Abstract
Vascular endothelial growth factor (VEGF) plays important roles in physiological and pathological angiogenesis. Recent studies have demonstrated that direct injection of VEGF protein, plasmid DNA, or an adenoviral vector encoding the VEGF gene into ischemic myocardium or limb can induce collateral blood vessel formation and improve perfusion of the ischemic areas. However, these approaches have limitations ranging from a short-lasting effect to angioma formation. In this study, we investigated the feasibility of using adeno-associated viral (AAV) vectors to deliver VEGF genes to mouse myocardium. A cytomegalovirus promoter was used to drive genes for a human VEGF isoform, VEGF165, and LacZ. A mouse myocardial ischemic model was generated by ligation of the anterior descending coronary artery. Approximately 1011 copies of the AAV-VEGF vector mixed with 1010 copies of AAV-LacZ were injected to one site of normal myocardium and a total of 1011 copies of AAV-VEGF were injected to multiple sites of myocardium around the ischemic region. LacZ gene expression was observed up to 3 months after the vector inoculation. After AAV-VEGF inoculation, neoangiogenesis was observed in the ischemic heart model but not in normal heart tissue. An inflammatory-cell infiltration was not observed in the AAV-VEGF- and AAV-LacZ-inoculated hearts, and angioma-like structure was not observed. These results indicated that injection of the AAV vector directly to myocardium could mediate efficient gene transfer and transgene expression and that VEGF gene delivered by AAV vector can induce angiogenesis in ischemic myocardium.
Coronary heart disease is a major cause of mortality and morbidity in humans. Despite preventive measures such as diet, cholesterol-lowering drugs, and treatment of obesity, diabetes, and hypertension, coronary disease remains a major health problem. Present treatments for severe coronary insufficiency include angioplasty or coronary bypass. Although these treatments are often helpful, restenosis of coronary vessels occurs in 30–35% of the patients (1). Therefore, alternate treatments are needed.
The discovery of growth factors that stimulate blood vessel formation has opened many approaches for the investigation of treatment of vascular insufficiencies (2–4). The growth factors that have been investigated for new blood vessel formations are the vascular endothelial growth factors (VEGF) (5–7), fibroblast growth factors (8, 9), and the angiopoietins (10). One of the most widely studied vascular growth factors is VEGF, a homodimeric heparin-binding glycoprotein of 34–46 kDa (11). VEGF occurs naturally in four isoforms that differ in the number of amino acids (121, 165, 189, and 206 aa) generated by alternative splicing (12, 13). VEGF121 and VEGF165 are soluble proteins, whereas VEGF189 and VEGF206 are bound to heparin-containing proteoglycans on the cell surface or in the basement membrane (14, 15). VEGF is one of the most specific vascular growth factors because of the abundant expression of its receptors in endothelial cells (16).
Angiogenic factors have been administered to ischemic hearts by various routes to improve coronary circulation. VEGF injected intravenously has been shown to improve myocardial blood flow, but it also results in hypotension as a side effect (17). Alternatively, VEGF and fibroblast growth factor have been injected directly into the coronary arteries (6, 8, 9). The angiogenic effect appears to be transient, because most of the angiogenic factors are not retained in the heart. In pig experiments, a single intracoronary administration of VEGF was efficacious in increasing coronary blood flow and resulted in functional improvement, despite the fact that only a small fraction of protein was localized to the ischemic area (18). However, brief exposure of humans to recombinant human VEGF165 (hVEGF165) was insufficient to trigger and maintain a therapeutically meaningful angiogenic response in clinical trials, especially if extensive atherosclerotic disease was present (4). Intramyocardial injection of growth factors also has been tried (19). Because proteins are likely to have limited life span, this approach is not expected to have prolonged effect.
