Abstract
We have investigated the dependence of the rate of lactic acid production on the rate of Na+ entry in cultured transformed rat Müller cells and in normal and dystrophic (RCS) rat retinas that lack photoreceptors. To modulate the rate of Na+ entry, two approaches were employed: (i) the addition of L-glutamate (D-aspartate) to stimulate coupled uptake of Na+ and the amino acid; and (ii) the addition of monensin to enhance Na+ exchange. Müller cells produced lactate aerobically and anaerobically at high rates. Incubation of the cells for 2–4 h with 0.1–1 mM L-glutamate or D-aspartate did not alter the rate of production of lactate. ATP content in the cells at the end of the incubation period was unchanged by addition of L-glutamate or D-aspartate to the incubation media. Na+-dependent L-glutamate uptake was observed in the Müller cells, but the rate of uptake was very low relative to the rate of lactic acid production. Ouabain (1 mM) decreased the rate of lactic acid production by 30–35% in Müller cells, indicating that energy demand is enhanced by the activity of the Na+–K+ pump or depressed by its inhibition. Incubation of Müller cells with 0.01 mM monensin, a Na+ ionophore, caused a twofold increase in aerobic lactic acid production, but monensin did not alter the rate of anaerobic lactic acid production. Aerobic ATP content in cells incubated with monensin was not different from that found in control cells, but anaerobic ATP content decreased by 40%. These results show that Na+-dependent L-glutamate/D-aspartate uptake by cultured retinal Müller cells causes negligible changes in lactic acid production, apparently because the rates of uptake are low relative to the basal rates of lactic acid production. In contrast, the marked stimulation of aerobic lactic acid production caused by monensin opening Na+ channels shows that glycolysis is an effective source of ATP production for the Na+–K+ ATPase. A previous report suggests that coupled Na+–L-glutamate transport stimulates glycolysis in freshly dissociated salamander Müller cells by activation of glutamine synthetase. The Müller cell line used in this study does not express glutamine synthetase; consequently these cells could only be used to examine the linkage between Na+ entry and the Na+ pump. As normal and RCS retinas express glutamine synthetase, the role of this enzyme was examined by coapplication of L-glutamate and NH4+ in the presence and absence of methionine sulfoximine, an inhibitor of glutamine synthetase. In normal retinas, neither the addition of L-glutamate alone or together with NH4+ caused a significant change in the glycolytic rate, an effect linked to the low rate of uptake of this amino acid relative to the basal rate of retinal glycolysis. However, incubation of the RCS retinas in media containing L-glutamate and NH4+ did produce a small (15%) increase in the rate of glycolysis above the rate found with L-glutamate alone and controls. It is unlikely that this increase was the result of conversion of L-glutamate to L-glutamine, as it was not suppressed by inhibition of glutamine synthetase with 5 mM methionine sulfoximine. It appears that the magnitude of Müller cell glycolysis required to sustain the coupled transport of Na+ and L-glutamate and synthesis of L-glutamine is small relative to the basal glycolytic activity in a rat retina.
Keywords: glutamine synthetase, lactate production, L-glutamate, monensin, rat retina, retinal Müller cells
Abbreviations used: GFAP, glial fibrillary acidic protein; GS, glutamine synthetase; MSO, methionine sulfoximine; SCMF, Dulbecco’s phosphate-buffered saline; TCA, trichloroacetic acid; THA, DL-threo-β-hydroxyaspartic acid
There is great interest in the processes and events that control the rate of ATP production in neurons and glial cells (Erecinska and Silver 1989; Erecinska and Dagani 1990; Erecinska and Silver 1994; Hertz and Dienel 2002). Recently, a focus has emerged that centers on the linkage between the L-glutamate released by neurons at excitatory synapses and its uptake by Na+-dependent L-glutamate transporters in cerebral astrocytes (Pellerin and Magistretti 1994; Takahashi et al. 1995; Sokoloff et al. 1996; Demestre et al. 1997; Pellerin et al. 1998; Sibson et al. 1998; Magistretti and Pellerin 1999; Magistretti et al. 1999; Deitmer 2000; but also see Hertz et al. 1999). The idea is that the entry of Na+ and L-glutamate into astrocytes should have metabolic consequences, since cellular homeostasis requires extrusion of the Na+ by the energy-dependent Na+–K+ ATPase.
Pellerin and Magistretti (1994) were the first to report using in vitro assays in cultured cells that the uptake of L-glutamate or D-aspartate by astrocytes prepared from mouse cerebral cortex stimulated glucose utilization, measured by the accumulation of labeled 2-deoxyglucose-6-phosphate (2-DG-6P) and by lactic acid production. This stimulation of glycolysis by L-glutamate was blocked by ouabain. Subsequently, Takahashi et al. (1995) and Sokoloff et al. (1996) observed similar effects of L-glutamate and ouabain on the accumulation of labeled 2-DG-6P in astroglia from rat cerebral cortex. However, other investigators have failed to confirm these initial observations (McKenna et al. 1996; Hertz et al. 1998; Peng et al. 2001; Qu et al. 2001).
Like astrocytes in the brain, Müller cells, which are the major glial cell in the retina, metabolize glucose to lactate (Poitry-Yamate et al. 1995; Winkler et al. 2000, 2003a). Recently, Poitry et al. (2000) reported that exposure of acutely dissociated salamander Müller cells to L-glutamate had no effect on lactic acid production but that ‘addition of NH4+ in the presence of glutamate induced a large increase in the concentration of lactate.’ These authors suggested that the increase in glycolysis caused by L-glutamate and NH4+ was not due to activation of the Na+ pump as a consequence of Na+ entry, as proposed in brain astrocytes, but rather was due to activation of glutamine synthetase (GS), an ATP-dependent enzyme localized exclusively to Müller cells (Riepe and Norenberg 1977). In support of this suggestion, they showed that inhibition of GS with methionine sulfoximine (MSO, Rowe and Meister 1970) blocked the L-glutamate- and NH4+-dependent stimulation of glycolysis.
