Abstract
Dominantly inherited mutations in the genes encoding presenilins (PS) and the amyloid precursor protein (APP) are the major causes of familial Alzheimer's disease (AD). The prevailing view of AD pathogenesis posits that accumulation of β-amyloid (Aβ) peptides, particularly Aβ42, is the central event triggering neurodegeneration. Emerging evidence, however, suggests that loss of essential functions of PS could better explain dementia and neurodegeneration in AD. First, conditional inactivation of PS in the adult mouse brain causes progressive memory loss and neurodegeneration resembling AD, whereas mouse models based on overproduction of Aβ have failed to produce neurodegeneration. Second, whereas pathogenic PS mutations enhance Aβ42 production, they typically reduce Aβ40 generation and impair other PS-dependent activities. Third, γ-secretase inhibitors can enhance the production of Aβ42 while blocking other γ-secretase activities, thus mimicking the effects of PS mutations. Finally, PS mutations have been identified in frontotemporal dementia, which lacks amyloid pathology. Based on these and other observations, we propose that partial loss of PS function may underlie memory impairment and neurodegeneration in the pathogenesis of AD. We also speculate that Aβ42 may act primarily to antagonize PS-dependent functions, possibly by operating as an active site-directed inhibitor of γ-secretase.
Alzheimer's disease (AD) is an age-related neurodegenerative dementia and is the most common cause of both neurodegeneration and dementia. Neurodegenerative dementias are characterized clinically by progressive impairment of cognitive abilities, which most prominently affects memory in AD. Neuronal and synaptic loss is the essential neuropathological feature common to different forms of neurodegenerative dementias, including AD, frontotemporal dementia (FTD) and Lewy body dementia (LBD). These diseases are distinguished neuropathologically by characteristic patterns of abnormal protein aggregation, such as the presence in the AD brain of cerebral cortical amyloid plaques and neurofibrillary tangles (NFTs). Extracellular amyloid plaques consist primarily of 40- to 42-residue β-amyloid (Aβ) peptides (Aβ40 and Aβ42) derived from proteolytic processing of the amyloid precursor protein (APP). NFTs are intraneuronal inclusions composed of hyperphosphorylated forms of the microtubule-associated protein tau.
Research on AD has been greatly stimulated by the identification of causative mutations in the genes encoding APP and presenilins (PS1 and PS2). Dominantly inherited missense mutations in APP increase the production of Aβ peptides and account for ≈10% of mutations identified in familial AD (FAD). PSs harbor ≈90% of identified FAD mutations, and many of these mutations increase the relative production of Aβ42 peptides. The prevailing amyloid hypothesis posits that accumulation of Aβ peptides, particularly the more hydrophobic and aggregation-prone Aβ42, triggers a pathogenic cascade, leading to neurodegeneration in AD (1). However, amyloid accumulation is not an obligatory feature of dementia or neurodegeneration because neurodegenerative dementias lacking amyloid pathology (e.g., FTD) have been well described. Accordingly, the regional distribution of amyloid plaques correlates poorly with the pattern and severity of dementia in AD, whereas synaptic loss correlates well with these clinical features (2). More surprisingly, mouse models overexpressing mutant human APP have reproduced overproduction of Aβ peptides and progressive amyloid deposition, but they have largely failed to reproduce neurodegeneration (e.g., see ref. 3).
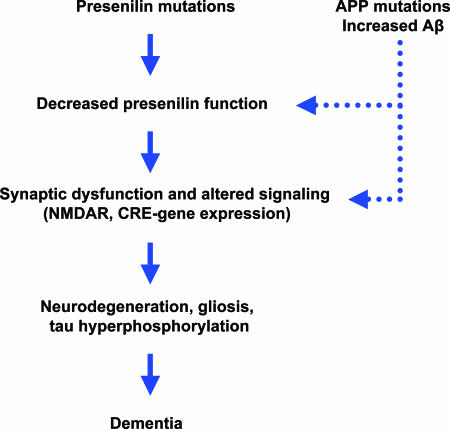
The presenilin hypothesis (Fig. 1) was prompted by our recent studies of conditional knockout mice in which PSs are selectively inactivated in the adult cerebral cortex (4). These mice develop age-related, progressive neurodegeneration characterized by hallmarks of AD neuropathology, including synaptic loss, neuronal cell death, astrogliosis and tau hyperphosphorylation (Fig. 2). In these conditional mutant mice, inactivation of PS expression occurs at 4 weeks of age postnatally, and neurodegeneration becomes evident by 4 months of age. By the age of 9 months, 24% of cortical neurons and 35% of cortical volume are lost. Neurodegeneration is preceded by memory loss, synaptic plasticity impairments, reductions in NMDA receptor-mediated synaptic responses, and decreases in cAMP-response element (CRE)-dependent gene expression (e.g., BDNF, c-fos), suggesting that these molecular defects mediate the subsequent neurodegeneration. Among mouse models of AD, conditional PS knockout mice are the only mutant mice derived from genetic manipulation of AD genes that reproduce the central features of AD, namely neurodegeneration and dementia.
Fig. 1.
The presenilin hypothesis. This diagram depicts the cascade of events leading to neurodegeneration and dementia in AD, as proposed by the presenilin hypothesis. Pathogenic mutations in PS partially impair γ-secretase-dependent and -independent activities through a dominant-negative mechanism. Elevated levels of Aβ, particularly Aβ42, resulting from pathogenic mutations in APP or PS, or in association with sporadic AD, may act to inhibit PS function, mimicking the effect of PS mutations. Because production of Aβ42 is enhanced by partial loss of PS and γ-secretase activity, Aβ42-mediated inhibition may create a vicious cycle leading to progressively greater impairment of PS function. Loss of PS activity results in synaptic dysfunction, such as deficits in synaptic plasticity, and alterations in molecular signaling events, including impairment of NMDA receptor-mediated functions and reduction in CRE-dependent gene expression. Loss of PS function ultimately leads to age-related, progressive neurodegeneration characterized by loss of synapses, dendrites, and neurons; astrogliosis; and tau hyperphosphorylation.
