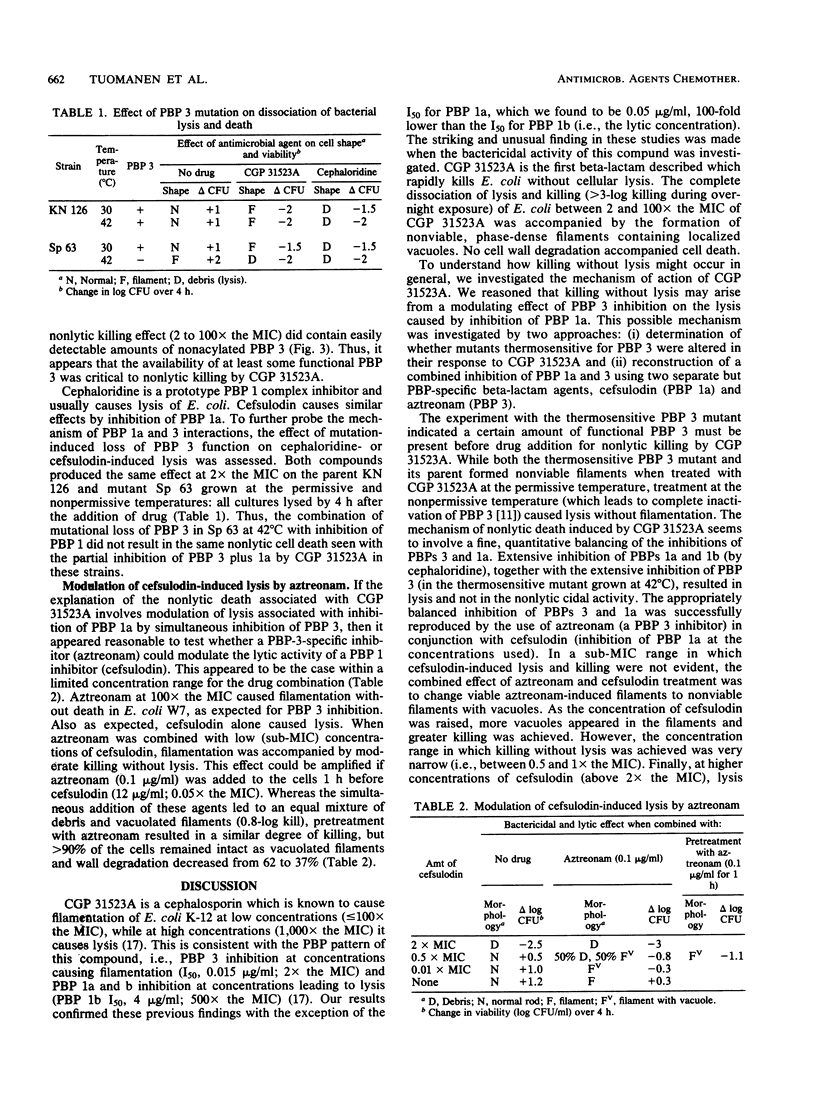
Abstract
Escherichia coli characteristically lyses upon treatment with most beta-lactam antimicrobial agents. In contrast, an investigational aminothiazole cephem, CGP 31523A, produced a new combination of antibacterial effects: it was highly bactericidal without causing cell wall degradation or lysis. Killing was associated with the formation of vacuolated filaments. Because the compound bound to penicillin-binding proteins (PBPs) 1a and 3, we investigated the role of PBP 3 in modulation of lysis caused by inhibition of PBP 1a. A temperature-sensitive mutant with a nonfunctional PBP 3 lysed when treated with CGP 31523A. The combination of a PBP 1 inhibitor (cefsulodin) and a PBP 3 inhibitor (aztreonam) also caused filamentation and death without lysis of wild-type cells over a narrow concentration range. We conclude that cooperative effects between PBPs in E. coli can lead to a dissociation of bacterial killing and lysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broome-Smith J. K., Spratt B. G. Deletion of the penicillin-binding protein 6 gene of Escherichia coli. J Bacteriol. 1982 Nov;152(2):904–906. doi: 10.1128/jb.152.2.904-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H. A., Fuller C., Reynolds P. E. The role of penicillin-proteins in the action of cephalosporins against Escherichia coli and Salmonella typhimurium. Eur J Biochem. 1981 Jul;117(2):301–310. doi: 10.1111/j.1432-1033.1981.tb06337.x. [DOI] [PubMed] [Google Scholar]
- Cozens R. M., Tuomanen E., Tosch W., Zak O., Suter J., Tomasz A. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob Agents Chemother. 1986 May;29(5):797–802. doi: 10.1128/aac.29.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., Turner K. A., White A. J. Inhibition of penicillin-binding protein 3 of Escherichia coli K-12. Effects upon growth, viability and outer membrane barrier function. J Antimicrob Chemother. 1985 Sep;16(3):287–296. doi: 10.1093/jac/16.3.287. [DOI] [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Dec;16(6):838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. S., Botta G., Park J. T. Effects of furazlocillin, a beta-lactam antibiotic which binds selectively to penicillin-binding protein 3, on Escherichia coli mutants deficient in other penicillin-binding proteins. J Bacteriol. 1981 Jan;145(1):632–637. doi: 10.1128/jb.145.1.632-637.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Kitzis M. D., Acar J. F. An evaluation of the bacteriolytic and biochemical properties of ceftiolene (42980RP). J Antimicrob Chemother. 1984 Dec;14(6):581–593. doi: 10.1093/jac/14.6.581. [DOI] [PubMed] [Google Scholar]
- Wise R., Cross C., Andrews J. M. In vitro activity of CGP 31523A, a broad-spectrum cephalosporin, in comparison with those of other agents. Antimicrob Agents Chemother. 1984 Dec;26(6):876–880. doi: 10.1128/aac.26.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif S. Y., Broome-Smith J. K., Spratt B. G. Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985 Oct;131(10):2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]