We have chosen to examine the relationship between coupled Na+–L-glutamate/D-aspartate transport, activity of GS and lactic acid production in transformed cultured rat Müller cells (Newman and Reichenbach 1996; Sarthy and Ripps 2001), in normal rat retinas and in photoreceptorless (RCS) rat retinas (Bourne et al. 1938; Dowling and Sidman 1962). Müller cells and retinal neurons have Na+-dependent L-glutamate transporters (Barbour et al. 1988, 1991; Derouiche and Rauen 1995; Reichelt et al. 1997; Rauen et al. 1998; Li and Puro 2002) and, like cerebral astrocytes (Walz and Mukerji 1988; Dringen et al. 1993; Swanson and Bennington 1996), the retina and cultured cells produce lactate aerobically (Poitry-Yamate et al. 1995; Winkler 1995; Winkler et al. 2000; Winkler et al. 2003a). Indeed, it has long been known that lactate, rather than CO2, is the major product of aerobic glucose metabolism in rat, rabbit and cat retinas (Cohen and Noell 1960; Winkler 1981, 1995; Ames et al. 1992; Wang et al. 1997). Calculations in these studies indicate that approximately 70–90% of the glucose consumed aerobically are used to produce lactate. Moreover, inhibition of the activity of the Na+–K+ ATPase in isolated rat and rabbit retinas causes a 60% decrease in aerobic lactic acid production (Winkler 1981; Ames et al. 1992), a result consistent with a major fraction of aerobic glycolysis being used to support active Na+ extrusion. Thus, these preparations should serve as excellent models for studying the linkage between Na+ entry stimulated by coupled uptake with L-glutamate (D-aspartate) or by monensin (Pressman 1976; Pressman and Fahim 1982; Yarowsky et al. 1986; Erecinska et al. 1991), stimulation of glutamine synthetase by L-glutamate and pathways of glucose metabolism.
Materials and methods
Materials
Dulbecco’s modified Eagle’s bicarbonate-buffered medium (DMEM, catalogue number 31600) was purchased from Invitrogen Life Technologies (Grand Island, NY, USA). Ouabain, Antimycin A, DL-threo-β-hydroxyaspartic acid (THA), amino acids and reagents used in the lactic acid dehydrogenase kit (826-A) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Solutions for the Pierce protein assay were purchased from Pierce (Rockford, IL, USA). Solutions and reagents for measuring ATP were obtained from Turner Systems (Sunnyvale, CA, USA). Isotopically labeled D-aspartate and L-glutamate were purchased from PerkinElmer Life Sciences, Inc. (Boston, MA, USA). All reagents and chemicals were of the highest analytical grade. Antimycin A and monensin were made as concentrated stock solutions in ethanol and diluted 500-fold to obtain the desired final concentration.
Cell culture and tissue preparation
A transformed rat Müller cell line (rMC-1) was kindly sent to us by VJ Sarthy (Sarthy et al. 1998). rMC-1 cell cultures were grown in DMEM supplemented with 15% fetal bovine serum, as well as with a fungicide mixture and 0.5% gentamicin in a humidified atmosphere of 5% CO2/95% air. Medium was changed every 2–3 days, and cells were grown to confluence in a 150-mm dish. At confluence, cells were split into 60-mm dishes and when these cells were confluent, they were used in the metabolic experiments.
Retinas were obtained from 3-month old normal adult rats and from rats (Royal College of Surgeons, RCS) with hereditary visual cell degeneration at 6–8 months of age, when histological analysis shows these retinas lack completely their rod photoreceptor cells, but the inner nuclear and ganglion cell layers are well preserved (Dowling and Sidman 1962). The RCS rat is an animal model of recessively inherited degeneration that results in a progressive, postnatal loss of photoreceptor cells (Bourne et al. 1938). Following euthanasia of rats by carbon dioxide inhalation, retinas were isolated from other ocular tissues and incubated in a bicarbonate-buffered (pH = 7.4), glucose-containing media, as described in detail elsewhere (Winkler 1981; Winkler 1995). Retinas (1–4/flask) were placed in 25 mL Erlenmyer flasks (10 mL/flask), kept at 37°C, and the incubation media were equilibrated with 95% O2 and 5% CO2.
Metabolic measurements
At the start of an experiment with the cell cultures the serum containing DMEM was decanted and replaced by 4 mL of serum-free DMEM for 30 min. The control DMEM contained 4 mM L-glutamine, 1 mM pyruvate, and 5.5 mM glucose. After this initial 30 min in serum-free media, the various test media were introduced, i.e. with and without L-glutamate or D-aspartate, with and without 1 mM ouabain, and with and without 10−5M Antimycin A. Ouabain was used to inhibit the Na+–K+ ATPase and Antimycin A (0.01 mM) was used to inhibit mitochondrial electron transport. Antimycin A interferes with electron flow from cytochrome bH in Q-cytochrome c oxidoreductase. In this article, the terms mitochondrial inhibition and anaerobic condition are used interchangeably. It is understood, however, that cells and tissues exposed to Antimycin A are not anaerobic, as all incubations were carried out in the presence of oxygen. The pH of the bicarbonate-buffered media was 7.4 at the start of an experiment, and all media used in the cell culture experiments were equilibrated with air/5% CO2 throughout the incubations. Because the cultured cells produce lactate that accumulates in the media as a function of time, measurements were made of media pH after 4 h. There were only small decreases in pH, i.e. from 7.4 to 7.3, when rMC-1 were incubated under normal conditions. To minimize changes in media pH when the duration of incubations was extended to 24 h, the volume of media was increased to 6 mL per well and fresh media was added every 8 h.
The accumulation of lactate in the media was estimated by taking 0.1 mL samples at timed intervals throughout the incubations. These samples were then used in the determination of lactate using a Sigma-supplied lactic acid dehydrogenase kit, as previously reported (Winkler 1981; Winkler et al. 2000). Lactic acid production was then calculated and expressed as micromoles of lactate/h/106 cells, based on a standard lactate curve that was produced in each experiment. ATP content was measured at the end of the incubation, using methods identical to those previously published (Winkler et al. 2000). Briefly, experimental media were aspirated, and the cells were rinsed twice with Dulbecco’s phosphate-buffered saline (SCMF). Following the second rinse and decanting, the cells were then scraped twice with 0.3 mL of 5% perchloric acid to obtain a total volume of 0.6 mL. Samples were sonicated, centrifuged, and the supernatant was diluted 200-fold. A 50 μL sample was used in the firefly luciferin-luciferase spectrofluorometric assay along with appropriate standards and blanks. Cell counts were made following trypsinization to release the cells (addition of 0.5 mL to each well), dilution with a counting solution (0.1 mL to 20 mL diluent), and measurement of cell numbers with a Coulter Counter (Coulter Electronics, Model ZM). For rat retinas, the procedures for sampling and assaying lactate have been described in detail previously (Winkler 1981; Winkler 1995), and are similar in most respects to the procedures described above for work with cell cultures.
Activity of glutamine synthetase (GS) was measured in cytosolic extracts of Müller cells and normal and RCS rat retinas using procedures described in detail previously (Winkler et al. 1999).
Results are expressed as means ± SD. Each mean value represents the number of independent experiments. For the cell cultures, triplicate dishes were employed per experiment per condition tested. Data were analyzed by Student’s t-test, and a p-value of 0.05 was considered to be statistically significant. Results are expressed as per 106 cells or per retina or per mg protein. The protein content, expressed per 106 cells, is 0.33 ± 0.02 mg for rMC-1 cells (n = 8). The total (homogenate) protein content of a normal rat retina is 0.88 ± 0.1 mg (n = 18) and cytosolic protein is 0.34 ± 0.03 mg (n = 18). An RCS retina contains 0.62 ± 0.04 mg total protein and 0.26 ± 0.02 cytosolic protein (n = 8).
