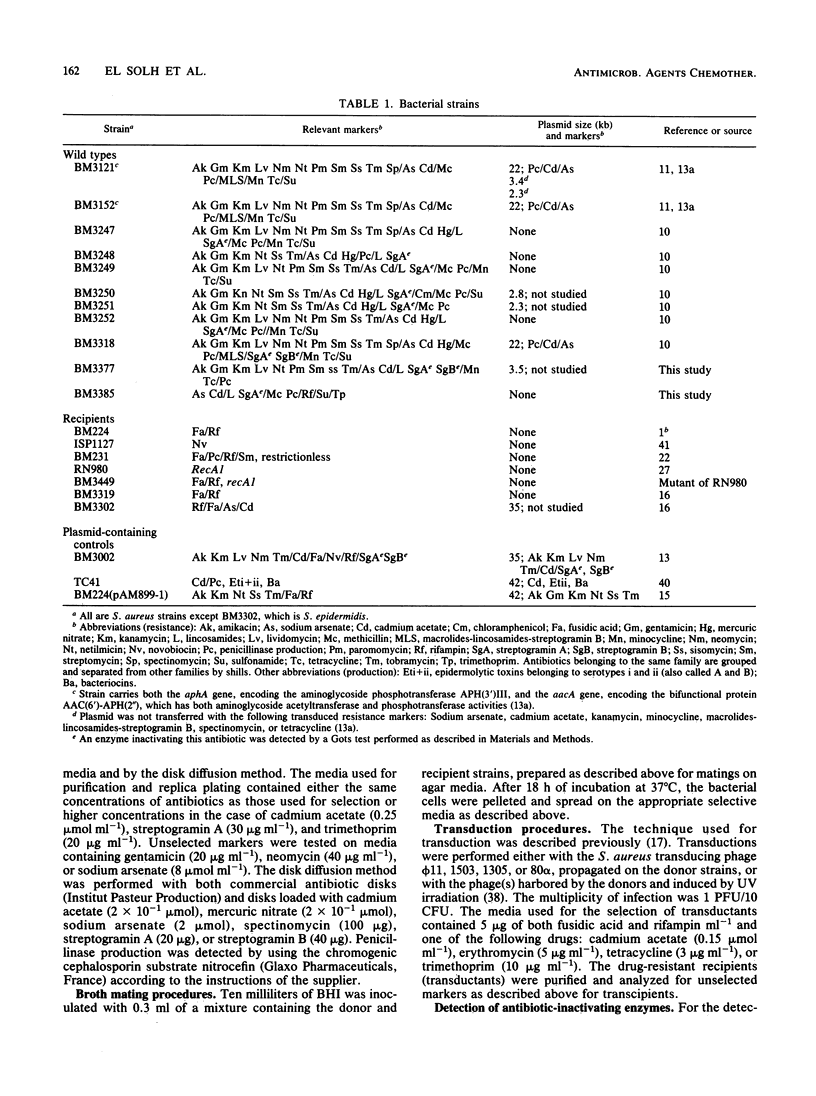
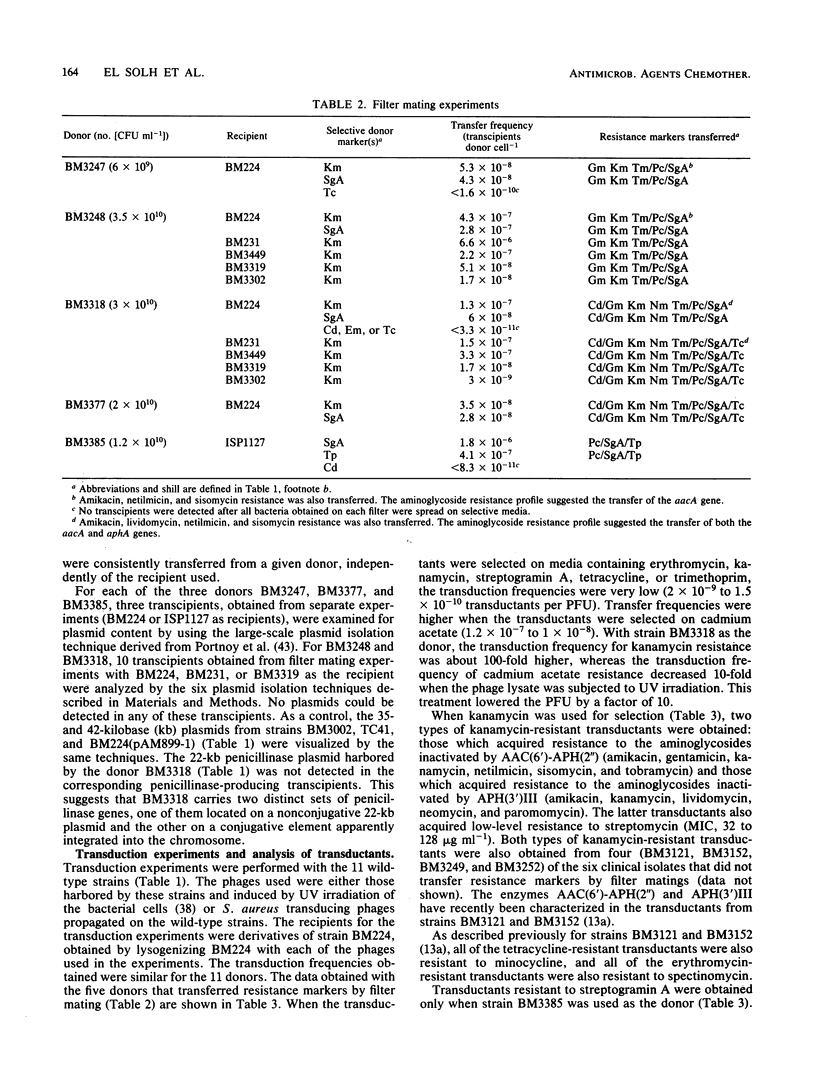
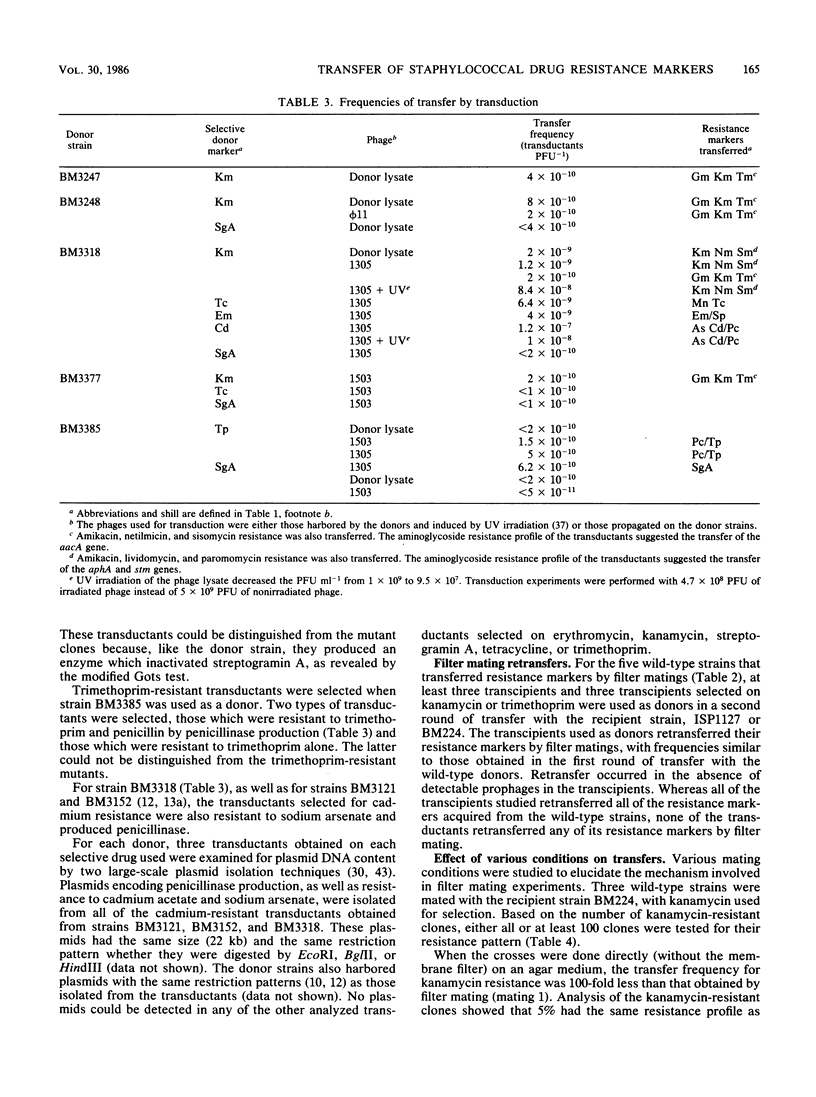
Eleven Staphylococcus aureus clinical isolates were tested for transfer of resistance markers by transduction and filter mating. The resistance markers of six of the strains could be transferred only by transduction; however, the five remaining strains transferred their resistance both by transduction and filter mating. The resistance markers that were cotransferred in filter matings (transfer of resistance to penicillin and streptogramin A was accompanied, in each case, by the transfer of one or more markers, i.e., resistance to aminoglycosides, cadmium, or tetracycline, depending on the donor) were not cotransduced. The filter mating transfers were recA independent and were observed with both Staphylococcus aureus and Staphylococcus epidermidis recipients. Experiments to elucidate the mechanism of transfer by filter mating suggested that conjugation requiring cell-to-cell contact may have been involved. These transfers occurred in the absence of detectable plasmid DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch D. K., Goering R. V., Ruff E. A. Isolation and preliminary characterization of a plasmid mutant derepressed for conjugal transfer in Staphylococcus aureus. Plasmid. 1984 Nov;12(3):197–202. doi: 10.1016/0147-619x(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Horodniceanu T. Conjugative transfer of multiple antibiotic resistance markers in Streptococcus pneumoniae. J Bacteriol. 1980 Jul;143(1):313–320. doi: 10.1128/jb.143.1.313-320.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson S., Brumfitt W., Hamilton-Miller J. M. Stability of aminoglycoside resistance in vitro in gentamicin-resistant Staphylococcus aureus. J Hyg (Lond) 1984 Aug;93(1):43–49. doi: 10.1017/s0022172400060915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A., Grandi G., Grandi R., Gryczan T. J., Shivakumar A. G., Dubnau D. Naturally occurring macrolide-lincosamide-streptogramin B resistance in Bacillus licheniformis. J Bacteriol. 1981 Jan;145(1):129–137. doi: 10.1128/jb.145.1.129-137.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Iandolo J. J. Autogenous transduction of phi 11de in Staphylococcus aureus: transfer and genetic properties. J Bacteriol. 1984 May;158(2):689–695. doi: 10.1128/jb.158.2.689-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Lee C. Y., Iandolo J. J. Generation of transducing particles in Staphylococcus aureus. J Bacteriol. 1985 Jan;161(1):91–95. doi: 10.1128/jb.161.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Solh N., Bismuth R., Allignet J., Fouace J. M. Résistance à la pristinamycine (ou virginiamycine) des souches de Staphylococcus aureus. Pathol Biol (Paris) 1984 May;32(5):362–368. [PubMed] [Google Scholar]