A more prolonged effect may be achieved by injecting DNA encoding the genes for angiogenic factors. Intramuscular injection of plasmids encoding VEGF has been shown to be effective in animal experiments and in some patients with peripheral vascular disease (20–23). Plasmid injection for treatment of coronary artery disease has been tested (24). The therapeutic usefulness of this approach is limited by the low efficiency of cardiomyocyte transduction (0.1–1% of cardiomyocytes in the area of injection) (25). However, Schwarz et al. (24) showed that injection of a high dose of VEGF plasmids into an ischemic heart caused angioma formation. Recent experiments indicated that plasmid-mediated VEGF gene transfer to ischemic myocardium augmented perfusion of ischemic myocardium of patients with chromic myocardial ischemia (26). Adenoviral delivery of angiogenic factors has the advantage of providing high-level expression of transgenes (27–31). However, gene expression is transient in immunocompetent hosts. Adenoviral vectors have direct cytopathic effects, can elicit an immune response to viral and foreign transgene proteins, and can cause significant inflammation of myocardium and elimination of virally transduced cells (32–35).
Adeno-associated viral (AAV) vectors possess properties that may be advantageous when used to deliver angiogenic factors for coronary diseases. (i) AAV vector is nonpathogenic (36–39) and elicits no inflammatory response (25, 40). (ii) When injected into skeletal muscle, the expression of secretory proteins, such as erythropoietin (41, 42) and factor IX (43), can reach therapeutic levels that last for years in animals. Experiments with reporter genes have shown that AAV vectors can transduce myocardium efficiently by direct injection (44, 45). So far, the effects of AAV-vector-delivered VEGF in ischemic myocardium have not been investigated. In this study, we directly injected AAV vectors carrying the VEGF cDNA into the mouse myocardium and demonstrated angiogenesis in the ischemic mouse heart.
Materials and Methods
Cell Culture and AAV Vector Infection.
The 293 cells (American Type Culture Collection) were maintained in DMEM supplemented with 10% FBS. Mouse cardiomyocytes were isolated from the neonatal mice. Hearts were collected and cut into small pieces, and the pieces were digested with trypsin (1 mg/ml) and DNase (0.8 μg/10 ml) for 5–10 min at room temperature. The supernatant was collected and centrifuged at 1,000 rpm to collect the separated cells. Cells were preplated in MEM with Hanks' salts for 2 h in an atmosphere of 1% CO2/99% air. Unattached cells were collected and replated in the same medium with cytosine arabinonucleoside (25 mM) to inhibit the growth of fibroblasts and cultured for 24 h before infection. To infect the cultured cells, AAV vectors were added to tissue culture dishes and incubated with the cells for 24 h at 37°C in 5% CO2/95% air. Transgene expression was analyzed 48 h after viral vector transduction.
rAAV Vector Construction.
hVEGF165 cDNA was provided by Judith Abraham (Scios Nova, Mountain View, CA). AAV-VEGF vector was generated by cloning hVEGF165 into an AAV vector, pAVLL1.3. This vector has two left inverted terminal repeats of the AAV vector and a 1.3-kb SalI fragment of pCEP4 (Invitrogen) that contained the cytomegalovirus promoter, multiple cloning sites, and a simian virus 40 polyadenylation signal. hVEGF165 was inserted in the multiple cloning sites between the cytomegalovirus promoter and simian virus 40 polyadenylation signal. The AAV-LacZ plasmid was provided by Avigen (Alameda, CA).
AAV Vector Production.
Large quality of AAV vectors were prepared by using the three-plasmid cotransfection system (46). Briefly, AAV vector was cotransfected with two helper plasmids (provided by Avigen) into 293 cells by the calcium phosphate method. One helper plasmid, pLadeno5, has the adenoviral VA, E2A, and E4 regions that mediate AAV vector replication. The other, pHLP19, has AAV rep and cap genes. Cell lysate was produced by using three freeze and thaw cycles 3 days after the transfection. AAV vectors were purified by CsCl2 centrifugation. Viral titers were determined by dot-blot analysis. The purified vectors were tested for their infectious ability and transgene expression by infecting 293 cells.
Ischemic Heart Model and AAV Vector Inoculation in Vivo.
