Abstract
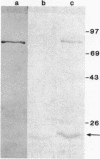
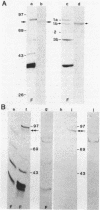
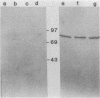
Penicillin-binding proteins (PBPs) from Haemophilus influenzae RD purified by a combination of affinity chromatography, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electroelution were used to immunize rabbits to obtain specific antisera. Antisera directed against PBP 1 (1b) of H. influenzae cross-reacted with representative organisms of the family Pasteurellaceae and with many members of the family Enterobacteriaceae but not with other gram-negative organisms. Immunization with purified PBP 3 of H. influenzae produced antisera that reacted with PBP 1 (1b) of H. influenzae and showed the same cross-reactive pattern with other species as the anti-PBP 1 antiserum. A 24,000-molecular-weight polypeptide of H. influenzae, not radiolabeled by [35S]penicillin, reacted with antisera against purified PBPs 1 (1a, 1b), 2, and 3. The results suggest that antigenic epitopes are shared among similar PBPs from related species and even among different PBPs within the same species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Edelman A., Yousif S., Spratt B. G. The nucleotide sequences of the ponA and ponB genes encoding penicillin-binding protein 1A and 1B of Escherichia coli K12. Eur J Biochem. 1985 Mar 1;147(2):437–446. doi: 10.1111/j.1432-1033.1985.tb08768.x. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Hedge P. J., Spratt B. G. Production of thiol-penicillin-binding protein 3 of Escherichia coli using a two primer method of site-directed mutagenesis. EMBO J. 1985 Jan;4(1):231–235. doi: 10.1002/j.1460-2075.1985.tb02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. Deletion of the penicillin-binding protein 6 gene of Escherichia coli. J Bacteriol. 1982 Nov;152(2):904–906. doi: 10.1128/jb.152.2.904-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Strominger J. L. Purification of penicillin-binding protein 2 of Escherichia coli. J Bacteriol. 1981 Jan;145(1):398–403. doi: 10.1128/jb.145.1.398-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984 Feb 24;67(1):1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]