Uptake measurements
For the evaluation of uptake of L-glutamate or D-aspartate into cells, the radioisotope stock specific activities were 16.2 Ci/mmol for D-[2,3–3H]-aspartate and 293 mCi/mmol for L-[U-14C]-glutamate. For D-aspartate uptake, rMC-1 cells were incubated in 2 mL media/dish containing 2 μCi of D-[2,3-3H]-aspartate and 0.2 or 1 mM unlabeled D-aspartate. The concentration of labeled (hot) D-aspartate was 0.06 μM which yielded 55 000 dpm/25 μL of media with specific activities of 11 × 106 dpm/μmol for 0.2 mM D-aspartate (55 000/0.005) and 2.2 × 106 dpm/μmole for 1 mM D-aspartate (55 000/0.025). For L-[U-14C]-glutamate uptake, rMC-1 cells were incubated in 2 mL media/dish containing 0.2 μCi of L-[U-14C]-glutamate and 0.2 or 1 mM unlabeled L-glutamate. The concentration of labeled (hot) L-glutamate was 0.34 μM, yielding 5550 dpm/25 μL of media with specific activities of 1.1 × 106 dpm/μmole for 0.2 mM L-glutamate (5550/0.005) and 222 000 dpm/μmole for 1 mM L-glutamate (5550/0.025).
Confluent dishes of rMC-1 cells were rinsed twice in serum-free DMEM, then preincubated 30 min in serum-free DMEM ± 1 mM THA, a potent linear competitive inhibitor of L-glutamate uptake (Balcar and Johnston 1972) and a substrate for the transport system (Barbour et al. 1991). Following preincubation, cells were incubated in serum-free DMEM with either 0.2 or 1 mM L-glutamate or D-aspartate, with and without 1 mM THA, and with respective radioisotope. Unlabeled THA was used to inhibit the Na+-dependent L-glutamate transporter. Cells were incubated for 1–3 h in a humidified atmosphere of 5% CO2/95% air. At the end of the incubations, the radioactive media were removed, and each dish was rinsed with 5 mL (three consecutive rinses) of ice-cold normal media (lacking L-glutamate and D-aspartate). Each dish was scraped into 0.3 mL of cold 5% trichloroacetic acid (TCA). Scraped cells were homogenized and sonicated, spun for 10 min at 20 000 × g and 0.25 mL of the radioactive supernatant was counted for uptake of labeled compounds. The counts obtained from cells incubated in media containing THA (‘blank values’) were subtracted from the counts obtained from cells incubated in media lacking THA.
For the evaluation of uptake of D-aspartate into isolated rat retinas, the radioisotope stock specific activity was 16.2 Ci/mmol. Four retinas were incubated in 10 mL media/flask containing 45 μCi of D-[2,3-3H]-aspartate and 1 mM unlabeled D-aspartate. The concentration of labeled (hot) D-aspartate was 0.3 μM, yielding 250 000 dpm/25 μL of media with a specific activity of 10 × 106 dpm/μmole for 1 mM D-aspartate (250 000/0.025). Tissues were preincubated in 10 mL of the standard incubation medium ± 5 mM THA for 30 min in a 37°C water bath with constant shaking. Flasks were continuously gassed with 95% O2/5% CO2. Retinas were then transferred to ‘hot’ flasks with 10 mL of 10 mM glucose, 1 mM D-aspartate ± 5 mM THA plus D-[2,3-3H]-aspartate. At the end of the radioactive incubation, each retina was placed in 9 mL ice-cold normal media and rinsed for 5 min. Each retina was rinsed twice more in fresh 9 mL of ice-cold normal media for 5 min. Each retina was placed in 0.3 mL cold 5% TCA, homogenized, sonicated, and spun for 10 min at 20 000 g and 0.025–0.1 mL of the radioactive supernatant was counted for uptake of radioactive D-[2,3-3H]-aspartate.
Immunocytochemistry
Enucleated eyes were fixed with 4% paraformaldehyde (in 0.1 M phosphate-buffered saline, PBS, pH = 7.4)) for 1 h at room temperature and embedded in OCT compound (Sakura Finetek USA, Torrance, CA, USA). Transverse, 10-μm thick retinal sections were cut and placed onto super-frost plus slides (Fisher Scientific, Pittsburgh, PA, USA). Retinal sections were blocked with 5% bovine serum albumin (in PBS containing 0.3% Triton X-100) for 1 h at room temperature, washed three times with PBS and incubated with antibodies against glial fibrillary acidic protein (GFAP,1 : 100 dilution, Santa Cruz, CA, USA) or glutamine synthetase (GS, 1 : 100 dilution, Chemicon, CA, USA) for 2 h at room temperature. Sections were washed three times with PBS and incubated with AlexaFluor-488 (for GFAP) or AlexaFluor-568 (for GS) secondary antibodies for 1 h at room temperature. Sections were washed again and mounted with a coverslip. Where indicated, Hoechst dye (0.1 mg/mL) was added to localize nuclei in the tissue. Sections were observed under a Nikon bright field microscope equipped with epifluorescence, and digitized images were obtained using a SPOT digital camera. Images were processed and compiled using Adobe Photoshop Software, versions 5.5 and 7.0 (Adobe system Incorporated, CA, USA).
Results
Cultured rat Müller cells (rMC-1)
In a previous study that used third and fifth passaged human Müller cells, we found that these cells did not express glutamine synthetase (GS) activity (Winkler et al. 2000). We then tested whether rMC-1 cells, which express cellular-retinaldehyde-binding protein (CRALBP), a marker for Müller cells in the adult rat retina (Sarthy et al. 1998), would express GS. Unfortunately, GS activity was not detected in the rMC-1 cultures (n = 12). Consistent with this lack of enzymatic activity, we also failed to detect any immunocytochemical stain with a monoclonal antibody against GS. We received two additional cell lines of retinal Müller cells from colleagues, but neither of these lines expressed GS. It was recognized therefore that these cells are not be suitable models to investigate the proposed linkage between L-glutamate stimulation of GS activity and glycolysis. Nevertheless, we reasoned that rMC-1 cells could provide useful information about the relationship between the coupled uptake of L-glutamate and Na+, the Na+–K+ pump, and rate of glycolysis.