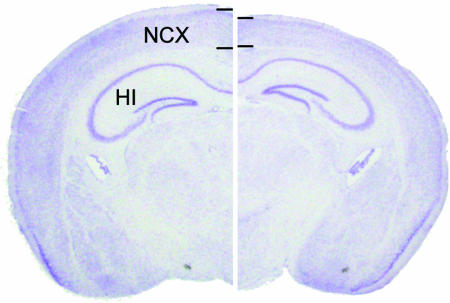
Fig. 2.
Loss of PS function in the adult cerebral cortex causes striking neurodegeneration. Coronal sections of control (Left) and PS conditional double knockout (cDKO) (Right) brains at 9 months of age are shown to illustrate the extent of neurodegeneration in PS cDKO mice. Thin lines mark the boundaries of cortical layers and show the thickness of the cerebral cortex. Note the diffuse thinning of the cerebral cortex and underlying hippocampal atrophy. Labels indicate the locations of the neocortex (NCX) and hippocampus (HI).
The fact that loss of PS function in the mouse brain phenocopies the essential manifestations of AD raised the possibility that FAD-linked mutations in PS may cause the disease by means of the partial loss of essential PS functions. Indeed, substantial experimental evidence supports the view that pathogenic PS mutations cause partial impairment of PS-mediated activities. These findings provided the initial impetus to rethink how PS and APP may be involved in AD. Below, we will summarize accumulating evidence for the presenilin hypothesis and discuss how it can explain familial and sporadic AD.
FAD-Linked PS Mutations Impair γ-Secretase-Dependent and -Independent PS Activities
PSs are essential components of γ-secretase, a multisubunit protease complex that catalyzes the intramembranous cleavage of a number of type I transmembrane proteins, including Notch, APP, and cadherins. Notch is a key physiological substrate of γ-secretase, as evidenced by similar developmental phenotypes exhibited by PS and Notch mutant mice (5), and the dependence of Notch signaling on the γ-secretase-mediated release of its intracellular domain (NICD). The APP intracellular domain (AICD), which is similarly released by γ-secretase-mediated cleavage, has been implicated in transcriptional regulation (6). Cadherins seem to undergo similar γ-secretase-dependent cleavages, although the physiological significance of this cleavage is unclear. One unusual feature of γ-secretase is its relaxed sequence specificity, as evidenced by lack of strong sequence similarity in its substrates and its tendency to cleave some substrates at a series of neighboring intramembranous residues. PSs also possess γ-secretase-independent activities, such as down-regulation of Wnt signaling through destabilization of β-catenin.
The first direct evidence that FAD-linked mutations impair the biological activity of PS came from genetic complementation studies in Caenorhabditis elegans (7). The C. elegans PS homolog, SEL12 (suppressor/enhancer of LIN12), was originally identified through its ability to revert the phenotype caused by constitutive activation of the Notch homolog LIN12. Loss-of-function mutations in SEL12, which exhibits ≈50% sequence identity to PS1 and PS2, reduce LIN12 activity and confer an egg-laying defective phenotype (Egl). Transgenic expression of wild-type human PS1 and PS2 rescued the Egl phenotype caused by a strongly hypomorphic SEL12 mutation to a level comparable with that of wild-type (wt) SEL12. In contrast, six different FAD-linked mutations reduced the ability of PS1 to substitute functionally for SEL12 to varying degrees (Table 1). Importantly, the ability of mutant PSs to suppress the Egl phenotype is dose-dependent, with higher levels of expression of some mutants resulting in full rescue, but lower levels of expression revealing a significant impairment. More recent studies in Drosophila have also shown that FAD-associated mutations reduce the ability of fly PS to complement Notch-like phenotypes exhibited by PS−/− mutants (8).
Table 1.
Pathogenic PS mutations impair γ-secretase-dependent activities
| Presenilin | Aβ40 | Aβ42 | NICD | AICD | sel12 |
|---|---|---|---|---|---|
| PS1 | |||||
| A79V | ↓ | ↔ | |||
| ΔI83/ΔM84 | ↓ | ||||
| C92S | ↔ | ↓ | |||
| Y115H | ↓ | ||||
| N135D | ↓ | ↑ | |||
| I143T | ↓ | ↑ | ↓ | ||
| M146L | ↔ | ↑ | ↔ | ↔ | ↓ |
| M146V | ↔ | ↑ | ↓ | ↓ | |
| H163R | ↔ | ↑ | ↓ | ||
| L166P | ↓ | ↑/↔ | ↓ | ↓ | |
| L166R | ↓ | ↓ | |||
| G206A | ↔ | ↑ | ↓ | ↓ | |
| G209V | ↔ | ↑ | ↓ | ↔ | |
| I229F | ↔ | ↓ | |||
| A231V | ↓ | ↔ | |||
| M233L | ↔ | ↓ | |||
| M233T | ↓ | ↑ | ↔ | ↓ | |
| M233V | ↔ | ↓ | |||
| F237I | ↔ | ↓ | |||
| A246E | ↔ | ↑ | ↓ | ||
| P264L | ↓ | ↑ | |||
| L286V | ↔ | ↓ | |||
| Δex9 | ↓ | ↑/↔ | ↓ | ↓ | ↓ |
| insR352* | ↓ | ↓ | ↓ | ||
| G384A | ↓ | ↑ | ↓ | ||
| L392V | ↔ | ↑ | ↓ | ↓ | |
| C410Y | ↓ | ↑ | ↓ | ↓ | ↓ |
| PS2 | |||||
| T122P | ↓ | ↑ | ↓ | ↓ | |
| N141I | ↓ | ↑/↔ | ↓ | ↓ | |
| M239V | ↓ | ↑ | ↔ | ↓ | |
| M239I | ↓ | ↑ | ↔ | ↓ |
This table summarizes the reported effects of FAD-linked PS mutations on the γ-secretase-dependent production of Aβ40, Aβ42, and the intracellular domains of APP (AICD) and Notch (NICD), as well as activity in complementing SEL12 deficiency in C. elegans. Increases and decreases are indicated by up (↑) and down (↓) arrows, respectively, and results in which the level of γ -secretase product was found to be unchanged are indicated by a sideways arrow (↔). The asterisk after insR352 indicates its association with familial FTD rather than AD. Consensus data are tabulated from published studies (7, 9–19, 45, 58–60).