CD1 mice (Charles River Breeding Laboratories) were anesthetized with 15–16 μl of 2.5% Avertin per g of body weight by intraperitoneal injection. AAV vectors in 50 μl of PBS were injected to normal hearts at the apex through the diaphragm via an incision on the upper abdomen (subdiaphragmatic approach). To produce ischemic myocardium, we exposed the trachea through a midline incision of the neck, placed a tube in the trachea by using an Angiocath (Becton Dickinson) [24 gauge, 0.75 inch (1 inch = 2.54 cm)], and connected the tube to a Small Animal Volume Controlled Ventilator (Harvard Rodent Ventilator, model 683, Harvard Apparatus, South Natick, MA). After respiration of the animal was controlled by the ventilator, a thoracotomy incision was made in the second intercostal space, and a small retractor was placed in the incision to expose the heart. The anterior descending coronary artery was ligated permanently with a 6–0 nonabsorbable surgical suture. Viral vectors in 50 μl of PBS were injected directly to multiple sites of the myocardium on the left ventricular wall around the ischemic region, which had a pale color. After viral inoculation, a small tube (0.7 mm × 19 mm) connected to a syringe was placed in the incision to suck out air in the thoracic cavity to restore negative pressure before closing the incision with sutures. The tube in the trachea was gently retracted after the voluntary respiration was restored and the incision on the neck was closed.
5-Bromo-4-Chloro-3-Indolyl β-d-Galactoside (X-Gal) Staining.
AAV-LacZ transduced cells were rinsed with 150 mM sodium phosphate pH 7.4 (PBS), fixed in 2% formaldehyde and 0.2% glutaraldehyde, and overlaid with X-Gal at 1 mg/ml for 4–12 h at 37°C. Hearts were collected at various times, fixed in 4% paraformaldehyde for 60 min, and incubated in X-Gal staining solution at 30°C for 16 h.
Histological Analysis.
Hearts were fixed in 10% formalin, embedded in paraffin, and sectioned. We routinely stained the sections with hematoxylin/eosin (H&E). An antibody against von Willebrand factor (vWF; NovoCastra, Newcastle, U.K.) was used for immunohistochemical staining to define the endothelium. Inflammatory cells were detected with monoclonal antibodies directed against CD4, CD8, B cells (CD40), and natural killer cells (CD56) (NovoCastra). An ABC kit (NovoCastra) was used for immunohistochemical staining.
ELISA.
hVEGF in tissue culture supernatants and mouse serum was measured by using an ELISA kit for hVEGF (Quantikine Immunoassay, R&D Systems, Minneapolis). Cells were cultured in a complete serum-free medium for 16 h before supernatant was collected. Mouse serum was collected by retroorbital bleeding. Each sample was measured in duplicate.
Statistical Analysis.
All results were expressed as mean ± SEM. Statistical significance was evaluated by unpaired Student's t test for comparisons of two means.
Results
AAV Vector Can Infect Cardiomyocytes Efficiently in Vitro and in Vivo.
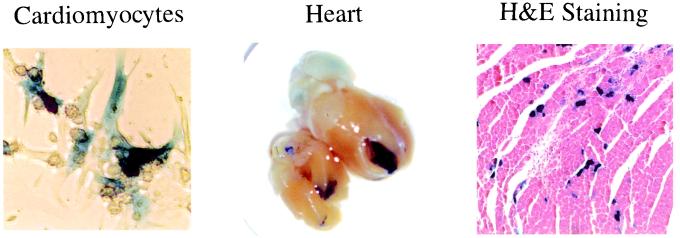
The ability of the AAV vector to infect mouse cardiomyocytes was tested with an AAV-LacZ vector. Cardiomyocytes were isolated from neonatal mouse hearts and infected with AAV-LacZ vector (multiplicity of infection = 10). LacZ staining was carried out 2 days after infection. About 30% of cardiomyocytes were infected (Fig. 1 Left). The AAV-LacZ vector also was used to infect adult mouse hearts by direct injection into the myocardium at the apex of the heart (1011 copies in 50 μl per heart) via the subdiaphragmatic approach. The hearts were collected 1, 2, and 3 months after AAV-LacZ inoculation; two hearts were collected each time. The apexes of the infected hearts appeared blue after X-Gal staining (Fig. 1 Middle). The needle injection sites were identified as fibrous scar tissues on H&E-stained slides. We found 40% of cardiomyocytes infected with AAV-LacZ vector within a diameter of 1 mm around the needle injection sites (Fig. 1 Right). The LacZ expression in the myocardium was present up to 3 months after the transduction when the experiment was stopped. No inflammatory cell was found in the AAV-infected myocardium by observing the slides stained with H&E (Fig. 1 Right) and immunohistochemically stained with antibodies against CD4, CD8, CD40, or CD56 (data not shown).