Figure 1 shows lactic acid production in cultured rMC-1 cells over 4 h under normal (Fig. 1a) and mitochondrial-inhibited (Fig. 1b, with Antimycin A) conditions, and also when the cells were exposed to 1 mM L-glutamate and/or 1 mM ouabain. The control rate of aerobic glycolysis (in Fig. 1a) was 0.55 ± 0.1 μmol lactate produced/h/106 cells (n = 25). When rMC-1 were incubated in media containing 1 mM ouabain (• in Fig. 1a), the Na+–K+ ATPase was completely inhibited within 10 min (data not shown). Also, the rate of aerobic glycolysis (n = 25) decreased by 35% relative to the control rate, i.e. to 0.36 ± 0.1 μmol lactate produced/h/106 cells. The addition of 1 mM L-glutamate to control media (□ in Fig. 1a) and to ouabain-containing media (▪ in Fig. 1a) did not change the respective rates of aerobic glycolysis. To further explore the effects of L-glutamate on glucose metabolism, rMC-1 were incubated in media containing 0.01, 0.1 and 0.5 mM L-glutamate for 4 h. The rates of lactate production in the presence of these three concentrations of L-glutamate were not significantly different from the control rate (data not shown). Incubation of cells in media containing both 1 mM L-glutamate and 0.5 mM NH4Cl did not alter the control rate of glycolysis, a result that is consistent with the absence of GS in these cells.
Fig. 1.
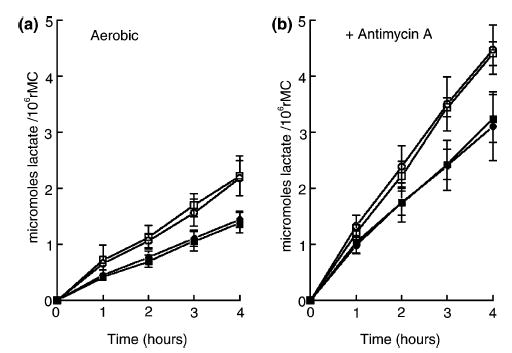
Effects of L-glutamate and ouabain on the rates of lactic acid production in cultured rat Müller cells (rMC) under control (left side, a) and Antimycin A-inhibited (right side, b) conditions. For both (a) and (b) the symbols are as follows: ○, control medium; □, with 1 mM L-glutamate; •, with 1 mM ouabain; ▪, with 1 mM ouabain and 1 mM L-glutamate. Each symbol is the average of at least 15 experiments, accompanied by standard deviations.
Hertz et al. (1998) reported that under anoxic conditions (nitrogen + carbon dioxide) the accumulation of labeled 2-DG − 6P in mouse astrocytes was stimulated twofold by 1 mM L-glutamate. We therefore tested the effects of L-glutamate on rMC-1 following incubation of the cells in media containing Antimycin A, an inhibitor of mitochondrial activity (Winkler 1995; Winkler et al. 2000). As shown in Fig. 1(b), the rate of lactic acid production following inhibition of the mitochondria (○, no added L-glutamate) was 1.12 ± 0.1 μmol of lactate produced/h/106 cells (n = 16). Comparing this rate with the rate of aerobic lactic acid production yields a Pasteur effect of 2.0; this effect is a measure of the compensatory increase in the rate of glycolysis in response to inhibition of the mitochondria. When 1 mM ouabain was added to the media containing Antimycin A (•), the rate of glycolysis fell from 1.12 to 0.69 ± 0.1 μmol lactate produced/h/106 cells, representing a 38% decline. Addition of 0.01–1 mM L-glutamate did not significantly alter the rate of anaerobic glycolysis in the control (□) or ouabain-treated condition (▪).
Since rMC-1 cells, like human Müller cells passaged in culture (Winkler et al. 2000), lose the expression of GS and do not exhibit activity of this enzyme in a biochemical assay (Winkler et al. 1999, 2000), we were concerned that the absence of a stimulatory effect of L-glutamate on the rate of glycolysis could be due to the absence of GS. We considered that the inability of rMC-1 to convert L-glutamate to L-glutamine could result in a build-up of L-glutamate intracellularly, which could limit the rate of the Na+-dependent uptake of L-glutamate. As Pellerin and Magistretti (1994) showed that D-aspartate, a transportable but non-metabolizable analog of glutamate, exhibited an action comparable to L-glutamate in stimulating aerobic glycolysis in astrocytes prepared from mouse cerebral cortex, we incubated rMC-1 cells in media containing 0.1–1 mM D-Aspartate and measured the rates of glycolysis and the content of ATP in the cells. Figure 2(a,b) shows that the addition of 1 mM D-aspartate (□) to media bathing rMC-1 cells did not stimulate the rates of aerobic (Fig. 2a) and anaerobic glycolysis (Fig. 2b) relative to the rate of glycolysis measured in rMC-1 incubated in D-aspartate-free media. The rates of aerobic and anaerobic glycolysis were similar in cells incubated in media containing ouabain in the presence and absence of D-aspartate (• versus ▪). Though not shown, addition of less than 1 mM D-aspartate to incubation media did not alter the rates of aerobic and anaerobic glycolysis.
Fig. 2.
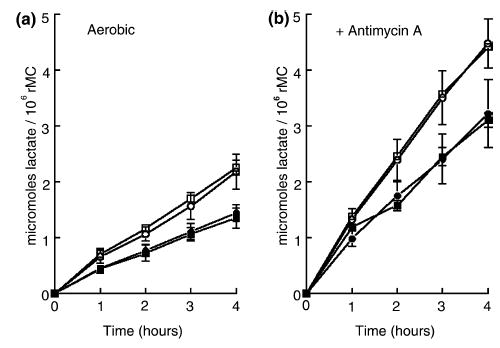
Effects of D-aspartate and ouabain on the rates of lactic acid production in cultured rat Müller cells (rMC) under control (left side, a) and Antimycin A-inhibited (right side, b) conditions. For both (a) and (b) the symbols are as follows: ○, control medium; □, with 1 mM D-aspartate; •, with 1 mM ouabain; ▪, with 1 mM ouabain and 1 mM D-aspartate. Each symbol is the average of eight experiments, accompanied by standard deviations.
The aerobic and anaerobic content of ATP in cultured Müller cells in the presence and absence of L-glutamate and ouabain is shown in Table 1. The data clearly show that relative to the control amount of ATP at the end of 4 h, e.g. 6.6 nmol/106 cells, there were only small differences (not greater than 15%) between this value and the amounts of ATP found in the cells after incubation in media containing either ouabain or L-glutamate, or with ouabain and L-glutamate. Anaerobic ATP content in control cells (no added ouabain or L-glutamate) after 4 h was similar to the control level of ATP under the aerobic condition. Cells treated with ouabain, however, showed a nearly 40% loss in anaerobic ATP content, an effect likely linked to the ouabain-induced reduction in the rate of anaerobic glycolysis, resulting in ATP synthesis failing to keep up with demand. However, exposure of cells to 1 mM L-glutamate under the anaerobic condition did not affect the level of ATP in control or ouabain-treated cells. Aerobic and anaerobic ATP content in rMC-1 was also not significantly altered after 4 h of incubation in D-aspartate-containing media.
Table 1.