Although initial investigations of FAD-linked PS mutations in mammalian systems focused on enhancement of Aβ42 production, it has now become clear that a large number of pathogenic mutations cause impairments in other PS activities (Table 1). Of the major cleavages of APP mediated by γ-secretase, PS mutations typically increase the production of Aβ42 (and Aβ43), but can impair the normally predominant γ-cleavage following Aβ residue 40 and the more distal “ε cleavage” following residue 49, resulting in significantly reduced generation of Aβ40 and AICD, respectively. Similarly, impairment of the γ-secretase-dependent S3 cleavage of Notch and consequent reduction in production of NICD have been well documented with a variety of PS mutations. The first study in a vertebrate system to demonstrate reduction of PS activity by pathogenic mutations analyzed the effects on NICD generation of six FAD-linked PS1 mutations distributed across the coding sequence (Y115H, I143T, M146V, G209V, G384A, and C410Y) (9). All six mutations caused reductions in proteolytic release of NICD ranging from 40% to >90% relative to wild-type PS1. Subsequent studies confirmed the reduction in NICD generation conferred by several of these mutations, and identified a number of additional FAD-linked mutations in both PS1 (V96F, L166P, L166R, G206A, Δexon9, and L392V) and PS2 (T122P and N141I) that substantially impair NICD production (10–13).
Interestingly, the vast majority of PS mutations that impair NICD production also impair AICD production, indicating a general impairment of γ-secretase-dependent function that is not limited to a single substrate (Table 1) (11–15). This correspondence of the effects of mutations on liberation of NICD and AICD may reflect mechanistic similarities between the S3 and ε cleavages of Notch and APP, respectively, which occur at similar intramembranous positions near the cytoplasmic face of the plasma membrane. Conversely, most mutations that interfere with normal AICD generation also reduce NICD generation.
Perhaps more surprisingly, numerous mutations in PS1 (e.g., N135D, L166P, M233T, P264L, G384A, and C410Y) and PS2 (e.g., T122P, N141I, M239V, and M239I) can cause significant reductions in the production of Aβ40, often despite a concomitant increase in the production of Aβ42 (12–14, 16–19). The differential effect of PS mutations on alternative cleavage positions in the APP transmembrane domain seems not to represent a simple shift in substrate preference or specificity, because the degree of elevation in Aβ42 levels with individual mutations does not correlate with the degree of depression in Aβ40 or AICD levels (e.g., ref. 13). Thus, a concerted effect of many FAD-linked PS mutations on γ-secretase activity seems to be enhancement of cleavage following Aβ residue 42, accompanied by inhibition of cleavage at other possible positions in the APP transmembrane domain, with the magnitude of the effects varying with the specific mutation.
FAD-linked PS mutations also impair γ-secretase-dependent proteolysis of substrates other than APP and Notch. N-cadherin undergoes a PS- and γ-secretase-dependent ε-cleavage analogous to that of APP, and a series of FAD-linked mutations (Y115H, M146L, A246E, E280A, E280G, G384A, and Δexon9) uniformly suppressed this cleavage (20). In addition, FAD-linked mutations have been reported to cause a variable but general impairment of “presenilinase” activity. One of the first reports describing PS1 endoproteolysis noted inhibition of this activity by two FAD-linked mutations (M146V and A246E) (21). In a survey of 29 distinct FAD-linked PS1 mutations, PS1 endoproteolysis was significantly reduced in all cases, with the most severe impairments (>80% reduction) observed with the V96F, E280G, and C410Y mutations (22). Reduced PS1 endoproteolysis was also observed in mice bearing a targeted germ-line P264L missense mutation (23). The functional significance of deficient presenilinase activity is unclear, as PS1 mutants (e.g., Δexon9) refractory to endoproteolysis still support γ-secretase activity, and whether PS endoproteolysis is in fact autocatalytic remains unresolved (24, 25).
PS also possess γ-secretase-independent activities, such as their down-regulation of the Wnt signaling pathway through interaction with and destabilization of β-catenin. Interestingly, FAD-linked mutations (M146L, Δexon9, C263R, and P246L) have been found to impair this γ-secretase-independent activity as well, resulting in enhanced β-catenin stability and β-catenin-dependent signaling (26, 27). Recently, PS holoproteins were found to function as passive ER calcium leak channels independent of γ-secretase activity, and two FAD-associated mutations (PS1 M146V and PS2 N141L) impaired this function (28). Collectively, these observations strongly suggest that pathogenic mutations cause a general impairment of PS function affecting both γ-secretase-dependent and -independent activities. Given the large number of pathogenic mutations that do not localize to a particular domain, the deleterious functional impact of pathogenic mutations may reflect a general destabilization of PS structure.
The ability of PS1 bearing the FAD-linked A246E mutation to rescue the phenotypic defects of PS1−/− mice has been taken as evidence that mutant PS possess normal biological activity, and that pathogenic PS1 mutations do not act through a loss-of-function mechanism (21, 29). However, PS1+/− mice with only one functional PS1 allele are phenotypically normal, indicating that 50% of the normal PS1 dosage is sufficient to rescue the phenotype of PS1−/− mice (30). Therefore, even if the A246E mutation caused a 50% reduction in PS1 activity, expression at a level equal to the normal PS1 expression level would yield a phenotypic rescue; greater levels of overexpression would compensate for greater decrements in activity. Consistent with this view, analysis of transgene expression levels in embryos in one study revealed that the degree of phenotypic rescue correlated with the extent of overexpression of mutant PS1 (21). Moreover, the A246E mutation exhibits reduced ability to rescue phenotypes caused by PS homologue deficiency in C. elegans and Drosophila, further arguing that this mutation does indeed compromise the biological activity of PS1 (7, 8).