Figure 1.
AAV-LacZ infection of cultured mouse cardiomyocytes and adult mouse heart. (Left) Infected cardiomyocytes. (Middle) X-Gal staining of a whole heart. (Right) H&E staining of the myocardium. Note the lack of any inflammatory changes surrounding the cells expressing LacZ.
Neovascular Formation Is Absent in Nonischemic Mouse Myocardium After AAV-Mediated VEGF Gene Transfer.
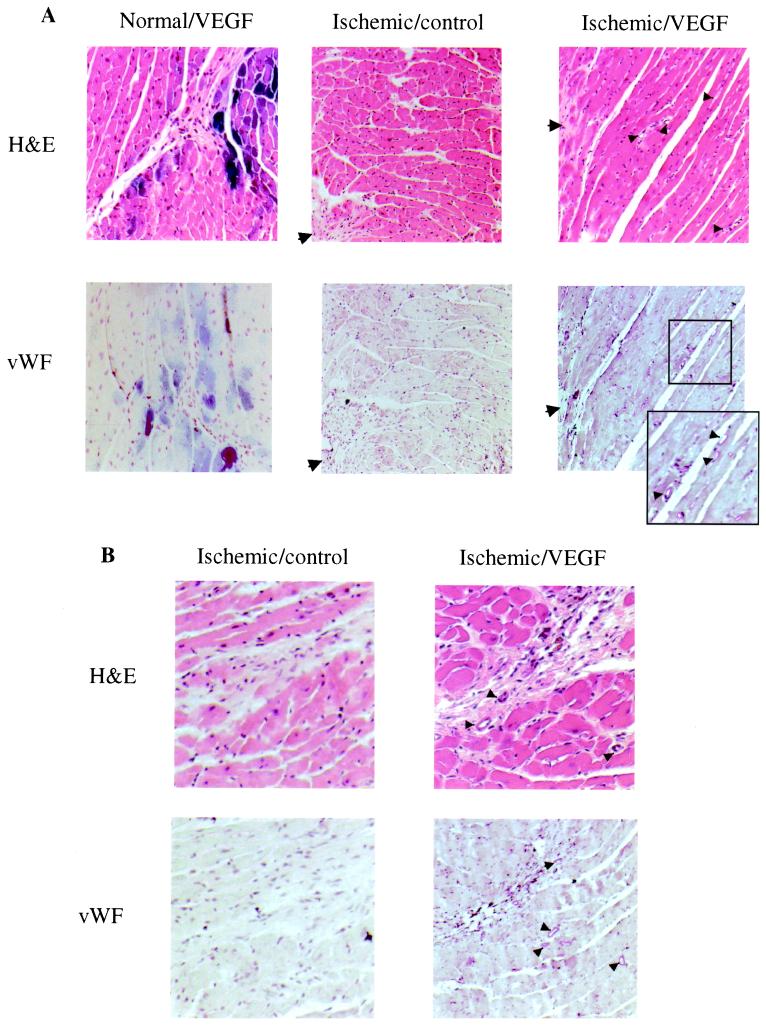
An AAV-VEGF vector was generated by insertion of VEGF165 cDNA between two inverted terminal repeats of AAV vector. We used a cytomegalovirus promoter to control the expression of hVEGF165. The gene expression mediated by this vector was tested by infecting 293 cells. The amount of VEGF protein in cell culture supernatant was about 3 ng per 107 cells per 24 h of culture. Approximately 1011 copies of AAV-VEGF and 1010 copies of AAV-LacZ were coinjected to the myocardium of six mice via the subdiaphragmatic approach. The 10% AAV-LacZ vector in the viral stock was used to mark the injection sites and indicate whether the gene transduction was successful. Hearts were collected 1, 2, and 3 months after the viral vector inoculation with two hearts at each time point. LacZ gene expression was detected at the apexes of the hearts. The needle tracks were identified as fibrous scar tissues on H&E-stained slides, with 30–40% of cardiomyocytes within a diameter of 1 mm around the needle tracks expressing LacZ, indicating a successful AAV transduction. The endothelium of blood vessels was stained with the antibody against vWF. The numbers of blood vessel around the injection site and an uninjected region were the same, and no neovascular formation was observed in these nonischemic hearts (Fig. 2A Left).