ATP content in cultured rat Müller cells (rMC-1)a
| Condition | Antimycin A | nanomoles of ATP/106 cells |
|---|---|---|
| Control | − | 6.6 ± 1.0 (17) |
| Control | + | 6.3 ± 1.1 (12) |
| + 1 mM L-glutamate | − | 6.3 ± 1.3 (8) |
| + 1 mM L-glutamate | + | 5.8 ± 0.8 (8) |
| + 1 mM ouabain | − | 5.4 ± 0.7 (14)* |
| + 1 mM ouabain | + | 3.7 ± 1.1 (11)* |
| + L-glutamate + ouabain | − | 5.7 ± 0.8 (8) |
| + L-glutamate + ouabain | + | 3.8 ± 1.3 (8)* |
| + 1 mM D-aspartate | − | 6.6 ± 0.3 (6) |
| + 1 mM D-aspartate | + | 6.3 ± 0.4 (3) |
Values are expressed as means ± SD and number of experiments is given in parentheses. Incubation duration was 4 h.
indicates values that are statistically significant (p = 0.02) from the control values (+ and − Antimycin A).
To further probe the dependence of glucose utilization in rMC-1 cells on Na+ entry, we used the Na+ ionophore monensin (0.01 mM) to open Na+ channels (Pressman 1976; Pressman and Fahim 1982; Yarowsky et al. 1986; Erecinska et al. 1991; Takahashi et al. 1995). Figure 3(a) shows that monensin stimulated the rate of aerobic glycolysis by twofold, from 0.55 to 1.16 μmol lactate produced/h/106 cells (n = 3). Ouabain completely inhibited this stimulation (n = 2, data not shown). Figure 3(b) shows that 0.01 mM monensin did not significantly affect the rate of anaerobic glycolysis above that found in the presence of Antimycin A alone (n = 3); similar results were obtained with 0.1 mM monensin. Monensin did not alter aerobic ATP content in rMC-1 cells, but reduced the anaerobic content of ATP by approximately 40%, from 7.3 ± 0.3 to 4.5 ± 0.5 nmol/106 cells (n = 3).
Fig. 3.
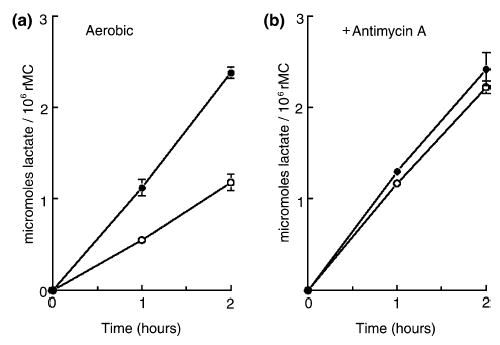
Effects of 0.01 mM monensin on the rates of lactic acid production in cultured rat Müller cells (rMC) under control (left side, a) and Antimycin A-inhibited (right side, b) conditions. For both (a) and (b), the symbols are as follows: ○, control medium; •, with 0.01 mM monensin. Each symbol is the average of at least three experiments, accompanied by standard deviations, shown only when they were larger than the size of the symbol.
The morphologic appearance of rMC-1 was examined after incubations lasting 4 h in media containing Antimycin A, ouabain and/or L-glutamate. As shown in Fig. 4, the cells were well maintained in these different conditions for 4 h. When the duration of the cultures was extended to 24 h, cells treated with either Antimycin A or 1 mM L-glutamate also appeared healthy, but there was substantial cell death in those cultures incubated in media containing ouabain in the presence and absence of L-glutamate (data not shown).
Fig. 4.

Morphological appearance of rat Müller cells incubated for 4 h under aerobic (left column) and anaerobic (right column, with Antimycin A) conditions in the presence either of 1 mM L-glutamate (second row), 1 mM ouabain (third row) or 1 mM L-glutamate and 1 mM ouabain (bottom row). Top pair is the aerobic and anaerobic control panels. Note excellent preservation of cells under all conditions.
Uptake measurements in cultured cells
Since the mechanism of the stimulation of aerobic lactic acid production by L-glutamate and D-aspartate involves a L-glutamate transporter (Pellerin and Magistretti 1994) and since the present findings have failed to confirm this result in rMC-1, it is possible that the cultured retinal cells used in this study do not take up the amino acids. Measurements of uptake of labeled L-glutamate and D-aspartate in the presence and absence of 1 mM THA were carried out with rMC-1. The specific rates of uptake (linear over 3 h) were as follows: in rMC-1 cells incubated with 0.2 mM D-aspartate, the rate of uptake was 5.40 ± 0.17 nmol/h/106 cells (n = 4); with 1 mM D-aspartate, it was 6.02 ± 0.24 nmol/h/106 cells (n = 4); corresponding rates with 0.2 and 1 mM L-glutamate were, respectively, 2.17 ± 0.06 and 2.62 ± 0.64 nmol/h/106 cells (n = 4, each condition). That uptake of L-glutamate and D-aspartate involved a Na+-dependent transporter in rMC-1 cells was shown by the 90% inhibition of uptake when cells were incubated in media containing 1 mM THA.
Isolated rat retina
In order to test the hypothesis of Poitry et al. (2000), namely that the effect of L-glutamate on glycolysis in retinal Müller cells depends on a stimulation of GS, it is necessary to perform experiments to demonstrate that L-glutamate enhances lactic acid production when applied with NH4+. Such an experiment requires a preparation that expresses GS. The isolated rat retina, a tissue that has a high specific activity of GS (Winkler et al. 1999) and produces lactate aerobically at a high rate (Winkler 1981; Winkler 1995) was chosen for these experiments. Two preparations were employed: normal rat retinas containing all cellular elements, and RCS retinas that lack photoreceptor cells (Bourne et al. 1938; Dowling and Sidman 1962). The specific activity of GS in normal rat retinas was 3.44 ± 0.23 μmol/min/mg protein (n = 18) and in RCS retinas activity was 4.66 ± 0.49 μmol/min/mg protein (n = 5). When expressed on a per tissue basis (see protein values for each tissue in Methods), the activity was similar in both tissues (1.17 versus 1.21 μmol/min/retina), indicating that activity of the GS is preserved in RCS retinas that have experienced widespread degeneration of photoreceptors (Organisciak et al. 1998). Figure 5 shows that the immunocytochemical distribution of GS in both normal and RCS rat retinas is consistent with localization to Müller cells (Riepe and Norenberg 1977). It can also be seen in Fig. 5 that in normal rat retinas, GFAP is expressed in astrocytes at the vitreal border of the retina and not in Müller cells, while in RCS retinas enhanced expression of GFAP is seen in Müller cells (Marc et al. 2003).
Fig. 5.