Thus, studies of pathogenic PS mutations have revealed that a large number of mutations confer a partial loss of function (Table 1). Indeed, such analyses have shown that impairment of PS activities by pathogenic mutations is the rule rather than the exception. Because these studies typically involve overexpression of mutant PS, the observed reductions in PS-dependent activities are likely to represent underestimates; in cases in which PS-dependent activities were not apparently impaired by mutations, overexpression may have obscured a partial loss of activity, as observed in the C. elegans studies described above. Comparison of the effects of pathogenic PS mutations with those of inactivating or null mutations has confirmed that the reductions in γ-secretase-dependent and γ-secretase-independent activities represent a partial loss of function. The sole discrepancy is the relative increase in production of Aβ42 caused by pathogenic mutations, which has been generally interpreted as a gain of function, because genetic inactivation of PS impairs all γ-secretase-mediated cleavages of the APP transmembrane domain (but see next section). Recent analysis of a series of FAD-linked PS mutations in cultured human cells and Drosophila has further shown that the degree of reduction in PS-dependent activity correlates well with the corresponding clinical severity, as indicated by age of disease onset (8, 18). These observations lend additional support to the view that impaired PS function plays an important role in disease pathogenesis.
γ-Secretase Inhibitors Mimic the Effects of Pathogenic PS Mutations
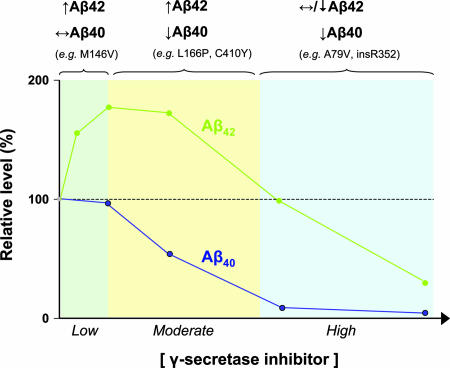
Peptidomimetic compounds of several structural classes act as small molecule inhibitors of γ-secretase (reviewed in ref. 31). These compounds, many of which are based on the APP substrate sequence surrounding the Aβ42 cleavage site, occupy the enzyme's active site and function as analogs of the transition state intermediate in aspartic protease catalysis. An intriguing property shared by γ-secretase inhibitors is their paradoxical ability to enhance Aβ42 production while blocking other γ-secretase-dependent cleavages, thus mimicking the effects of PS mutations (Fig. 3) (32–41). Low to moderate doses of essentially all γ-secretase inhibitors used to date increase Aβ42 production, whereas higher doses produce the expected inhibition of Aβ42 production. In contrast to this biphasic effect on Aβ42, γ-secretase inhibitors cause a progressive inhibition of Aβ40 production. Enhancement of Aβ42 production often occurs even at subinhibitory concentrations, arguing that the increase in Aβ42 levels is not simply a consequence of increased substrate availability owing to reduced Aβ40 generation. In addition, observation of this phenomenon in both cell culture systems and partially purified membrane preparations makes it unlikely that increased Aβ42 production is a result of inhibitory effects on other intracellular processes (36, 41). Interestingly, the degree of enhancement of Aβ42 production elicited by γ-secretase inhibitors is strongly correlated with their inhibitory potency (42), suggesting that increased Aβ42 generation is mechanistically related to active site-directed inhibition.
Fig. 3.
γ-Secretase inhibitors mimic the effects of pathogenic PS mutations. The graph depicts schematically the effects of increasing concentrations of γ-secretase inhibitors on Aβ40 and Aβ42 production, based on data from published reports (32–41). Similar findings have been reported with inhibitors of different structural classes, assayed in either cell culture systems or partially purified membrane preparations. Three distinct patterns of change in the levels of Aβ40 and Aβ42 production are observed in response to increasing concentrations of γ-secretase, as indicated above the graph: (i) increased Aβ42 and unchanged Aβ40; (ii) increased Aβ42 and decreased Aβ40; and (iii) unchanged or decreased Aβ42 and decreased Aβ40. Pathogenic PS mutations can be classified into similar patterns based on their effect on Aβ42 and Aβ40 (representative mutations are shown for each pattern), with most mutations corresponding to the intermediate pattern. Thus, the impact of PS mutations on γ-secretase activity can be equated with the effects of varying concentrations of an active site-directed γ-secretase inhibitor. Note that the Aβ42/Aβ40 ratio is consistently increased across all concentrations of γ-secretase inhibitor, suggesting that this ratio provides a more reliable index of γ-secretase inhibition than the individual levels of Aβ42 or Aβ40.
The fact that γ-secretase inhibitors can mimic the effects of pathogenic PS mutations (i.e., increase Aβ42 production while inhibiting other γ-secretase activities) supports the hypothesis that pathogenic mutations cause a partial loss of PS function, equivalent to the effects of low to moderate doses of γ-secretase inhibitors (Fig. 3). Thus, the elevated Aβ42 production caused by PS mutations may represent a symptom of a “sick” and otherwise impaired γ-secretase. The opposing effect on Aβ42 production relative to other substrate cleavages exerted by γ-secretase inhibitors and PS mutations is not easily reconciled with a monomeric enzyme structure, and suggests instead a multimeric enzyme subject to allosteric regulation. This view is consistent with evidence for PS dimerization and dominant-negative effects of PS mutations (13). Interestingly, substrate or transition state analogs can have biphasic effects on the activity of allosteric enzymes, similar to the effect of γ-secretase inhibitors on Aβ42 production. This phenomenon has been well documented with the classic allosteric enzyme aspartate transcarbamylase: l-aspartate analogs (i.e., dicarboxylic acids such as maleate) perform as activators at low concentrations and inhibitors at higher concentrations (43, 44).