Figure 2.
Photomicrographs of normal and ischemic hearts inoculated with AAV-VEGF (Normal/VEGF and Ischemic/VEGF) and ischemic heart not injected with AAV vector (Ischemic/control). Arrows, infarcted regions; arrowheads, small blood vessels. (A) Area around the infarcted regions. (Upper) H&E staining. (Lower) vWF staining. An increase in vessel formation is observed in the Ischemic/VEGF hearts. An inset in the micrograph of the vWF-stained ischemic/VEGF heart presents an enlarged area to show blood vessels more clearly. (B) Area around scar tissues formed by needle injections. H&E and vWF stains of cardiac myocardium of both the Ischemic/control and Ischemic/VEGF mice. Note the new blood vessel formation around the scar caused by the needles in the AAV-VEGF-injected heart but not in the control without injection of AAV.
AAV-VEGF Induces Neovascular Formation in Ischemic Myocardium.
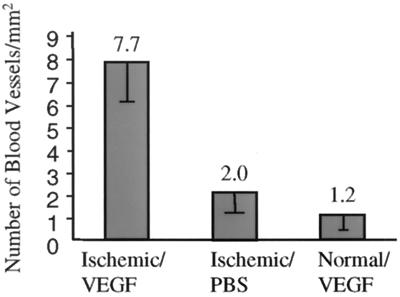
To test whether AAV-vector-mediated VEGF gene transfer can induce new blood vessel formation in ischemic myocardium, we generated an ischemic myocardium model by ligation of anterior descending coronary artery of the mouse. Approximately 1011 copies of AAV-VEGF were injected to the myocardium around the ischemic region at multiple sites. PBS was injected in control hearts. Hearts were collected 2 months after the inoculation, two hearts in each group. There was no discernable difference in the size and gross appearance of the hearts that were injected with AAV-VEGF or PBS. The infarct areas were located on the anterior region of the septum ventriculorum and the ventricular wall in front of that region as indicated by the fibrous scar tissues on H&E-stained slides. The sizes of the infarct were about 1.5 mm3. In the hearts that were inoculated with AAV-VEGF, many new blood vessels were observed around the infarct myocardium region and the needle tracks (Fig. 2 A Right and B Right). In contrast, mice that were not injected with AAV-VEGF showed fewer blood vessels in the same areas (Fig. 2 A Middle and B Left). The newly formed small vessels consisted of endothelial cells surrounded by a few layers of smooth muscles. The number of small blood vessels was counted with a ×20 microscopic objective in 10 randomly selected fields around the scar tissues formed by the infarct and the needle injection. The mean densities of the small blood vessels were 1.2 ± 0.9 vessels per mm2 in normal hearts transduced with AAV-VEGF/AAV-LacZ, 2 ± 0.9 vessels per mm2 in ischemic hearts without AAV transduction, and 7.7 ± 2.7 vessels per mm2 in ischemic hearts transduced with AAV-VEGF (Fig. 3). Differences in the means of small blood vessel densities analyzed by the Student's t test were statistically significant between AAV-VEGF transduced and untransduced ischemic hearts (P < 0.01) and AAV-VEGF transduced ischemic and normal heart (P < 0.01). No angioma-like structure was observed. hVEGF was not detected by ELISA in the serum of any of the mice that had been inoculated with AAV-VEGF vector.
Figure 3.
Densities of small blood vessel. Bars: Ischemic/VEGF, ischemic heart injected with AAV-VEGF; Ischemic/PBS, ischemic heart injected with PBS; Normal/VEGF, normal heart injected with AAV-VEGF/AAV-LacZ. The mean is shown above each bar; error bars are the SEM. Differences between the ischemic/VEGF and ischemic /PBS groups and between the ischemic/VEGF and normal/VEGF groups are statistically significant (P < 0.01).
Discussion
In this study, we investigated the effects of direct AAV-VEGF viral vector injection in normal and ischemic mouse myocardium. Our findings are as follows: (i) the AAV vector can infect mouse cardiac myocyte efficiently in vitro and in vivo; (ii) AAV-mediated hVEGF165 gene transfer induced angiogenesis in ischemic myocardium without evidence of angioma formation; (iii) AAV-mediated hVEGF165 gene transfer did not induce angiogenesis in normal nonischemic hearts; and (iv) hVEGF was not detected in mouse serum after intramyocardium inoculation of AAV-VEGF.