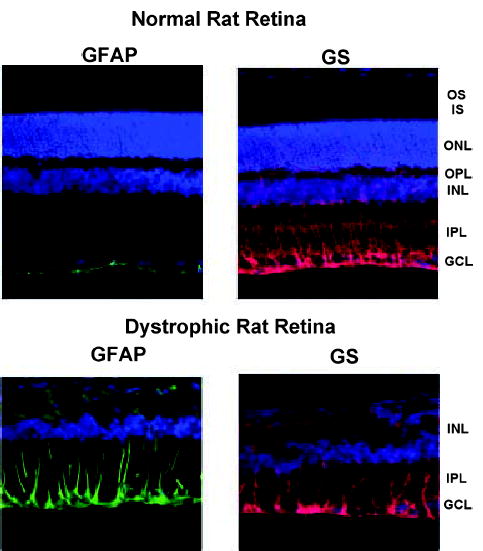
Rat retinas processed for glial fibrillary acidic protein (GFAP, left column) and glutamine synthetase (GS, right column) immuno-reactivity. Top pair: Normal retinas. Bottom pair: Dystrophic (RCS) retinas that lack photoreceptors. Staining as follows: green, GFAP; red, GS; blue, nuclear stain. Retinal layers denoted as follows: GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segments of photoreceptors; OS, outer segments of photoreceptors. Note that RCS retinas lack the ONL, IS and OS layers.
To test whether L-glutamate stimulates glycolysis in rat retinas and whether such a stimulation depends on the activity of GS in Müller cells, measurements were made of the rate of lactic acid production during incubation of normal rat retinas for 2 h in the following media: (i) control; (ii) with 1 mM L-glutamate; and (iii) with 1 mM L-glutamate and 5 mM MSO (Fig. 6). The experiments were divided into two phases. During the first phase (0–60 min), all media lacked NH4Cl and in the second phase (60–120 min) all media contained 0.5 mM NH4Cl. In this way, the effects of L-glutamate and MSO were tested in the presence and absence of NH4Cl. As shown in Fig. 6, exposure of control retinas to 0.5 mM NH4Cl caused a small (10%) decrease in the rate of glycolysis. The control rate of glycolysis in the absence of NH4Cl (0–60 min) was 1.60 ± 0.14 μmol/retina/h (n = 12) and in the presence of NH4Cl (60–120 min) the rate was 1.44 ± 0.17 μmol/retina/h (n = 12). On the basis of unpaired statistical analyses, considering the average of each rate independently, this difference is not significant at the p = 0.05 level. However, the 10% decrease in lactate production is significant at the p = 0.02 level when the value of t is calculated according to paired statistical analysis (t = average difference/SE). This procedure is justified since each pair of values (before and after NH4Cl) was obtained from the same retina. Addition of 1 mM L-glutamate to NH4Cl-free media had no significant effect on the rate of glycolysis in comparison to the control rate, i.e. 1.62 ± 0.17 (n = 7) versus 1.60 ± 0.14 (n = 12) μmol/retina/h. The addition of NH4Cl to L-glutamate-containing media caused a small, but insignificant (paired analysis) decrease in the rate of glycolysis in comparison to the rate found with L-glutamate alone i.e. 1.47 ± 0.10 (n = 7) versus 1.62 ± 0.17 (n = 7) μmol/retina/h. When 5 mM MSO was added to L-glutamate-containing media the rate of glycolysis was not significantly different from the rate with L-glutamate alone in the presence and absence of NH4Cl. It was previously shown that incubation of isolated rat retinas with 5 mM MSO resulted in complete inhibition of GS activity within 5–10 min (Winkler et al. 1999). The rates of aerobic and anaerobic (+ Antimycin A) lactic acid production from retinas incubated with 1 mM D-aspartate were not statistically different from the rates in control retinas (data not shown).
Fig. 6.
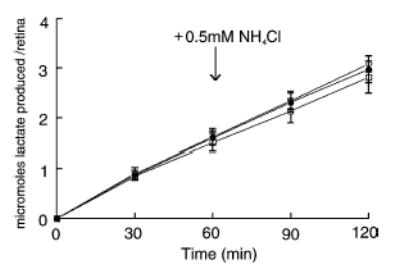
Aerobic lactic acid production from normal rat retinas that were incubated under the following conditions: (i) control media, •, n = 12; (ii) + 1 mM L-glutamate, ○, n = 6; and (iii) + 1 mM L-glutamate and 5 mM methionine sulfoximine (MSO), □, n = 9. At t = 60 min (immediately after withdrawal of an aliquot from the media for lactate determination) 0.5 mM NH4Cl (final concentration) was added to each media, and the incubations continued for a second hour. Each symbol is the average together with its standard deviation.
A similar series of protocols involving measurements of lactic acid production were carried out on isolated RCS retinas that were incubated in the presence and absence of NH4Cl under the same conditions as for the normal retinas. The use of RCS retinas was based on the assumption that the relative contribution of Müller cells to total retinal glycolysis would be greater in the absence of photoreceptors, as these cells normally produce lactic acid at a high rate (Graymore et al. 1959; Winkler 1995). Indeed, as shown in Fig. 7, the control rate of glycolysis in RCS retinas in the absence of NH4Cl was 0.53 ± 0.08 μmol/retina/h (n = 5), a value that is about 33% of that found in the normal rat retina (see above; Winkler 1995). In RCS retinas, the addition of NH4Cl to control media resulted in a small, statistically insignificant (p = 0.41; paired analysis) increase in the rate of glycolysis, i.e. from 0.53 to 0.59 ± 0.17 μmol/retina/h (n = 5). When 1 mM L-glutamate was added to control media, a 15% increase in the rate of glycolysis (to 0.61 ± 0.07 μmol/retina/h, n = 5) was observed, an effect that was not statistically significant (p = 0.14; unpaired analysis) when compared to the control rate of glycolysis. The addition of NH4Cl to L-glutamate-containing media increased the rate of glycolysis by 15% (p = 0.01, paired analysis), i.e. from 0.61 ± 0.07 (n = 5) to 0.70 ± 0.07 (n = 5) μmol/retina/h. However, a similar stimulatory effect of NH4Cl was observed when it was added to media containing L-glutamate and 5 mM MSO, i.e. from 0.57 ± 0.05 (n = 5) to 0.70 ± 0.06 (n = 5) μmol/retina/h.
Fig. 7.
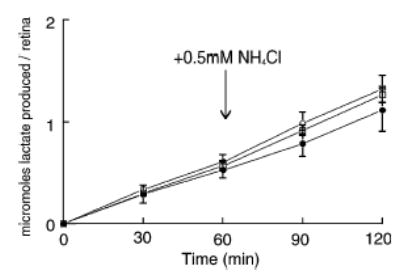
Aerobic lactic acid production from dystrophic (RCS) rat retinas that lack photoreceptor cells. Incubation conditions were identical to those in Fig. 6 for the normal rat retinas. The conditions were (i) control media, •, n = 5; + 1 mM L-glutamate, ○, n = 5; + 1 mM L-glutamate and 5 mM methionine sulfoximine (MSO), □ n = 5. Each symbol is the average, together with its standard deviation.