PS Mutations Can Cause Neurodegenerative Dementia in the Absence of Aβ Accumulation
Three PS1 mutations (L113P, G183V, and insR352) have been identified in families with FTD, a common neurodegenerative dementia that lacks amyloid pathology (45–47). The absence of Aβ accumulation in FTD implies that these PS1 mutations may confer a stronger loss of function than those causing familial AD, equivalent to high doses of γ-secretase inhibitor. Indeed, one of these FTD-associated PS1 mutations (insR352) strongly impairs γ-secretase activity in a dominant-negative manner (46). The remaining two mutations occur at exon-intron boundaries, probably giving rise to both full-length transcripts encoding PS1 bearing the identified missense mutation as well as aberrantly spliced transcripts encoding markedly truncated PS fragments (46, 47). Thus, PS1 mutations can cause neurodegeneration and dementia in humans without increasing Aβ production, possibly by imposing a generalized reduction in PS activity. Moreover, PS mutations also occur in families in which early-onset AD is associated with cortical Lewy bodies, which contain α-synuclein aggregates (48–50). Collectively, these observations further suggest that the variable protein aggregates associated with PS mutations do not play an essential role in the pathogenic mechanism leading to neurodegeneration and dementia.
The Diffuse Distribution of PS Mutations Is Most Compatible with a Loss of Function
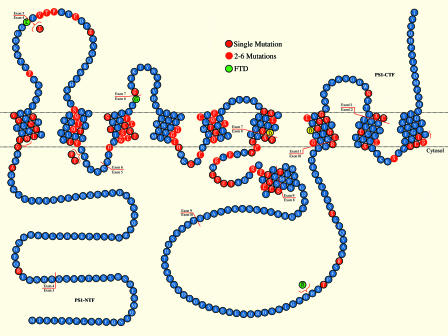
In contrast to pathogenic mutations in APP, which cluster around sites of proteolytic cleavage, PS mutations are scattered throughout the protein's extracellular, cytosolic and transmembrane domains, occurring at ≈20% of the amino acid residues (Fig. 4). This suggests that “random” alterations of single amino acid residues in PS are sufficient to cause AD, highlighting the importance of normal PS functions in AD pathogenesis. The large number (>150) and diffuse distribution of PS mutations are most compatible with a loss of protein function, such as might be caused by a general destabilization of the folded protein structure. The absence of pathogenic mutations that would result in a complete loss of functional protein (e.g., non-sense and frame-shift mutations) suggests that PS mutations are unlikely to act through a simple loss of function. Rather, these observations collectively suggest that pathogenic PS mutations may act through a dominant-negative mechanism, in which mutant PS protein with diminished activity and/or stability interferes with the function of wild-type protein. Such a mechanism would be consistent with the dominant inheritance of PS mutations, as discussed below.
Fig. 4.
Large numbers of pathogenic PS1 mutations are diffusely distributed throughout the coding sequence. This diagram shows the distribution of the missense, small insertion and deletion mutations in PS1. In addition to the depicted mutations, in-frame deletion of exon 9 has also been reported in FAD. Residues highlighted in red indicate the sites of identified FAD mutations, and the three residues highlighted in green (L113P, G183V, and insR352) indicate the sites of mutations identified in familial FTD. The two aspartates (D257 and D385) implicated as catalytic residues in the active site of γ-secretase are highlighted in yellow. PS endoproteolysis occurs within the protein sequence derived from exon 9.
Is a Pathogenic Mechanism Based on Loss of PS Function Compatible with AD Genetics?
One might question whether loss of PS function can provide a tenable explanation for AD pathogenesis, because APP mutations alone are sufficient to cause AD. APP mutations increase the production of Aβ, suggesting that Aβ itself can be pathogenic, as proposed by the amyloid hypothesis. How Aβ causes neurodegeneration, however, is presently unclear. We propose that increased levels of Aβ may cause AD by interfering with PS function and/or expression, resulting in a loss of PS-dependent activities. An interesting possibility that has not previously been considered is that elevated Aβ42 levels may inhibit γ-secretase activity through a product-based negative feedback mechanism, effectively mimicking the effects of PS mutations and γ-secretase inhibitors. Because of the relaxed substrate specificity of γ-secretase, longer forms of Aβ (i.e., Aβ42/43) contain potential cleavage sites for generation of shorter forms of Aβ (i.e., Aβ38/40). Such longer forms of Aβ, whose production is favored in both familial and sporadic AD, are likely to be ineffective substrates for cleavage, owing to the absence of downstream residues important for productive active site-substrate interactions. Thus, longer forms of Aβ may act as γ-secretase inhibitors by occupying the enzyme's active site nonproductively, in a manner similar to peptidomimetic active-site directed inhibitors (Fig. 5). These putative inhibitory forms of Aβ could remain kinetically associated with the γ-secretase active site after their production, or they could reassociate with γ-secretase after retention in or reinsertion into the plasma membrane.
Fig. 5.
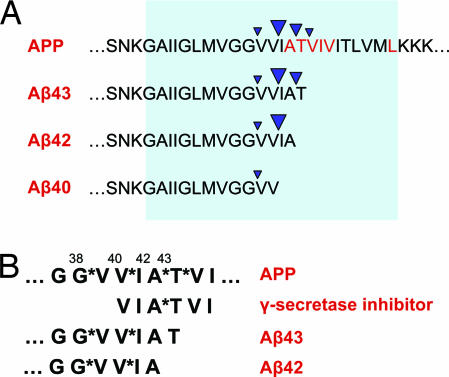
Longer forms of Aβ could act as competitive inhibitors of γ-secretase. (A) The diagram shows the APP amino acid sequence surrounding the sites of intramembranous cleavage by γ-secretase and the major Aβ peptides produced. The intramembranous portion of APP is indicated by the shaded region. Cleavage sites are indicated by inverted arrowheads, with the size of the arrowhead denoting the relative frequency of each cleavage site. Under normal circumstances, Aβ40 is the predominant species produced by γ-secretase cleavage, and Aβ peptides of 43, 42, and 38 residues in length are produced in lower amounts. FAD-linked mutations in PS and APP typically enhance the production of Aβ42 and Aβ43. APP residues at which FAD-linked mutations have been identified are highlighted in red. Interestingly, the longer forms of Aβ (Aβ42, Aβ43) retain the major cleavage site for generation of Aβ40. Although they may be capable of interacting with the enzyme active site, these longer forms of Aβ are unlikely to be efficient substrates for cleavage owing to the absence of distal residues, raising the possibility that they may occupy the active site nonproductively after their generation. Thus, Aβ42 and Aβ43 may act as γ-secretase inhibitors, and their increased production in familial and sporadic AD may result in inhibition of γ-secretase. (B) The amino acid sequence of APP surrounding the multiple intramembranous γ-secretase cleavage sites is diagrammed at top, with the cleavage sites designated by asterisks; the length of the corresponding Aβ peptide is indicated above the sequence. The structure of a typical substrate-based γ-secretase inhibitor and the C-terminal sequences of Aβ43 and Aβ42 are shown below. Substrate-based γ-secretase inhibitors are peptide analogs typically derived from the amino acid sequence surrounding the Aβ42 cleavage site. Residues immediately flanking the cleavage site (A*T) are often modified to incorporate hydroxyethyl isostere or difluoroketone moieties, which mimic the transition state intermediate in aspartic protease catalysis. The C-terminal sequences of Aβ43 and Aβ42 resemble substrate-based peptide inhibitors directed against the Aβ38–Aβ40 cleavage sites.
