Abstract
Protein synthesis requires the pairing of amino acids with tRNAs catalyzed by the aminoacyl-tRNA synthetases. The synthetases are highly specific, but errors in amino acid selection are occasionally made, opening the door to inaccurate translation of the genetic code. The fidelity of protein synthesis is maintained by the editing activities of synthetases, which remove noncognate amino acids from tRNAs before they are delivered to the ribosome. Although editing has been described in numerous synthetases, the reaction mechanism is unknown. To define the mechanism of editing, phenylalanyl-tRNA synthetase was used to investigate different models for hydrolysis of the noncognate product Tyr-tRNAPhe. Deprotonation of a water molecule by the highly conserved residue βHis-265, as proposed for threonyl-tRNA synthetase, was excluded because replacement of this and neighboring residues had little effect on editing activity. Model building suggested that, instead of directly catalyzing hydrolysis, the role of the editing site is to discriminate and properly position noncognate substrate for nucleophilic attack by water. In agreement with this model, replacement of certain editing site residues abolished substrate specificity but only reduced the catalytic efficiency of hydrolysis 2- to 10-fold. In contrast, substitution of the 3′-OH group of tRNAPhe severely impaired editing and revealed an essential function for this group in hydrolysis. The phenylalanyl-tRNA synthetase editing mechanism is also applicable to threonyl-tRNA synthetase and provides a paradigm for synthetase editing.
Keywords: proofreading, translation, phenylalanine
Aminoacyl-tRNA synthetases (aaRSs) maintain fidelity during protein synthesis by attaching amino acids to their cognate tRNAs. Quality control by aaRSs is monitored at the aminoacylation step, with the active site distinguishing cognate from noncognate amino acids with great accuracy. Erroneous activation may become a risk to the cell when the cognate amino acid exhibits structural similarities to other natural compounds. In these aaRSs, an editing mechanism hydrolyzes the misactivated aminoacyl-adenylate (pretransfer editing) (1–3) or the mischarged aminoacyl-tRNA (aa-tRNA) (posttransfer editing) (4–10).
The aaRSs are divided into two structurally unrelated groups, classes I and II, both of which contain examples of enzymes with editing activities (11–13). Editing sites in the two classes share little in common. Class I aaRSs including isoleucyl-tRNA synthetase (IleRS) (14, 15), leucyl-tRNA synthetase (LeuRS) (9), and valyl-tRNA synthetase (ValRS) (16) harbor the editing activity in the well conserved CP1 domain, which is inserted in the catalytic domain. Class II aaRSs contain more diverse editing sites, none of which bears any resemblance to the class I CP1 domain. Within class II, threonyl-tRNA synthetase (ThrRS) and alanyl-tRNA synthetase editing domains share strong sequence similarities with each other (8, 17, 18) but are not homologous to that of prolyl-tRNA synthetase (19). Escherichia coli phenylalanyl-tRNA synthetase (PheRS) possesses a predominant posttransfer editing activity against the misaminoacylated species Tyr-tRNAPhe (10). The editing domain of this enzyme, and of its archaeal/eukaryal counterpart (20), is located in the B3/B4 region of the β-subunit and lacks structural features resembling any known editing domains (21).
Despite the emerging knowledge on the biological functions and structures of aaRS editing sites, little is known about the molecular mechanisms of either pretransfer or posttransfer editing. The recently resolved crystal structure of LeuRS complexed with a posttransfer editing analog suggested that the editing site binds the substrate and positions a catalytic water molecule (ref. 9, discussed in ref. 22). In contrast, structural studies of the ThrRS editing site suggested that two water molecules are specifically activated by editing site residues and subsequently hydrolyze the posttransfer editing substrate (18). Here we used available PheRS structures to develop different models for editing and probed the possible mechanisms using site-directed mutagenesis and biochemical analyses. We found that the moderate catalytic efficiency and specificity of PheRS editing are mainly achieved through substrate binding by several conserved residues, whereas the chemistry is not rate-limiting during the editing step. Hydrolysis is catalyzed by two water molecules, which are positioned by editing site residues, and most importantly an indispensable interaction with the 3′ OH of the terminal adenosine of tRNAPhe. The PheRS editing mechanism can also be applied to ThrRS and provides a paradigm for synthetase editing.
Results
Functional Domains in the E. coli PheRS Editing Site.
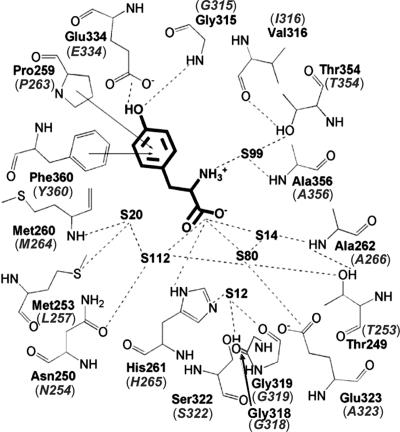
We previously found that the B3/B4 domain of the β-subunit of E. coli PheRS harbors an editing activity against misactivated Tyr (10). The recently resolved crystal structure of Thermus thermophilus PheRS complexed with Tyr supported this assignment of the B3/B4 domain (21), as did the absence of editing activity in mitochondrial PheRS, which lacks the β-subunit (23). To probe the roles of the different domains of the PheRS editing site we first replaced residues whose side chains form putative interactions with Tyr (Fig. 1) and a number of neighboring positions. To accurately determine kinetic parameters for editing, all replacements were made in the context of the αA294G variant, which has previously been shown to better accommodate Tyr in the active site than wild-type PheRS without affecting editing (10). Editing activities of the resulting PheRS variants were analyzed by using ATP consumption assays, which measure both pre- and posttransfer editing of misactivated Tyr (cis editing), and Tyr-tRNAPhe hydrolysis assays, which measure rates of trans editing of exogenous substrate (Table 1). Most replacements tested had little or no effect on editing, including βP263 and βY360, which were suggested to form edge-to-face interactions with the Tyr ring, and numerous other residues in the vicinity of the carboxyl group of Tyr (21). These results indicate significant differences in the binding of free Tyr and Tyr-tRNAPhe at the editing site, suggesting that the PheRS:Tyr complex depicts a postediting state different from the transition state. This finding is consistent with previous structural studies of ThrRS, where the product (Ser) and a substrate analog (SerA76) of posttransfer editing were shown to bind differently at the editing site (18).