To obtain an estimate of the uptake of L-glutamate in normal rat retinas, we employed D-aspartate, a transportable analog that is not metabolized and does not interact with L-glutamate receptors, to minimize metabolic contributions resulting from activation of L-glutamate receptors. Figure 8 shows that net uptake of D-aspartate was observed. The initial, maximal rate of uptake (linear over first 5 min) amounted to 3 ± 0.7 nmol/min/retina (n = 4). Retinas continued to accumulate labeled D-aspartate over the entire 2-h incubation period. Total accumulation over this duration amounted to more than 100 nmol/retina. THA (at 5 mM) inhibited uptake of labeled D-aspartate by 90%.
Fig. 8.
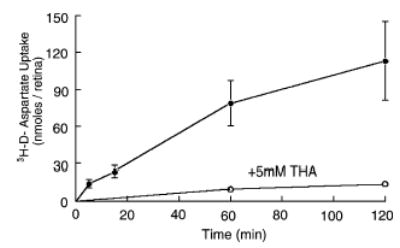
Accumulation of 3H-D-aspartate (nmol/retina) in isolated rat retinas as a function of time in the presence and absence of 5 mM THA. Each point represents the mean ± SD of three or four individual experiments. For THA data, SDs are smaller than the size of the symbol.
Discussion
The intent of the present experiments was to examine the linkage between the Na+-dependent L-glutamate transporter and lactic acid production in cultured rat Müller cells and rat retinas. In primary cultures of brain astrocytes, it has been demonstrated that the rise in the intracellular concentration of Na+ that occurs when the transporter is activated by incubation of cells in media containing L-glutamate is sufficient to stimulate the activity of the Na+–K+ ATPase, which in turn increases the rate of glycolysis (Pellerin and Magistretti 1994). The increase in glycolysis in astrocytes does not depend on subsequent metabolism of L-glutamate via GS, because uptake of D-aspartate causes a similar increase in glycolysis. In contrast, in freshly dissociated salamander Müller cells, Poitry et al. (2000) reported that an increase in lactic acid production was observed only if the cells were exposed to both L-glutamate and NH4Cl. They stated that ‘the increase in lactate production is linked to a reaction shared by the metabolism of both glutamate and NH4+.’ Because Poitry et al. (2000) found that the L-glutamate- and NH4+-induced increase in glycolysis in the Müller cells was suppressed by inhibiting GS with MSO, they concluded that the metabolic response results from an increased rate of GS. Taken together, the model of glycolytic activation by L-glutamate in cerebral astrocytes, though controversial (see introduction), may not apply to retinal Müller cells. We therefore set out to provide an independent test of the model of Poitry et al. (2000).
When our initial experiments with transformed Müller cells failed to find any stimulation of lactate production with increasing concentrations of L-glutamate, we were concerned that these cells might not express GS. This indeed turned out to be the case. As a result, the cells were not suitable for investigating all aspects of the Poitry et al. (2000) model, nor were two other Müller cell lines that also failed to exhibit GS activity. These results are in agreement with other studies showing that even primary confluent rabbit Müller cells early in culture lose the expression of GS (McGillem et al. 1998). Nevertheless, the present results with rMC-1 cells appear to confirm the suggestion of Poitry et al. (2000) that stimulation of the Na+–K+ pump by the coupled uptake of Na+ and L-glutamate is not sufficient by itself to cause a measurable increase in the rate of glycolysis in cultured Müller cells. Since the interaction between the cotransport of Na+/L-glutamate and energy metabolism depends on activation of the Na+–K+ ATPase, we examined the effects of ouabain on the rates of lactic acid production in control and Antimycin A-inhibited cells. Clearly, ouabain decreased both aerobic and anaerobic lactate production in rMC-1, indicating that the cultured retinal cells have a functioning Na+–K+ ATPase, as does the isolated rat retina (Winkler and Riley 1977; Winkler 1981).
Pellerin et al. (1997) suggested that the failure of L-glutamate to stimulate aerobic glycolysis in a cell line (DI TNC1) displaying several characteristics of astrocytes was because this cell line apparently lacked a Na+-dependent L-glutamate transporter. Because this possibility would also explain the present findings, it was necessary to show that the transformed rat Müller cells have the transporter. In the present experiments, estimates of the rates of Na+-dependent uptake were obtained with L-glutamate and with the glutamate analog D-aspartate. It is clear that rat Müller cells take up these compounds, but the maximal rates of uptake were very low relative to the rate of lactic acid production in the presence and absence of Antimycin A, a finding that likely accounts for the negligible changes in glycolysis. The same argument applies to passaged human Müller cells. These cells take up 1-14C-L-glutamate (1 mM) and convert it to 14CO2 (Winkler et al. 2000), but aerobic and anaerobic lactic acid production is not stimulated by this uptake (Winkler, unpublished results). However, the rate of production of 14CO2 from 1–14C-L-glutamate was only 8 nmol/mg protein/h, while the rate of aerobic glycolysis was 1.1 μmol/h/mg protein. In cultured human Müller cells, the capacity to carry out oxidative metabolism is very low, accounting for about 1% of total glucose utilization (Winkler et al. 2000), a result similar to that recently reported for astroglial cultures obtained from mesencephalon of fetal rats (Itoh et al. 2003). Moreover, coupled transport of Na+ and GABA in mouse cortical astrocytes, unlike the effects observed for L-glutamate transport, does not lead to an enhanced metabolic response, despite the fact that GABA is taken up with Na+ and causes a change in intracellular Na+ concentration (Chatton et al. 2003). Thus, the lack of effect of L-glutamate transport on lactic acid production in cultured retinal Müller cells appears to be due to insufficient activation of the Na+ pump.
It is of interest that the recent report of Voutsinos-Porche et al. (2003) provides data showing that in mouse cortical astrocytes the rates of uptake of 0.05 and 0.5 mM D-aspartate, respectively, are 17 and 33 nmol/mg protein/min and the rate of aerobic lactic acid production by cells incubated in media containing 0.2 mM D-aspartate is 45 nmol/mg protein/min. On this basis, it would appear that the contribution of L-glutamate (and D-aspartate) uptake to aerobic lactic acid production is far greater in cultured astrocytes than in cultured rMC-1 cells. However, this suggestion does not apply to the experiments of McKenna et al. (1996) who showed that exogenous L-glutamate was taken up by cerebral cortical astrocytes and converted to glutamine, aspartate and lactate, but that L-glutamate did not stimulate lactate production.
Opening Na+ channels with the ionophore monensin led to a marked stimulation of aerobic glycolysis in rMC-1 cells; this result is consistent with previous reports showing that monensin stimulated glucose uptake in cerebral astrocytes (Yarowsky et al. 1986; Erecinska et al. 1991; Peng et al. 2001). The stimulation of aerobic glycolysis by monensin in rMC-1 cells was completely blocked by ouabain, a result entirely consistent with a coupling between Na+ entry, its extrusion by the Na+–K+ ATPase and a stimulation of energy metabolism. Clearly, incubation of cells with monensin increased the rate of entry of Na+ sufficiently so that the change in intracellular Na+ activated the Na+ pump. It appears then that in these retinal cells the energy required to sustain the coupled transport of Na+ and L-glutamate is small (undetectable by our measurements) in comparison to the energy required to sustain a monensin-induced large passive influx of Na+ and to that of constitutive or basal Na+ flux (see effects of ouabain shown in Figs 1 and 2).