Increased Aβ levels may also inhibit the expression of normal levels of γ-secretase, because Aβ negatively regulates CRE-dependent gene expression, and expression of the genes encoding both PS1 and the essential γ-secretase subunit Pen-2 is CRE-dependent (51–53). In support of a role for reduced PS expression in AD pathogenesis, PSEN1 promoter polymorphisms that reduce PS1 expression have been reported as risk factors for sporadic AD (54, 55). Alternatively, loss of PS function and increased Aβ production may converge at common downstream signaling pathways that are required for synaptic plasticity and neuronal survival. For example, both PS inactivation and increased Aβ lead to reductions in synaptic NMDA receptors and CRE-dependent gene expression (4, 51, 52, 56).
The presenilin hypothesis must also be reconciled with the fact that mutation of a single PS allele is sufficient to cause AD, despite the presence of three remaining intact PS alleles. In contrast, inactivation of a single PS1 allele in mice, whether germ-line or conditional, is insufficient to cause neurodegeneration (30, 57). The most straightforward solution to this apparent dilemma is that a single mutant allele may be pathogenic because the resulting mutant protein can act in a dominant-negative manner to inhibit the activities of normal PS produced from the remaining wild-type PS alleles. In genetic terms, FAD-linked PS mutations are likely to be antimorphic, causing an intrinsic loss of function as well as a gain of negative function, thereby bringing about an overall loss of PS activity. Such a model would reconcile the dominant inheritance of PS mutations with the evidence for a loss-of-function pathogenic mechanism outlined above. Indeed, PS appear to form dimers within the γ-secretase complex (13), suggesting a possible allosteric mechanism that could allow for dominant-negative effects of PS mutations, as well as their capacity to enhance Aβ42 production while impairing other γ-secretase activities. Consistent with this view, dominantly inherited mutations causing a variety of familial human diseases have been found to act through a dominant-negative mechanism, causing both intrinsic and overall losses of protein function.
Conclusion
Based on several independent lines of evidence, we propose that loss of PS function may be a primary event triggering neurodegeneration in AD, and possibly in other forms of neurodegenerative dementia, such as FTD. The presenilin hypothesis derives from the following observations: (i) inactivation of PS function in the adult brain provides the only mouse model based on genetic manipulation of PS or APP that recapitulates dementia and widespread neurodegeneration; (ii) although FAD-linked PS mutations cause increased production of Aβ42, a large and increasing number of FAD-linked PS mutations have been shown to inhibit other PS activities; (iii) γ-secretase inhibitors can mimic the effects of PS mutations, stimulating Aβ42 production while inhibiting other γ-secretase activities; (iv) PS mutations have recently been identified in FTD, indicating that PS mutations can cause dementia and neurodegeneration in the absence of amyloid accumulation; and (v) the large number, diffuse distribution and missense nature of pathogenic PS mutations is most compatible with mutations causing a loss of PS function through a dominant-negative mechanism.
Our model does not discount an important role for elevated levels of Aβ peptides, particularly Aβ42, in the pathogenesis of AD. However, we suggest that increased Aβ levels may cause neurodegeneration and dementia primarily by interfering with PS-dependent activities, thereby causing an effective loss of PS function. As one possible mechanism, we speculate that Aβ42 may act as an inhibitor of γ-secretase through prolonged occupation of the enzyme's active site. Regardless of the specific mechanism, one would expect that indirect Aβ-mediated inhibition would have an inherently weaker influence on PS function than the more direct effect of PS mutations. In this respect, the presenilin hypothesis may help to explain several perplexing observations: (i) the paucity of pathogenic mutations in APP relative to PS; (ii) the failure of mutant mice overproducing Aβ to develop neurodegeneration, whereas mutant mice with complete loss of PS function exhibit striking neurodegeneration; and (iii) the earlier age of onset and more aggressive course of PS-linked FAD in comparison to APP-linked FAD, despite the fact that APP mutations often elicit a far greater elevation in Aβ levels. The proposed antagonistic effect of Aβ on PS-dependent functions could apply to any mechanism that increases the steady state levels of Aβ, including familial and sporadic forms of AD, although elevated Aβ42/Aβ43 production by γ-secretase may have the most deleterious impact.
Recent analysis of a PS conditional double knockout (cDKO) mouse has provided insight into the consequences of loss of PS function in the adult mammalian brain, outlining a putative pathogenic cascade in which loss of PS function compromises NMDA receptor function, synaptic plasticity and CRE-dependent gene expression, ultimately precipitating widespread and progressive neuronal atrophy and death (4). Neurodegeneration in the PS cDKO mouse displays many of the hallmark features of AD neuropathology, including synaptic and neuronal loss, astrogliosis and tau hyperphosphorylation. Although the PS cDKO mouse lacks the amyloid pathology characteristic of AD, the evidence outlined above suggests that loss of PS function may be a central pathogenic mechanism that operates downstream of Aβ accumulation in AD and independent of Aβ accumulation in FTD. Furthermore, complete PS inactivation models the proposed dominant-negative effects of a single partial loss of function mutant PS allele, which may lead to gradual and progressive inhibition of total PS activity over time, an effect that may be further exacerbated by overproduction of Aβ42. Genetic exaggeration of these effects through complete PS inactivation presumably facilitated the development of neurodegeneration within the short (2-year) life span of mice, whereas similar neuropathology becomes manifest only after 5–6 decades in FAD patients. Such genetic exaggeration has thus far been largely unsuccessful in producing neurodegeneration in a variety of transgenic mice overexpressing mutant human APP, possibly because the pathogenic effects of Aβ are less direct and/or less potent than PS mutations in causing neurodegeneration.