Because we used a cytomegalovirus promoter to drive VEGF expression, the AAV-VEGF-mediated VEGF gene expression should be in the same level in normoxic and hypoxic conditions. However, we were only able to induce angiogenesis in ischemic myocardium, but not in nonischemic myocardium, with intramyocardium injection of 1011 copies of AAV-VEGF. The difference may be caused by differences in the expression of VEGF receptors under normoxic and hypoxic conditions. It has been reported that hypoxia, in addition to inducing the expression of VEGF, also up-regulates the expression of VEGF receptors (Flt and Flk) (47–50). Previous studies of angiogenesis induced by VEGF were performed in the presence of ischemia. Therefore, it likely that VEGF receptors were up-regulated as well (7, 21, 23, 28, 51–54). In fact, when VEGF protein was administered intravenously to animals with one ischemic leg, new vessels were formed only in the ischemic limb (55). Thus, our study showing new blood vessel formation only in the presence of ischemia is comparable with these findings and implies that up-regulation of VEGF receptors in ischemia may be important for neoangiogenesis in the myocardium.
Potential problems associated with prolonged and high-level expression of VEGF are inappropriate angiogenesis resulting in adverse consequences in tissues, such as the retina and the synovium and in occult tumors (56), as well as the formation of hemagioma. Hemagioma has been seen in the heart injected with plasmid (24) and retroviral vector-mediated VEGF gene transfer (57, 58). Retroviruses can mediate transgene expression at high levels in transduced tissue (58), and high concentration of VEGF in local areas can induce exaggerated angiogenesis and angioma formation. Injection of a large dose of VEGF plasmid (500 μg of plasmid DNA) to ischemic rat heart induced angioma formation. No angioma was seen in a similar rat model when a smaller dose (125 μg of plasmid DNA) was used (24). Springer et al. (58) reported that high levels of serum VEGF (200 μg/ml) caused hemangiomas in adult skeletal muscles and that low serum levels (30 μg/ml) did not cause vascular malformations but were sufficient to induce angiogenesis in ischemic muscle. Hence, it is important to control the level of VEGF expression. Our experiment demonstrated that 1011 copies of AAV-VEGF was adequate to induce angiogenesis in the local ischemic environment. However, no angioma was observed in any of the normal and ischemic hearts, and no hVEGF was detected in the mouse serum. This result suggested that AAV-mediated VEGF expression was not as high as those mediated by adenoviral and retroviral vectors, and yet it is enough to induce new vascular formation in the ischemic myocardium. Thus, with proper dose, AAV may represent an ideal vector for VEGF delivery.
Erythropoietin and other secretory proteins delivered by AAV vectors may last for years in mice. It is possible that long-term expression of VEGF eventually could lead to angioma formation. Although the dose of AAV-VEGF used in this experiment did not induce angioma formation, a higher dose may still have the potential to generate side effects. A method of circumventing this possibility is to allow the expression of VEGF only in the presence of hypoxia. Hypoxia-inducible factor 1 is a heterodimeric basic helix–loop–helix protein (59, 60) that activates transcription of the human erythropoietin gene in hypoxic cells (61). It is also involved in the activation of VEGF transcription. Instead of using a constitutionally expressing promoter, a hypoxia response element can be incorporated into the construct to provide a regulated expression of VEGF in response to cardiac ischemia. Hence, adding the hypoxia response elements found in erythropoietin or VEGF genes to control the expression of VEGF may further minimize the side effect. AAV responds to regulatory elements for an extended time (44). Thus, AAV vectors combined with an inducible promoter could provide a safe delivery system for VEGF in clinical use.
Acknowledgments
We thank Dr. Peter Colosi for providing AAV-LacZ, pLadeno5, and pHLP19 plasmids, and Mr. Norm Honbo for preparing mouse cardiomyocytes.