Fig. 1.
T. thermophilus PheRS editing site complexed with Tyr; equivalent residues in E. coli PheRS are shown in parentheses (adapted from ref. 21).
Table 1.
Steady-state kinetics of editing for E. coli PheRS editing site replacements
| PheRS β-subunit* | ATP consumed†, kobs, min−1 | Loss of activity‡, fold | Tyr-tRNAPhe hydrolysis†, kcat/KM, μM−1·min−1 | Loss of activity§, fold |
|---|---|---|---|---|
| Wild type | 66 ± 1 | 1.0 | 55 ± 10 | 1.0 |
| Structure-based replacements | ||||
| T253A | 50 ± 2 | 1.3 | 51 ± 10 | 1.1 |
| N254A | 47 ± 4 | 1.4 | 33 ± 10 | 1.6 |
| Y255A | 45 ± 3 | 1.5 | 56 ± 10 | 1.0 |
| P263A | 50 ± 5 | 1.3 | 73 ± 10 | 0.7 |
| P263A/Y360A | 65 ± 3 | 1.0 | 46 ± 10 | 1.2 |
| H265A | 29 ± 2 | 2.2 | 17 ± 4 | 3.2 |
| F267A | 58 ± 6 | 1.1 | 47 ± 1 | 1.2 |
| S322A | 45 ± 2 | 1.5 | 38 ± 10 | 1.4 |
| E334A | 15 ± 4 | 4.3 | 12 ± 0.8 | 4.7 |
| T354V | 20 ± 1 | 3.3 | 16 ± 2 | 3.4 |
| A356W | 4.8 ± 0.8 | 14 | 1.0 ± 0.4 | 53 |
| Alignment-based replacements | ||||
| R244A | 39 ± 6 | 1.7 | 20 ± 3 | 2.8 |
| R244A/H265A | 7 ± 2 | 10 | 2.9 ± 0.6 | 19 |
| D251A | 64 ± 5 | 1.0 | 50 ± 10 | 1.1 |
| Q262A | 57 ± 5 | 1.2 | 36 ± 10 | 1.5 |
| D268A | 54 ± 4 | 1.2 | 55 ± 6 | 1.0 |
| G318A | 6.5 ± 2 | 10 | 7.3 ± 1 | 7.5 |
| G318W | 5.2 ± 1 | 13 | 0.7 ± 0.2 | 78 |
| R359A | 47 ± 4 | 13 | 45 ± 10 | 1.2 |
| 361A | 53 ± 6 | 1.2 | 75 ± 9 | 0.7 |
*PheRS concentrations used in ATP consumption and Tyr-tRNAPhe hydrolysis assays were normalized according to their respective phenylalanylation activities.
†Data are the means of at least three independent experiments with standard deviations indicated.
‡Loss of cis editing activity relative to wild type.
§Loss of trans editing activity relative to wild type.
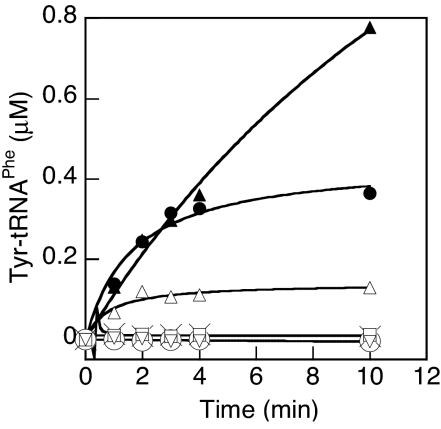
In an effort to find other domains of the editing site, we extended our search to include well conserved residues on the surface of the B3/B4 region [supporting information (SI) Fig. 8 and Table 1]. As with the structure-based replacements, the majority of the changes had little effect on editing. Taken together, the editing site replacements revealed that βR244, βH265, βG318, βE334, βT354, and βA356 are all involved in editing (Table 1). βG318 replacements, as with the previously described βA356 changes (10), hinder access to the editing site, leading to the most significant decreases in activity. βR244, βH265, βE334, and βT354 replacements all showed less dramatic changes and were further tested for editing defects with respect to their tyrosylation activities (Fig. 2). In agreement with the kinetic data, βG318W exhibited the best misacylation activity and βE334A PheRS, which had 5-fold reduced trans editing activity, could also tyrosylate tRNAPhe. Although the trans editing activities of βR244A, βH265A, and βT354V all dropped by ≈3-fold, no tyrosylation activity was detected, suggesting that they contain sufficient residual hydrolytic activity to prevent Tyr-tRNAPhe accumulation (Fig. 2). This was supported by the observation that βR244A/βH265A PheRS, which showed a 19-fold reduction in editing efficiency, was able to stably attach Tyr to tRNAPhe. Finally, to exclude the possibility that replacement of these residues either affects tRNA binding or induces global conformational changes that impair activity, we tested the affinity of the editing-defective PheRS variants for free tRNAPhe. None of the replacements led to a significant change in the KD for tRNAPhe (SI Table 2), which, in combination with their similar phenylalanylation activities (data not shown), indicated that the replacements at the editing site did not influence the global conformation of the enzyme.