The presenilin hypothesis of AD potentially reconciles important discrepancies in our current understanding of AD, thereby uniting a fragmented set of observations. Although this hypothesis derives in part from genetic observations, progressive loss of PS function, whether due to inhibitory effects of Aβ accumulation or to independent mechanisms, could similarly explain the pathogenesis of sporadic, late-onset AD. If correct, this hypothesis has significant therapeutic implications. It suggests that γ-secretase inhibitors would aggravate instead of ameliorate neurodegeneration and dementia, and that boosting PS-dependent pathways or inhibiting opposing pathways will offer the most promising therapeutic strategies for AD.
Acknowledgments
We thank Mike Brown and John Hardy for discussions and comments and Mary Wines-Samuelson for preparation of Fig. 2. Research in the authors' laboratories is supported by grants from the National Institutes of Health and the Alzheimer's Association. R.J.K. is a Pew Scholar in the Biomedical Sciences.
Abbreviations
- AD
Alzheimer's disease
- Aβ
β-amyloid
- FAD
familial AD
- FTD
frontotemporal dementia
- LBD
Lewy body dementia
- NFT
neurofibrillary tangle
- APP
amyloid precursor protein
- PS
presenilin
- NICD
Notch intracellular domain
- AICD
APP intracellular domain
- SEL12
suppressor/enhancer of LIN12
- cDKO
conditional double knockout.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 3.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, III, Kandel ER, Duff K, Kirkwood A, Shen J. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 5.Wines-Samuelson M, Shen J. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- 6.Cao X, Südhof TC. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 7.Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I. Proc Natl Acad Sci USA. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidner GA, Ye Y, Faraday MM, Alvord WG, Fortini ME. Curr Biol. 2006;16:1026–1033. doi: 10.1016/j.cub.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA. Proc Natl Acad Sci USA. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunkan AL, Martinez M, Wang J, Walker ES, Beher D, Shearman MS, Goate AM. J Neurochem. 2005;94:1315–1328. doi: 10.1111/j.1471-4159.2005.03278.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Gu Y, Hasegawa H, Ruan X, Arawaka S, Fraser P, Westaway D, Mount H, St George-Hyslop P. J Biol Chem. 2002;277:36521–36526. doi: 10.1074/jbc.M205093200. [DOI] [PubMed] [Google Scholar]
- 12.Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H. Proc Natl Acad Sci USA. 2002;99:8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeter EH, Ilagan MX, Brunkan AL, Hecimovic S, Li YM, Xu M, Lewis HD, Saxena MT, De Strooper B, Coonrod A, et al. Proc Natl Acad Sci USA. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker ES, Martinez M, Brunkan AL, Goate A. J Neurochem. 2005;92:294–301. doi: 10.1111/j.1471-4159.2004.02858.x. [DOI] [PubMed] [Google Scholar]
- 15.Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M. J Neurochem. 2005;94:1189–1201. doi: 10.1111/j.1471-4159.2005.03266.x. [DOI] [PubMed] [Google Scholar]
- 16.Qi Y, Morishima-Kawashima M, Sato T, Mitsumori R, Ihara Y. Biochemistry. 2003;42:1042–1052. doi: 10.1021/bi0267590. [DOI] [PubMed] [Google Scholar]
- 17.Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B. J Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar-Singh S, Theuns J, Van Broeck B, Pirici D, Vennekens K, Corsmit E, Cruts M, Dermaut B, Wang R, Van Broeckhoven C. Hum Mutat. 2006;27:686–695. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- 19.Siman R, Reaume AG, Savage MJ, Trusko S, Lin YG, Scott RW, Flood DG. J Neurosci. 2000;20:8717–8726. doi: 10.1523/JNEUROSCI.20-23-08717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, et al. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian S, Jiang P, Guan XM, Singh G, Trumbauer ME, Yu H, Chen HY, Van de Ploeg LH, Zheng H. Neuron. 1998;20:611–617. doi: 10.1016/s0896-6273(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 22.Murayama O, Murayama M, Honda T, Sun X, Nihonmatsu N, Takashima A. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:905–913. doi: 10.1016/s0278-5846(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 23.Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R, Scott RW. Neurobiol Aging. 2002;23:335–348. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 24.Steiner H, Romig H, Grim MG, Philipp U, Pesold B, Citron M, Baumeister R, Haass C. J Biol Chem. 1999;274:7615–7618. doi: 10.1074/jbc.274.12.7615. [DOI] [PubMed] [Google Scholar]
- 25.Nyabi O, Bentahir M, Horre K, Herreman A, Gottardi-Littell N, Van Broeckhoven C, Merchiers P, Spittaels K, Annaert W, De Strooper B. J Biol Chem. 2003;278:43430–43436. doi: 10.1074/jbc.M306957200. [DOI] [PubMed] [Google Scholar]
- 26.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killick R, Pollard CC, Asuni AA, Mudher AK, Richardson JC, Rupniak HT, Sheppard PW, Varndell IM, Brion JP, Levey AI, et al. J Biol Chem. 2001;276:48554–48561. doi: 10.1074/jbc.M108332200. [DOI] [PubMed] [Google Scholar]