Abbreviations
- VEGF
vascular endothelial growth factor
- AAV
adeno-associated viral
- h
human
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- vWF
von Willebrand factor
- H&E
hematoxylin/eosin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250488097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250488097
References
- 1.Libby P, Schwartz D, Brogi E, Tanaka H, Clinton S K. Circulation. 1992;86:III47–III52. [PubMed] [Google Scholar]
- 2.Folkman J. Circulation. 1998;97:628–629. doi: 10.1161/01.cir.97.7.628. [DOI] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, Martin J F. Lancet. 2000;355:213–222. doi: 10.1016/S0140-6736(99)04180-X. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Alitalo K. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 5.Losordo D W, Vale P R, Symes J F, Dunnington C H, Esakof D D, Maysky M, Ashare A B, Lathi K, Isner J M. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman J D, Hibberd M G, Chuang M L, Harada K, Lopez J J, Gladstone S R, Friedman M, Sellke F W, Simons M. Nat Med. 1995;1:1085–1089. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita S, Pu L Q, Stein L A, Sniderman A D, Bunting S, Ferrara N, Isner J M, Symes J F. Circulation. 1994;90:II228–II234. [PubMed] [Google Scholar]
- 8.Giordano F J, Ping P, McKirnan M D, Nozaki S, DeMaria A N, Dillmann W H, Mathieu-Costello O, Hammond H K. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 9.Harada K, Grossman W, Friedman M, Edelman E R, Prasad P V, Keighley C S, Manning W J, Sellke F W, Simons M. J Clin Invest. 1994;94:623–630. doi: 10.1172/JCI117378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shyu K G, Manor O, Magner M, Yancopoulos G D, Isner J M. Circulation. 1998;98:2081–2087. doi: 10.1161/01.cir.98.19.2081. [DOI] [PubMed] [Google Scholar]
- 11.Gospodarowicz D, Abraham J A, Schilling J. Proc Natl Acad Sci USA. 1989;86:7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houck K A, Ferrara N, Winer J, Cachianes G, Li B, Leung D W. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 13.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes J C, Abraham J A. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 14.Houck K A, Leung D W, Rowland A M, Winer J, Ferrara N. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 15.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Houck K, Jakeman L, Leung D W. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 17.Hariawala M D, Horowitz J R, Esakof D, Sheriff D D, Walter D H, Keyt B, Isner J M, Symes J F. J Surg Res. 1996;63:77–82. doi: 10.1006/jsre.1996.0226. [DOI] [PubMed] [Google Scholar]
- 18.Lopez J J, Laham R J, Stamler A, Pearlman J D, Bunting S, Kaplan A, Carrozza J P, Sellke F W, Simons M. Cardiovasc Res. 1998;40:272–281. doi: 10.1016/s0008-6363(98)00136-9. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher B, Pecher P, von Specht B U, Stegmann T. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 20.Bauters C, Asahara T, Zheng L P, Takeshita S, Bunting S, Ferrara N, Symes J F, Isner J M. Am J Physiol. 1994;267:H1263–H1271. doi: 10.1152/ajpheart.1994.267.4.H1263. [DOI] [PubMed] [Google Scholar]
- 21.Takeshita S, Tsurumi Y, Couffinahl T, Asahara T, Bauters C, Symes J, Ferrara N, Isner J M. Lab Invest. 1996;75:487–501. [PubMed] [Google Scholar]
- 22.Tsurumi Y, Kearney M, Chen D, Silver M, Takeshita S, Yang J, Symes J F, Isner J M. Circulation. 1997;96:II-382–388. [PubMed] [Google Scholar]
- 23.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow S T, Passeri J, Horowitz J R, Symes J F, Isner J M. Circulation. 1996;94:3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz E R, Speakman M T, Patterson M, Hale S S, Isner J M, Kedes L H, Kloner R A. J Am Coll Cardiol. 2000;35:1323–1330. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 25.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 26.Vale P R, Losordo D W, Milliken C E, Maysky M, Esakof D D, Symes J F, Isner J M. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- 27.Laitinen M, Makinen K, Manninen H, Matsi P, Kossila M, Agrawal R S, Pakkanen T, Luoma J S, Viita H, Hartikainen J, et al. Hum Gene Ther. 1998;9:1481–1486. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- 28.Mack C A, Patel S R, Schwarz E A, Zanzonico P, Hahn R T, Ilercil A, Devereux R B, Goldsmith S J, Christian T F, Sanborn T A, et al. J Thorac Cardiovasc Surg. 1998;115:168–176. doi: 10.1016/s0022-5223(98)70455-6. , and discussion 176–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlhauser J, Merrill M J, Pili R, Maeda H, Bacic M, Bewig B, Passaniti A, Edwards N A, Crystal R G, Capogrossi M C. Circ Res. 1995;77:1077–1086. doi: 10.1161/01.res.77.6.1077. [DOI] [PubMed] [Google Scholar]