Fig. 2.
Tyr-tRNAPhe synthesis by PheRS variants (100 nM). □, wild-type β-subunit; ▴, βG318W; ▵, βE334A; ○, βH265A; ×, βR244A; ●, βR244A/βH265A; ▿, βT354V. Plots represent the average of three independent experiments.
Active-Site Residues Do Not Directly Catalyze Editing.
To investigate the roles in the editing reaction of the residues identified above, we constructed several docking models of the PheRS editing site in complex with Tyr-A76. The model most consistent with our data is shown in Fig. 3, in which βE334 interacts with the hydroxyl group of Tyr, βR244 lies near the tRNA backbone, whereas βT354 is close to the aminoacyl-ester bond. The crystal structure of the PheRS-Tyr complex suggested that βH265 may also be in the vicinity of the ester bond (21). In a recent model proposed for the ThrRS editing mechanism, a critical histidine deprotonates a catalytic water molecule, which then performs a nucleophilic attack on the ester bond (18). To test whether βH265 of PheRS performs a similar role in catalysis, we attempted to rescue the editing defects of the corresponding PheRS variants using imidazole. Previous studies have demonstrated that imidazole is able to partially rescue catalytic defects resulting from the replacement of essential His residues in certain enzymes (24–27). Imidazole did not restore editing activity in any of the PheRS tested (SI Fig. 9A), suggesting that βH265 does not catalyze hydrolysis directly but rather plays a structural role. This finding is consistent with the modest change in editing efficiency seen when βH265 was replaced and in contrast to the dramatic changes previously reported when a catalytic histidine was replaced in other enzymatic systems (24–27). The lack of a direct role in catalysis for βH265 is also supported by the similar profiles for the pH dependence of Tyr-tRNAPhe hydrolysis upon replacement with Ala (SI Fig. 9B), indicating that the protonation state of βH265 does not significantly affect editing. These findings, together with the docking model, suggest that, rather than driving catalysis, the imidazole ring of βH265 contributes to substrate binding most likely through stacking interactions with Tyr, replacing the roles of βP263 and βY360 in the postediting complex.
Fig. 3.
Structural modeling of the PheRS editing site complexed with Tyr-A76.
In addition to βH265, structural modeling also identified βT354 as a potential catalytic residue. One potential mechanism of catalysis would involve nucleophilic attack of the ester bond by the side chain hydroxyl group of βT354, similar to the catalytic mechanism of many proteases. However, as with βH265A, the βT354V replacement only moderately diminished both cis and trans editing, which does not support the possible role of this residue as a nucleophile. To clarify this point, we replaced βT354 with serine and cysteine and characterized the resulting variants. The thiol group of cysteine is a better nucleophile but a poorer hydrogen bonder than the hydroxyl group of serine, which is a naturally occurring variant at this position in bacterial PheRSs (SI Fig. 8). Replacement of βT354 with serine resulted in a 2-fold decrease in cis editing, whereas the cysteine variant showed a 3.5-fold decrease, similar to valine (Fig. 4). These data clearly demonstrate that βT354 does not participate in the hydrolysis reaction as a nucleophile and suggest that it is involved in either substrate binding or hydrogen bonding with a catalytic water molecule.
Fig. 4.
Impact of PheRS βT354 replacements on ATP consumption. □, wild-type β-subunit; ○, βT354V; ×, βT354C; ▵, βT354S.
Tyr-tRNAPhe Hydrolysis Is Not Rate-Limiting in Posttransfer Editing.
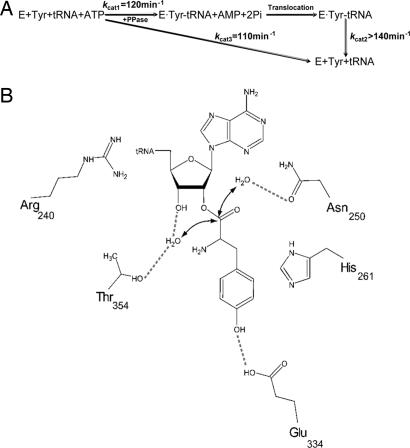
The PheRS variants βR244A, βH265A, and βT354V all showed an ≈3-fold decrease in Tyr-tRNAPhe hydrolysis activity but could not tyrosylate tRNAPhe, indicating that aminoacylation may be much slower than the hydrolysis step of editing. To address this question, we probed the kinetics of cis and trans editing, as well as tyrosylation. Cis editing kinetic parameters were directly determined with wild-type β PheRS by using the ATP consumption assay, which revealed a kcat of 110 min−1 and a KM of 852 μM for Tyr (SI Table 3). The KM for Tyr was assigned to the aminoacylation step. Trans editing was tested with wild-type β PheRS by using the Tyr-tRNAPhe hydrolysis assay. Because of experimental limitations, we were not able to saturate the enzyme with the substrate. The upper limit of Tyr-tRNAPhe used was 5.2 μM, which gave an observed hydrolysis rate of 140 min−1 (SI Fig. 10). By fitting the data to the Michalis–Menten equation, we estimated that the kcat was ≈270 min−1 and the KM for Tyr-tRNAPhe was 4.7 μM. This kcat is ≈104-fold higher than the uncatalyzed hydrolysis rate. Previously we determined that the kcat value of αA294G PheRS was ≈120 min−1 in phenylalanylation (10), which serves as a useful approximation for the kcat of tyrosylation given that Tyr and Phe were previously shown to have the same kcat in amino acid activation by E. coli PheRS (28). A simplified kinetic scheme for posttransfer editing by PheRS is shown in Fig. 5A, which suggests that Tyr-tRNAPhe hydrolysis is not the rate-limiting step; the slowest step is either tyrosylation or translocation, as previously proposed for IleRS (29). Next we tested whether proton transfer is involved in editing. The catalytic efficiency of Tyr-tRNAPhe hydrolysis was measured in 75% deuterium oxide (D2O) and compared with hydrolysis in H2O. No significant solvent isotope effect was observed (SI Fig. 11), indicating that proton transfer is not rate-limiting during Tyr-tRNAPhe hydrolysis.