- El Solh N., Fouace J. M., Pillet J., Chabbert Y. A. Plasmid DNA content of multiresistant Staphylococcus aureus strains. Ann Microbiol (Paris) 1981 Sep-Oct;132B(2):131–156. [PubMed] [Google Scholar]
- Fitzgerald G. F., Clewell D. B. A conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun. 1985 Feb;47(2):415–420. doi: 10.1128/iai.47.2.415-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes B. A., Schaberg D. R. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983 Feb;153(2):627–634. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouace J., Ferré V. Transduction chez le staphylocoque: modifications des lysotypes observées chez des souches du groupe 3 après acquisition de certains caractères de réstistance aux antibiotiques. Ann Microbiol (Paris) 1973 Apr;124(3):381–392. [PubMed] [Google Scholar]
- Fouace J. Mixed cultures of Staphylococcus aureus: some observations concerning transfer of anitbiotic resistance. Ann Microbiol (Paris) 1981 Nov-Dec;132 B(3):375–386. [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., Bieth G. Conjugative transfer of multiple-antibiotic resistance markers in beta-hemolytic group A, B, F, and G streptococci in the absence of extrachromosomal deoxyribonucleic acid. Plasmid. 1981 Mar;5(2):127–137. doi: 10.1016/0147-619x(81)90014-7. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Delbos F., Bieth G. High-level aminoglycoside resistance in group A, B, G, D (Streptococcus bovis), and viridans streptococci. Antimicrob Agents Chemother. 1982 Jan;21(1):176–179. doi: 10.1128/aac.21.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Buu-Hoï A., Le Bouguenec C., Bieth G. Narrow host range of some streptococcal R plasmids. Plasmid. 1982 Sep;8(2):199–206. doi: 10.1016/0147-619x(82)90057-9. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Burdett V. Structural organization of a 67-kilobase streptococcal conjugative element mediating multiple antibiotic resistance. J Bacteriol. 1985 Feb;161(2):620–626. doi: 10.1128/jb.161.2.620-626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L., Sykes R. B., Grinsted J., Saunders J. R., Richmond M. H. A transmissible resistance element from a strain of Pseudomonas aeruginosa containing no detectable extrachromosomal DNA. J Gen Microbiol. 