- 30.Lee L Y, Patel S R, Hackett N R, Mack C A, Polce D R, El-Sawy T, Hachamovitch R, Zanzonico P, Sanborn T A, Parikh M, et al. Ann Thorac Surg. 2000;69:14–23. doi: 10.1016/s0003-4975(99)01102-9. , and discussion 23–24. [DOI] [PubMed] [Google Scholar]
- 31.Lazarous D F, Shou M, Stiber J A, Hodge E, Thirumurti V, Goncalves L, Unger E F. Cardiovasc Res. 1999;44:294–302. doi: 10.1016/s0008-6363(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 32.Barr E, Carroll J, Kalynych A M, Tripathy S K, Kozarsky K, Wilson J M, Leiden J M. Gene Ther. 1994;1:51–58. [PubMed] [Google Scholar]
- 33.Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, Karpati G. Hum Mol Genet. 1994;3:579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Ertl H C, Wilson J M. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Haecker S E, Su Q, Wilson J M. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 36.Berns K I, Linden R M. BioEssays. 1995;17:237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- 37.Kotin R M. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 38.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzyczka N. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 40.Vincent-Lacaze N, Snyder R O, Gluzman R, Bohl D, Lagarde C, Danos O. J Virol. 1999;73:1949–1955. doi: 10.1128/jvi.73.3.1949-1955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder R O, Spratt S K, Lagarde C, Bohl D, Kaspar B, Sloan B, Cohen L K, Danos O. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- 43.Herzog R W, Hagstrom J N, Kung S H, Tai S J, Wilson J M, Fisher K J, High K A. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee L Y, Zhou X, Polce D R, El-Sawy T, Patel S R, Thakker G D, Narumi K, Crystal R G, Rosengart T K. J Thorac Cardiovasc Surg. 1999;118:26–34. doi: 10.1016/S0022-5223(99)70137-6. , and discussion 34–35. [DOI] [PubMed] [Google Scholar]
- 45.Svensson E C, Marshall D J, Woodard K, Lin H, Jiang F, Chu L, Leiden J M. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- 46.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman G J, Iwaki Y, Colosi P. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 47.Plate K H, Breier G, Weich H A, Risau W. Nature (London) 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 48.Plate K H, Breier G, Millauer B, Ullrich A, Risau W. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 49.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 50.Marti H H, Risau W. Proc Natl Acad Sci USA. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banai S, Jaklitsch M T, Shou M, Lazarous D F, Scheinowitz M, Biro S, Epstein S E, Unger E F. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 52.Harada K, Friedman M, Lopez J J, Wang S Y, Li J, Prasad P V, Pearlman J D, Edelman E R, Sellke F W, Simons M. Am J Physiol. 1996;270:H1791–H1802. doi: 10.1152/ajpheart.1996.270.5.H1791. [DOI] [PubMed] [Google Scholar]
- 53.Isner J M, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes J F. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 54.Takeshita S, Zheng L P, Brogi E, Kearney M, Pu L Q, Bunting S, Ferrara N, Symes J F, Isner J M. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauters C, Asahara T, Zheng L P, Takeshita S, Bunting S, Ferrara N, Symes J F, Isner J M. J Vasc Surg. 1995;21:314–324. doi: 10.1016/s0741-5214(95)70272-5. and discussion 324–325. [DOI] [PubMed] [Google Scholar]
- 56.Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 57.Lee R J, Springer M L, Blanco-Bose W E, Shaw R, Ursell P C, Blau H M. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 58.Springer M L, Chen A S, Kraft P E, Bednarski M, Blau H M. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 59.Wang G L, Jiang B H, Rue E A, Semenza G L. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang G L, Semenza G L. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 61.Jiang B H, Rue E, Wang G L, Roe R, Semenza G L. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]