Fig. 5.
Mechanism of posttransfer editing by PheRS. (A) Kinetic scheme for PheRS editing. (B) Model for Tyr-tRNAPhe hydrolysis at the PheRS editing site (T. thermophilus numbering; see Fig. 3).
Discrimination of Cognate Phe-tRNAPhe at the Editing Site.
Structural and modeling studies indicated that the side chain of βE334 forms a hydrogen bond with the para hydroxyl group of the Tyr moiety during substrate recognition (Fig. 3). The role of this residue in modulating substrate specificity was supported by the finding that replacing βE334 with Ala induced significant editing of cognate Phe-tRNAPhe while reducing activity against Tyr-tRNAPhe (Fig. 6 and Table 1). Increasing the hydrophobicity of the editing site binding pocket by replacing βE334 with Ile further increased editing of Phe-tRNAPhe. These findings demonstrate that βE334 determines substrate specificity both by direct recognition of the hydroxyl group of Tyr and by hydrophilic exclusion of Phe. In addition to the βE334 variants, βP263A/βY360A PheRS also displayed a relaxed specificity toward Phe-tRNAPhe, indicating that edge-to-face interactions with the Tyr ring contribute to editing site specificity (Fig. 1).
Fig. 6.
Editing of PheRS variants against cognate Phe. (A) ATP consumption in the presence of Phe (10 mM), tRNAPhe (10 μM), and PheRS (1 μM). (B) Phe-tRNAPhe hydrolysis by PheRS variants (0.5 μM). (C) Phe-tRNAPhe synthesis by PheRS variants (2 nM). ○, no enzyme; □, wild-type β-subunit; ▵, βE334A; ▴, βE334I; ×, βP263A/βY360A.
3′ Hydroxyl Group of A76 Is Critical for Editing.
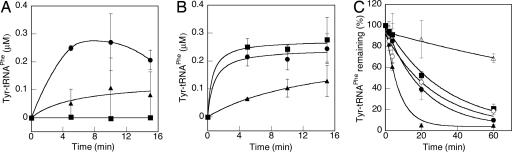
Previous studies indicated that the 3′-OH group is critical for the editing activities of IleRS and ValRS (30). IleRS requires transacylation of the mischarged amino acid from the 2′-OH to the 3′-OH before deacylation, whereas in ValRS it was proposed that the 3′-OH might be more directly involved in catalyzing hydrolysis (30). Interestingly, studies of the closely related LeuRS revealed that the original 2′ linked substrate analog (Nva2AA), but not the 3′ linked analog (Nva3AA), can inhibit LeuRS editing, suggesting that transacylation is not required by LeuRS (9). Our studies of the PheRS editing site did not reveal a residue directly involved in catalysis, prompting us to further investigate the role of the neighboring hydroxyl group. We modified tRNAPhe at the 2′-OH and 3′-OH of A76 by replacing them each with a proton, respectively. The resulting 2′-dA76 tRNAPhe could be charged with neither phenylalanine nor tyrosine (data not shown), whereas 3′-dA76 tRNAPhe was tyrosylated by both wild-type β-subunit and βG318W PheRSs (Fig. 7 A and B). The low tyrosylation efficiency indicates that replacing the 3′-OH with H slows down the aminoacylation step, because the editing-defective βG318W PheRS exhibited the same charging profile. In contrast, under the same conditions, A76 tRNAPhe could be mischarged only by βG318W but not by wild type, demonstrating that the 3′-OH is crucial for the editing activity. To clarify whether the 3′-OH plays a role in transacylation or in catalysis, we prepared Tyr-3′-dA76 tRNAPhe and tested its hydrolysis in the presence and absence of wild-type β-subunit PheRS. As shown in Fig. 7C, PheRS significantly enhances the rate of hydrolysis, indicating that transacylation is not required for editing. However, replacing the 3′-OH with H resulted in an ≈300-fold decrease in kcat/KM (0.19 ± 0.02 μM−1·min−1) compared with wild type, indicating that the 3′-OH plays a far more critical role in catalysis than any PheRS residues tested in this study. To further probe the function of the 3′-OH, we substituted it with a fluorine atom. Fluorine is highly electronegative but unable to donate a hydrogen bond. As with 3′-dA76 tRNAPhe, 3′-F-A76 tRNAPhe could be tyrosylated by both wild-type β-subunit and βG318W PheRSs (Fig. 7A). The hydrolysis of Tyr-3′-F-A76 tRNAPhe was not significantly different in the presence or absence of 0.5 μM wild-type β-subunit PheRS (Fig. 7C). These results indicate that the role of the 3′-OH cannot be substituted by fluorine, suggesting that this position acts as a hydrogen bond donor helping with water molecule positioning or with substrate binding in the editing site. Our results also demonstrated that the 3′-OH plays a role in uncatalyzed hydrolysis. The spontaneous hydrolysis rate of Tyr-3′-dA76 tRNAPhe was ≈5-fold slower than those of Tyr-3′-A76 tRNAPhe and Tyr-3′-F-A76 tRNAPhe (Fig. 7C) probably because of the inductive effects (−I) of the electronegative hydroxyl and fluoride groups, which destabilize the aa-tRNA by withdrawing electrons from the ester linkage (22).