- 28.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis JA, Naruse S, Chen H, Eckman C, Younkin S, Price DL, Borchelt DR, Sisodia SS, Wong PC. Neuron. 1998;20:603–609. doi: 10.1016/s0896-6273(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 30.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe MS. J Med Chem. 2001;44:2039–2060. doi: 10.1021/jm0004897. [DOI] [PubMed] [Google Scholar]
- 32.Citron M, Diehl TS, Gordon G, Biere AL, Seubert P, Selkoe DJ. Proc Natl Acad Sci USA. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durkin JT, Murthy S, Husten EJ, Trusko SP, Savage MJ, Rotella DP, Greenberg BD, Siman R. J Biol Chem. 1999;274:20499–20504. doi: 10.1074/jbc.274.29.20499. [DOI] [PubMed] [Google Scholar]
- 34.Klafki H, Abramowski D, Swoboda R, Paganetti PA, Staufenbiel M. J Biol Chem. 1996;271:28655–28659. doi: 10.1074/jbc.271.45.28655. [DOI] [PubMed] [Google Scholar]
- 35.Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato T, Dohmae N, Qi Y, Kakuda N, Misonou H, Mitsumori R, Maruyama H, Koo EH, Haass C, Takio K, et al. J Biol Chem. 2003;278:24294–24301. doi: 10.1074/jbc.M211161200. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe MS, Citron M, Diehl TS, Xia W, Donkor IO, Selkoe DJ. J Med Chem. 1998;41:6–9. doi: 10.1021/jm970621b. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski B, Rahmati T, Donkor IO, Selkoe DJ. Biochemistry. 1999;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki T, Haass C, Saido TC, Omura S, Ihara Y. Biochemistry. 1997;36:8377–8383. doi: 10.1021/bi970209y. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Song L, Parker EM. J Biol Chem. 1999;274:8966–8972. doi: 10.1074/jbc.274.13.8966. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Song L, Terracina G, Liu Y, Pramanik B, Parker E. Biochemistry. 2001;40:5049–5055. doi: 10.1021/bi0028800. [DOI] [PubMed] [Google Scholar]
- 42.Moore CL, Diehl TS, Selkoe DJ, Wolfe MS. Ann NY Acad Sci. 2000;920:197–205. doi: 10.1111/j.1749-6632.2000.tb06922.x. [DOI] [PubMed] [Google Scholar]
- 43.Gerhart JC, Pardee A. Cold Spring Harbor Symp Quant Biol. 1963;28:491–96. [Google Scholar]
- 44.Jacobson GR, Stark GR. J Biol Chem. 1975;250:6852–6860. [PubMed] [Google Scholar]
- 45.Amtul Z, Lewis PA, Piper S, Crook R, Baker M, Findlay K, Singleton A, Hogg M, Younkin L, Younkin SG, et al. Neurobiol Dis. 2002;9:269–273. doi: 10.1006/nbdi.2001.0473. [DOI] [PubMed] [Google Scholar]
- 46.Dermaut B, Kumar-Singh S, Engelborghs S, Theuns J, Rademakers R, Saerens J, Pickut BA, Peeters K, van den Broeck M, Vennekens K, et al. Ann Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 47.Raux G, Gantier R, Thomas-Anterion C, Boulliat J, Verpillat P, Hannequin D, Brice A, Frebourg T, Campion D. Neurology. 2000;55:1577–1578. doi: 10.1212/wnl.55.10.1577. [DOI] [PubMed] [Google Scholar]
- 48.Snider BJ, Norton J, Coats MA, Chakraverty S, Hou CE, Jervis R, Lendon CL, Goate AM, McKeel DW, Jr, Morris JC. Arch Neurol. 2005;62:1821–1830. doi: 10.1001/archneur.62.12.1821. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa A, Piao YS, Miyashita A, Kuwano R, Onodera O, Ohtake H, Suzuki M, Nishizawa M, Takahashi H. Ann Neurol. 2005;57:429–434. doi: 10.1002/ana.20393. [DOI] [PubMed] [Google Scholar]
- 50.Houlden H, Crook R, Dolan RJ, McLaughlin J, Revesz T, Hardy J. Neurosci Lett. 2001;313:93–95. doi: 10.1016/s0304-3940(01)02254-6. [DOI] [PubMed] [Google Scholar]
- 51.Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Proc Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong L, Thornton PL, Balazs R, Cotman CW. J Biol Chem. 2001;276:17301–17306. doi: 10.1074/jbc.M010450200. [DOI] [PubMed] [Google Scholar]
- 53.Mitsuda N, Ohkubo N, Tamatani M, Lee YD, Taniguchi M, Namikawa K, Kiyama H, Yamaguchi A, Sato N, Sakata K, et al. J Biol Chem. 2001;276:9688–9698. doi: 10.1074/jbc.M006153200. [DOI] [PubMed] [Google Scholar]
- 54.Theuns J, Remacle J, Killick R, Corsmit E, Vennekens K, Huylebroeck D, Cruts M, Van Broeckhoven C. Hum Mol Genet. 2003;12:869–877. doi: 10.1093/hmg/ddg098. [DOI] [PubMed] [Google Scholar]
- 55.Theuns J, Del-Favero J, Dermaut B, van Duijn CM, Backhovens H, Van den Broeck MV, Serneels S, Corsmit E, Van Broeckhoven CV, Cruts M. Hum Mol Genet. 2000;9:325–331. doi: 10.1093/hmg/9.3.325. [DOI] [PubMed] [Google Scholar]
- 56.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 57.Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, et al. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 58.Zhang DM, Levitan D, Yu G, Nishimura M, Chen F, Tandon A, Kawarai T, Arawaka S, Supala A, Song YQ, et al. NeuroReport. 2000;11:3227–3230. doi: 10.1097/00001756-200009280-00035. [DOI] [PubMed] [Google Scholar]
- 59.Steiner H, Revesz T, Neumann M, Romig H, Grim MG, Pesold B, Kretzschmar HA, Hardy J, Holton JL, Baumeister R, et al. J Biol Chem. 2001;276:7233–7239. doi: 10.1074/jbc.M007183200. [DOI] [PubMed] [Google Scholar]
- 60.Sun X, Beglopoulos V, Mattson MP, Shen J. Neurodegener Dis. 2005;2:6–15. doi: 10.1159/000086426. [DOI] [PubMed] [Google Scholar]