1972 Sep;72(2):269–279. doi: 10.1099/00221287-72-2-269. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Homberger F., Devaud M. Aminocyclitol-modifying enzymes specified by chromosomal genes in Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):766–772. doi: 10.1128/aac.19.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski J. J., Murphy E., Novick R. P., Rush M. G. Site-specificity of the chromosomal insertion of Staphylococcus aureus transposon Tn554. J Mol Biol. 1981 Oct 15;152(1):19–33. doi: 10.1016/0022-2836(81)90093-0. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Gerbaud G., Courvalin P. Translocation of sequences encoding antibiotic resistance from the chromosome to a receptor plasmid in Salmonella ordonez. Mol Gen Genet. 1981;182(3):390–408. doi: 10.1007/BF00293927. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Lord V. L. Transfer of gentamicin resistance between cultures of Staphylococcus aureus in nutrient broth, serum and urine. J Med Microbiol. 1980 Aug;13(3):411–421. doi: 10.1099/00222615-13-3-411. [DOI] [PubMed] [Google Scholar]
- Le Bouguenec C., Horaud T., Bieth G., Colimon R., Dauguet C. Translocation of antibiotic resistance markers of a plasmid-free Streptococcus pyogenes (group A) strain into different streptococcal hemolysin plasmids. Mol Gen Genet. 1984;194(3):377–387. doi: 10.1007/BF00425548. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Tally F. P. Mechanisms of drug-resistance transfer in Bacteroides fragilis. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):59–75. doi: 10.1093/jac/8.suppl_d.59. [DOI] [PubMed] [Google Scholar]
- Mays T. D., Smith C. J., Welch R. A., Delfini C., Macrina F. L. Novel antibiotic resistance transfer in Bacteroides. Antimicrob Agents Chemother. 1982 Jan;21(1):110–118. doi: 10.1128/aac.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell R. W., Sweeney H. M., Cohen S. Conjugational transfer of gentamicin resistance plasmids intra- and interspecifically in Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1983 Jan;23(1):151–160. doi: 10.1128/aac.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L. TRANSDUCTION BY STAPHYLOCOCCAL BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1959 May;45(5):722–727. doi: 10.1073/pnas.45.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., Dougan G., Foster T. J., Arbuthnott J. P. Plasmids in epidermolytic strains of Staphylococcus aureus. J Gen Microbiol. 1981 May;124(1):99–107. doi: 10.1099/00221287-124-1-99. [DOI] [PubMed] [Google Scholar]