Fig. 7.
The role of the 3′-OH of A76 in editing. (A and B) Tyrosylation of A76 tRNAPhe (squares), 3′-dA76 tRNAPhe (triangles), and 3′-F-A76 tRNAPhe (circles) by 1 μM wild-type PheRS (A) or 1 μM βG318W PheRS (B). (C) Deacylation of 0.2–0.4 μM tyrosylated A76 tRNAPhe, 3′-dA76 tRNAPhe, and 3′-F-A76 tRNAPhe in the presence (filled symbols) or absence (open symbols) of 0.5 μM wild-type β-subunit PheRS.
Discussion
Mechanism of Posttransfer Editing by PheRS.
Previous studies of the editing sites of IleRS (31) and LeuRS (32) did not identify catalytic residues. Instead, the structure of LeuRS complexed with a posttransfer editing analog suggested that the editing site binds the substrate and positions a catalytic water molecule for hydrolysis of the ester bond (9). A different water-mediated hydrolytic mechanism has also been proposed based on structures of ThrRS with and without editing analogs (18), although this mechanism has not yet been tested experimentally. Functional analyses of the PheRS editing site defined several important residues, none of which performs a nucleophilic attack at the ester bond of Tyr-tRNAPhe. Analyses of available PheRS crystal structures showed that divalent species such as magnesium or manganese were found only at the interface between the α- and β-subunits but not at the editing site, excluding a direct role for metal ions in hydrolysis (21, 33–35). This finding is consistent with both ThrRS (18) and alanyl-tRNA synthetase (36), where divalent ions are unlikely to play a role in posttransfer editing. Our structural modeling revealed two water molecules (S99 and S112 in Fig. 1) that are correctly positioned as candidates to catalyze hydrolysis (Fig. 3). These two water molecules are well conserved among available PheRS crystal structures, including the apo form and the PheRS:Tyr complex. S112 is positioned by βT253 and βN254, and S99 is hydrogen-bonded by the 3′-OH of A76, βT354, and βA356, all of which contribute to editing. The 3′-OH of A76, previously implicated in editing by LeuRS, IleRS, and ValRS (9, 30), was shown to be the most critical functional group for editing by PheRS, whereas changes of βT253, βN254, βT354, and βA356 produced more modest losses in catalytic efficiency.
Taken together, our data provide strong support for a substrate-assisted mechanism of posttransfer editing (Fig. 5B). After Tyr-tRNAPhe synthesis at the active site, the CCA-Tyr end translocates to the B3/B4 domain editing site, as implicated by other studies (7, 15, 16). The editing site binds the substrate in such a configuration that the ester bond is positioned close to two catalytic water molecules. βR244 interacts with the base of C75; the side chain of βH265 stacks with the Tyr ring; and the carboxyl group of βE334 hydrogen bonds with the hydroxyl group of Tyr, which ensures editing site specificity. The water positioned by βT253 and βN254 (S112) performs a nucleophilic attack on the ester bond, whereas the other water hydrogen-bonded by the 3′-OH, βT354, and βA356 (S99) donates a proton to stabilize the leaving group and complete the reaction. The catalytic power is mainly contributed by the 3′-OH via activation of the water molecule. Such a mechanism, where the pivotal role of the enzyme is to determine specificity rather than to drive catalysis, may be broadly applicable to posttransfer editing given the generally moderate effects on hydrolysis seen upon replacing editing site residues (31, 32). Structural and functional studies of LeuRS suggest that the 3′-OH might also play a role in catalysis by activating a water molecule (9, 30), which is consistent with the study of PheRS presented here. In the closely related IleRS, the misacylated amino acid transacylates from the 2′-OH to the 3′-OH before being hydrolyzed (30). The posttransfer editing then initiates subsequent pretransfer editing (37), but exactly how the 2′-OH participates in catalysis is not clear. On the other hand, in the proposed ThrRS model, two water molecules hydrogen-bonded by editing site residues were proposed to mediate substrate hydrolysis (18), which is also analogous to PheRS, although contributions of individual ThrRS editing site residues have not yet been quantified. Reexamination of the E. coli ThrRS structure revealed that the 2′-OH of A76 lies close to one catalytic water, and the recent crystal structure of Pyrococcus abyssi ThrRS revealed that the 2′-OH indeed interacts with a candidate catalytic water molecule (38), raising the intriguing possibility that the 2′-OH of tRNA may contribute to ThrRS editing in much the same way the 3′-OH assists PheRS (39). The high similarity in the editing mechanisms of PheRS and ThrRS, both class II aaRSs, would not seem to extend to class I aaRSs such as IleRS and LeuRS, which may partly reflect the general observation that the rate-determining step differs between the two enzyme classes (40).
Comparison of Aminoacyl- or Peptidyl-tRNA Esterases.
Aside from aaRSs, D-Tyr-tRNATyr deacylase (DTD) and peptidyl-tRNA hydrolase (PTH) represent other examples of enzymes that catalyze the hydrolysis of aminoacyl- or peptidyl-tRNA ester bonds. A recent study showed that the archaeal P. abyssi ThrRS editing domain shares a marked structural similarity to DTD (41), suggesting that the two enzymes might share a common mechanism for aa-tRNA hydrolysis. The kcat (6 s−1) and rate enhancement (3 × 104) of D-Tyr-tRNATyr hydrolysis by DTD are similar to those for L-Tyr-tRNAPhe hydrolysis by PheRS (42). The hydrolytic mechanism of DTD has not yet been determined, nor has any critical residue been identified. It is possible that DTD employs a similar mechanism as aaRSs, in which the vicinal hydroxyl group of A76 plays a critical role in hydrolyzing the ester bond. This mechanism, however, is less likely to be used by PTH; replacements of critical residues at the PTH active site resulted in >100-fold decreases in kcat without affecting KM (43), suggesting that these residues play more crucial roles in catalysis than do editing site residues in aaRSs and revealing a key difference between aa-tRNA and peptidyl-tRNA hydrolysis.
Role of Editing in Translational Quality Control.
The editing site has apparently evolved to maintain moderate catalytic efficiency sufficient for the hydrolysis of noncognate species while minimizing the hydrolysis of cognate aa-tRNA. Our proposed substrate-assisted mechanism of editing, wherein the enzyme simply accelerates the rate of spontaneous hydrolysis of aa-tRNA by ≈104, also suggests that selectively retaining misaminoacylated species is in fact the critical step in aaRS proofreading. The KM of Tyr-tRNAPhe for wild-type β-subunit PheRS is within the micromolar range; however, elongation factor Tu (EF-Tu) binds aa-tRNAs several orders of magnitude more tightly (44–46). Previously we showed that overexpressing βH265A or βE334A PheRS resulted in Tyr misincorporation at Phe codons in vivo (10), suggesting that EF-Tu promptly sequesters the mischarged Tyr-tRNAPhe upon its synthesis and that there is no efficient proofreading mechanism once Tyr-tRNAPhe leaves PheRS. Recently, it has been found that low levels of mischarged tRNAs can lead to protein misfolding and neurodegeneration in mice (47). Our present findings show that a loss of quality control follows a relatively minor loss in editing activity, emphasizing the fine balance that exists between a tolerable error rate and a level of misaminoacylation that critically impacts translational fidelity.
Materials and Methods
Strains, Plasmids, Site-Directed Mutagenesis, and General Methods.
Proteins and tRNAs were prepared as described previously (23). All PheRS variants described in this work contain an A294G replacement in the α-subunit. 2′- and 3′-dA76 tRNAPhe and 3′-F-A76 tRNAPhe were prepared as described (10), and 3′-fluro ATP was purchased from IBA (Göttingen, Germany). 32P-labeled tRNA pA76 transcripts were synthesized as described previously (23) and used to determine the KD for PheRS by using filter-binding assays (48). Structural modeling was performed with Autodock 3.0 (49).
Aminoacylation Assays.
Aminoacylation was performed as described (10) with the addition of 2 mM ATP, 20 μM [14C] Phe (273 cpm/pmol), 6 mg/ml total tRNA (from E. coli MRE 600; Roche), and 2 nM PheRS. Tyrosylation was performed with 2 units/ml yeast pyrophosphatase (Roche), 2 mM ATP, 10 μM E. coli tRNAPhe transcript, 50 μM [3H]Tyr (464 cpm/pmol), and 0.1 μM PheRS. Yeast pyrophosphatase, added to mimic conditions in the ATP consumption assay, had no effect on tyrosylation by βG318W PheRS (data not shown).
ATP Consumption Assay.
The ATP consumption assay was used to measure cis editing by PheRS. A 15-μl reaction mix contained 2 units/ml yeast pyrophosphatase (Roche), 2 mM Tyr or 10 mM Phe, 10 μM tRNAPhe, 2 mM [γ-32P]ATP (5 cpm/pmol), 0.1 M Na-Hepes (pH 7.2), 30 mM KCl, 10 mM MgCl2, and 1 μM PheRS. Steady-state kinetics were determined by using 20–3,000 μM Tyr or 1–32 μM tRNAPhe. In imidazole rescue experiments, 0.1 M imidazole (pH 7.2) was added into the above reaction mix.
Preparation of Tyr-tRNAPhe and Phe-tRNAPhe.
Tyr- and Phe-tRNAPhe were prepared as described (10) except that 2 μM βG318W PheRS was used. After a 10-min incubation at 37°C, the reaction was stopped by addition of 56 mM potassium acetate (pH 4.5) and 250 mM KCl, followed by phenol/chloroform extraction and ethanol precipitation. The aa-tRNA pellet was dried and resuspended in DEPC water with 2 mM MgCl2. The charging level was ≈25% as determined by radioactivity retained on 3-mm filter discs.
Tyr-tRNAPhe and Phe-tRNAPhe Hydrolysis Assays.