- Pattee P. A., Neveln D. S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975 Oct;124(1):201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Botta G., Sebald M. Macrolide, lincosamide, streptogramin and tetracycline transferable resistance in the Bacteroides fragilis group. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):87–94. doi: 10.1093/jac/8.suppl_d.87. [DOI] [PubMed] [Google Scholar]
- ROUNTREE P. M. The role of certain electrolytes in the adsorption of staphylococcal bacteriophages. J Gen Microbiol. 1951 Oct;5(4):673–680. doi: 10.1099/00221287-5-4-673. [DOI] [PubMed] [Google Scholar]
- Schwesinger M. D., Novick R. P. Prophage-dependent plasmid integration in Staphylococcus aureus. J Bacteriol. 1975 Aug;123(2):724–738. doi: 10.1128/jb.123.2.724-738.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Markowitz S. M., Macrina F. L. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents Chemother. 1981 Jun;19(6):997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Welch R. A., Macrina F. L. Two independent conjugal transfer systems operating in Bacteroides fragilis V479-1. J Bacteriol. 1982 Jul;151(1):281–287. doi: 10.1128/jb.151.1.281-287.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Momoh M., Grubb W. B. Distribution of plasmid-borne resistance to nucleic acid binding compounds in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1985 Apr;15(4):417–434. doi: 10.1093/jac/15.4.417. [DOI] [PubMed] [Google Scholar]
- Witte W., Dünnhaupt K. Occurrence of a nonplasmid-located determinant for gentamicin resistance in strains of Staphylococcus aureus. J Hyg (Lond) 1984 Aug;93(1):1–8. doi: 10.1017/s0022172400060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst J., Hardegger U. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob Agents Chemother. 1983 May;23(5):784–786. doi: 10.1128/aac.23.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Solh N., Fouace J. M., Shalita Z., Bouanchaud D. H., Novick R. P., Chabbert Y. A. Epidemiological and structural studies of Staphylococcus aureus R plasmids mediating resistance to tobramycin and streptogramin. Plasmid. 1980 Jul;4(1):117–120. doi: 10.1016/0147-619x(80)90087-6. [DOI] [PubMed] [Google Scholar]
- el Solh N., Moreau N., Ehrlich S. D. Molecular cloning and analysis of Staphylococcus aureus chromosomal aminoglycoside resistance genes. Plasmid. 1986 Mar;15(2):104–118. doi: 10.1016/0147-619x(86)90047-8. [DOI] [PubMed] [Google Scholar]