Tyr-tRNAPhe hydrolysis was performed in 0.1 M Na-Hepes (pH 7.2), 30 mM KCl, and 10 mM MgCl2. The hydrolysis rate–substrate concentration profile was determined with 2 nM wild-type β PheRS and 0.7–5.2 μM Tyr-tRNAPhe. kcat/KM values of PheRS variants for Tyr-tRNAPhe hydrolysis were determined with 0.6–0.9 μM substrate and 2–100 nM enzyme. Reaction mixtures were incubated at 37°C, and 2- to 9-μl aliquots were periodically spotted on 3-mm filter discs presoaked with 5% TCA, followed by extensive washing in 5% TCA, drying, and scintillation counting. Tyr-tRNAPhe hydrolysis was also monitored in the absence of PheRS at each substrate concentration as a control, and these rates subtracted from the enzyme catalyzed values to determine initial velocities. To determine the pH dependence of Tyr-tRNAPhe hydrolysis, 1–250 nM wild-type β PheRS and 5–500 nM βH265A PheRS were used. pH values determined were those of the final reaction mix before Tyr-tRNAPhe addition. Investigation of solvent isotope effects was performed in the presence of 75% deuterium oxide (D2O) and 2–10 nM wild-type β PheRS. The enzyme was preequilibrated in a buffer containing 75% D2O for 2 h on ice. Tyr-3′-dA76 tRNAPhe and Phe-tRNAPhe hydrolysis was performed in the presence of 0.4 μM substrate and 0.5 μM PheRS variants.
Supplementary Material
Acknowledgments
We thank D. Tirrell (California Institute of Technology, Pasadena, CA) and O. Uhlenbeck (Northwestern University, Evanston, IL) for strains and plasmids and S. Ataide, C. Hausmann, J. Levengood, N. Reynolds, and T. Rogers for critical reading of the manuscript. This work was supported by National Science Foundation Grant MCB-0344002.
Abbreviations
- aaRS
aminoacyl-tRNA synthetase
- aa-tRNA
aminoacyl-tRNA
- ValRS
valyl-tRNA synthetase
- IleRS
isoleucyl-tRNA synthetase
- LeuRS
leucyl-tRNA synthetase
- ThrRS
threonyl-tRNA synthetase
- PheRS
phenylalanyl-tRNA synthetase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606272104/DC1.
References
- 1.Baldwin AN, Berg P. J Biol Chem. 1966;241:839–845. [PubMed] [Google Scholar]
- 2.Fersht AR. In: Transfer RNA: Structure, Properties and Recognition. Schimmel PR, Söll D, Abelson JN, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1979. pp. 247–254. [Google Scholar]
- 3.Hale SP, Auld DS, Schmidt E, Schimmel P. Science. 1997;276:1250–1252. doi: 10.1126/science.276.5316.1250. [DOI] [PubMed] [Google Scholar]
- 4.Eldred EW, Schimmel PR. J Biol Chem. 1972;247:2961–2964. [PubMed] [Google Scholar]
- 5.Yarus M. Proc Natl Acad Sci USA. 1972;69:1915–1919. doi: 10.1073/pnas.69.7.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuning PJ, Musier-Forsyth K. Proc Natl Acad Sci USA. 2000;97:8916–8920. doi: 10.1073/pnas.97.16.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dock-Bregeon A, Sankaranarayanan R, Romby P, Caillet J, Springer M, Rees B, Francklyn CS, Ehresmann C, Moras D. Cell. 2000;103:877–884. doi: 10.1016/s0092-8674(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 8.Beebe K, Ribas De Pouplana L, Schimmel P. EMBO J. 2003;22:668–675. doi: 10.1093/emboj/cdg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lincecum TL, Tukalo M, Yaremchuk A, Mursinna RS, Williams AM, Sproat BS, Van Den Eynde W, Link A, Van Calenbergh S, Grøtli M, et al. Mol Cell. 2003;11:951–963. doi: 10.1016/s1097-2765(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 10.Roy H, Ling J, Irnov M, Ibba M. EMBO J. 2004;23:4639–4648. doi: 10.1038/sj.emboj.7600474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster T, Tsai H, Kula M, Mackie GA, Schimmel P. Science. 1984;226:1315–1317. doi: 10.1126/science.6390679. [DOI] [PubMed] [Google Scholar]
- 12.Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- 13.Cusack S, Berthet-Colominas C, Hartlein M, Nassar N, Leberman R. Nature. 1990;347:249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- 14.Nureki O, Vassylyev DG, Tateno M, Shimada A, Nakama T, Fukai S, Konno M, Hendrickson TL, Schimmel P, Yokoyama S. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 15.Silvian LF, Wang J, Steitz TA. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- 16.Fukai S, Nureki O, Sekine S, Shimada A, Tao J, Vassylyev DG, Yokoyama S. Cell. 2000;103:793–803. doi: 10.1016/s0092-8674(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 17.Sankaranarayanan R, Dock-Bregeon AC, Romby P, Caillet J, Springer M, Rees B, Ehresmann C, Ehresmann B, Moras D. Cell. 1999;97:371–381. doi: 10.1016/s0092-8674(00)80746-1. [DOI] [PubMed] [Google Scholar]
- 18.Dock-Bregeon AC, Rees B, Torres-Larios A, Bey G, Caillet J, Moras D. Mol Cell. 2004;16:375–386. doi: 10.1016/j.molcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Beuning PJ, Musier-Forsyth K. J Biol Chem. 2001;276:30779–30785. doi: 10.1074/jbc.M104761200. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki HM, Sekine S, Sengoku T, Fukunaga R, Hattori M, Utsunomiya Y, Kuroishi C, Kuramitsu S, Shirouzu M, Yokoyama S. Proc Natl Acad Sci USA. 2006;103:14744–14749. doi: 10.1073/pnas.0603182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotik-Kogan O, Moor N, Tworowski D, Safro M. Structure (London) 2005;13:1799–1807. doi: 10.1016/j.str.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Weinger JS, Strobel SA. Biochemistry. 2006;45:5939–5948. doi: 10.1021/bi060183n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy H, Ling J, Alfonzo J, Ibba M. J Biol Chem. 2005;280:38186–38192. doi: 10.1074/jbc.M508281200. [DOI] [PubMed] [Google Scholar]
- 24.Newmyer SL, de Montellano PR. J Biol Chem. 1996;271:14891–14896. doi: 10.1074/jbc.271.25.14891. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Tu SC. Biochemistry. 1997;36:14609–14615. doi: 10.1021/bi9722554. [DOI] [PubMed] [Google Scholar]
- 26.Venkataraman P, Lamb RA, Pinto LH. J Biol Chem. 2005;280:21463–21472. doi: 10.1074/jbc.M412406200. [DOI] [PubMed] [Google Scholar]
- 27.McCartney SA, Brignole EJ, Kolegraff KN, Loveland AN, Ussin LM, Gibson W. J Biol Chem. 2005;280:33206–33212. doi: 10.1074/jbc.M506876200. [DOI] [PubMed] [Google Scholar]
- 28.Ibba M, Kast P, Hennecke H. Biochemistry. 1994;33:7107–7112. doi: 10.1021/bi00189a013. [DOI] [PubMed] [Google Scholar]
- 29.Nomanbhoy TK, Hendrickson TL, Schimmel P. Mol Cell. 1999;4:519–528. doi: 10.1016/s1097-2765(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 30.Nordin BE, Schimmel P. J Biol Chem. 2002;277:20510–20517. doi: 10.1074/jbc.M202023200. [DOI] [PubMed] [Google Scholar]
- 31.Hendrickson TL, Nomanbhoy TK, De Crécy-Lagard V, Fukai S, Nureki O, Yokoyama S, Schimmel P. Mol Cell. 2002;9:353–362. doi: 10.1016/s1097-2765(02)00449-5. [DOI] [PubMed] [Google Scholar]
- 32.Mursinna RS, Lincecum TL, Jr, Martinis SA. Biochemistry. 2001;40:5376–5381. doi: 10.1021/bi002915w. [DOI] [PubMed] [Google Scholar]
- 33.Mosyak L, Reshetnikova L, Goldgur Y, Delarue M, Safro MG. Nat Struct Biol. 1995;2:537–547. doi: 10.1038/nsb0795-537. [DOI] [PubMed] [Google Scholar]
- 34.Reshetnikova L, Moor N, Lavrik O, Vassylyev DG. J Mol Biol. 1999;287:555–568. doi: 10.1006/jmbi.1999.2617. [DOI] [PubMed] [Google Scholar]
- 35.Fishman R, Ankilova V, Moor N, Safro M. Acta Crystallogr D Biol Crystallogr. 2001;57:1534–1544. doi: 10.1107/s090744490101321x. [DOI] [PubMed] [Google Scholar]
- 36.Sokabe M, Okada A, Yao M, Nakashima T, Tanaka I. Proc Natl Acad Sci USA. 2005;102:11669–11674. doi: 10.1073/pnas.0502119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordin BE, Schimmel P. Biochemistry. 2003;42:12989–12997. doi: 10.1021/bi035052q. [DOI] [PubMed] [Google Scholar]
- 38.Hussain T, Kruparani SP, Pal B, Dock-Bregeon AC, Dwivedi S, Shekar MR, Sureshbabu K, Sankaranarayanan R. EMBO J. 2006;25:4152–4162. doi: 10.1038/sj.emboj.7601278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igloi GL, Cramer F. FEBS Lett. 1978;90:97–102. doi: 10.1016/0014-5793(78)80306-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang CM, Perona JJ, Ryu K, Francklyn C, Hou YM. J Mol Biol. 2006;361:300–311. doi: 10.1016/j.jmb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Dwivedi S, Kruparani SP, Sankaranarayanan R. Nat Struct Mol Biol. 2005;12:556–557. doi: 10.1038/nsmb943. [DOI] [PubMed] [Google Scholar]
- 42.Soutourina J, Plateau P, Delort F, Peirotes A, Blanquet S. J Biol Chem. 1999;274:19109–19114. doi: 10.1074/jbc.274.27.19109. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt E, Mechulam Y, Fromant M, Plateau P, Blanquet S. EMBO J. 1997;16:4760–4769. doi: 10.1093/emboj/16.15.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaRiviere FJ, Wolfson AD, Uhlenbeck OC. Science. 2001;294:165–168. doi: 10.1126/science.1064242. [DOI] [PubMed] [Google Scholar]
- 45.Asahara H, Uhlenbeck OC. Proc Natl Acad Sci USA. 2002;99:3499–3504. doi: 10.1073/pnas.052028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asahara H, Uhlenbeck OC. Biochemistry. 2005;44:11254–11261. doi: 10.1021/bi050204y. [DOI] [PubMed] [Google Scholar]
- 47.Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 48.Roy H, Ibba M. Biochemistry. 2006;45:9156–9162. doi: 10.1021/bi060549w. [DOI] [PubMed] [Google Scholar]
- 49.Morris GM, Goodsell DS, Halliday RS, Hart WE, Belew RK, Olson AJ. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.