Interestingly, addition of Antimycin A caused a twofold increase in the rate of glycolysis (a Pasteur effect) but monensin had no additional stimulatory effect beyond the rate observed in the presence of Antimycin A alone. This is an important result because it suggests that complete inhibition of mitochondrial electron transport by Antimycin A results in a maximal rate of glycolysis. Under the anaerobic condition, the addition of monensin to the Antimycin A-containing media led to a 40% loss in ATP content in rMC-1 cells after 2 h. Thus, the inability of these cells to up-regulate the rate of anaerobic glycolysis when exposed to the added stress of a monensin-induced increase in membrane conductance to Na+ therefore places the cells at risk, since the production of ATP fails to keep pace with the increased demand for ATP by the Na+–K+ ATPase.
A final point concerns the attempt to evaluate effects of L-glutamate and NH4+ on lactic acid production in rat retinas, a tissue that expresses GS, contains Na+–L-glutamate (D-aspartate) cotransporters and expresses different types of L-glutamate receptors. Exposing retinas to 1 mM L-glutamate activates high and low affinity Na+-dependent L-glutamate transporters (Barbour et al. 1988, 1991; Rauen et al. 1998) and receptors (Ames and Li 1992) on Müller cells and retinal neurons. The results showed that the maximum initial rate of uptake of 1 mM D-aspartate was 3 nmol/min/retina, a value that is about threefold higher than that that reported by White and Neal (1976; see their Table 3, p. 89), but, nevertheless, is only 11% of the rate of aerobic lactic acid production (1.60 μmol/h/retina). A low rate of L-glutamate uptake relative to the resting rate of glycolysis in rat retinas may account for the absence of an L-glutamate-induced increase in the rate of glycolysis. Ames and Li (1992) previously found that incubation of isolated rabbit retinas in media containing 0.5 mM L-glutamate had no significant effect on the rate of lactic acid production. Although a glycolytic response upon enhancement of Na+ entry by coupled uptake with L-glutamate has been found in a number of cell types, it is not necessarily universal (see Introduction).
As the baseline rate of retinal glycolysis was unchanged by adding 1 mM L-glutamate to the incubation media bathing isolated rat retinas, the next step was to optimize conditions for activation of GS in Müller cells by supplying NH4+ to media containing L-glutamate; Poitry et al. (2000) have provided evidence in salamander retinas that NH4+ levels may drop severely after isolation of the tissues. However, the combination of L-glutamate and NH4+ failed to stimulate the rate of lactic acid production in normal rat retinas. As photoreceptors in a rat retina account for at least 50% of total volume (Cohen and Noell 1960) and at least 50% of retinal lactic acid production and glucose utilization (Graymore et al. 1959; Winkler 1995; Winkler et al. 2003b), it is reasonable to assume that this large background of glycolytic activity in normal rat retinas could mask detection of changes in lactate produced specifically by Müller cells. For this reason, RCS retinas that lack photoreceptors were incubated in media containing L-glutamate in the presence and absence of NH4+. We recognize that significant remodeling and reorganization takes place in these retinas in response to degeneration of the photoreceptors (see Fig. 5; Marc et al. 2003) and, thus, we are quite cautious in our interpretations and recognize the difficulty in the analysis. Nevertheless, RCS retinas were shown to retain high activity of GS, an obvious prerequisite for the in vitro incubations. While exposure of RCS retinas to 1 mM L-glutamate stimulated the basal rate of glycolysis by 10%, this effect was not statistically significant. When NH4+ was added to L-glutamate-containing media there was a statistically significant 15% stimulation in glycolysis. However, the increase in lactate production caused by NH4+ was not suppressed by addition of MSO. This suggests that the stimulatory effect of NH4+ in the presence of L-glutamate is not linked to activation of GS. Thus, NH4+ by itself appears to exert a small stimulatory effect on glycolysis in RCS retinas; further experiments will be required to analyze this effect. It should be noted that the present study employed mammalian (rat) cells and retinas incubated at 37°C in media buffered with 25 mM bicarbonate/5% CO2, while Poitry et al. (2000) used salamander glial cells incubated at 17°C in media buffered with 10 mM HEPES/1.0 mM bicarbonate. Thus, the extent of coupling between L-glutamine synthesis and glycolysis may differ in warm-blooded versus cold-blooded Müller cells. In this regard, Peng et al. (2001) found that incubation of mouse astrocytes in media containing L-glutamate and NH4+ did not stimulate glycolysis.
We leave open the possibility that the cotransport of Na+/L-glutamate and activation of GS in Müller cells may be linked to a stimulation of mitochondrial respiration (also see Poitry et al. 2000). A linkage between Na+-dependent L-glutamate cotransport and oxidative metabolism in cortical astrocytes has been reported (Eriksson et al. 1995; Peng et al. 2001). Peng et al. (2001) stated ‘glutamate carbon, after conversion of glutamate to alpha-ketoglutarate, replaced glucose carbon as oxidative substrate’ (p. 440). In other words, the use of L-glutamate could offset the need for enhancing glycolysis to support its uptake. Whether stimulation of mitochondrial activity by activation of GS in Müller cells involves oxidation of L-glutamate or another substrate, glucose being the likely choice, remains to be determined.
Acknowledgments
This work was supported in part by National Institutes of Health Grant EY 10015 (BSW) and by Vision Research Infrastructure Development Grant EY R24 014803. Michael Sauer was a recipient of an undergraduate research scholar’s award from Oakland University and a summer research fellowship from the Eye Research Institute. The authors thank Dr S.-C. Chen for assistance in the tissue culture facility and Dr Shravan Chintala and Mei Cheng for the immunocytochemistry. We are grateful to Dr. Dan Organisciak and Ruth Darrow for providing a colony of dystrophic (RCS) rats. The authors also thank the reviewers of this paper for many constructive and incisive comments that were incorporated into the revision.
References
- Ames A, III, Li YY. Energy requirements of glutamatergic pathways in rabbit retina. J Neurosci. 1992;12:4234–4242. doi: 10.1523/JNEUROSCI.12-11-04234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A, III, Li YY, Heher EC, Rising Kimble C. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcar VJ, Johnston GAR. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972;19:2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;335:433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne MC, Campbell DA, Tansley K. Hereditary degeneration of the rat retina. Br J Ophthalmol. 1938;22:613–622. doi: 10.1136/bjo.22.10.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: Implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Noell WK. Glucose catabolism of rabbit retina, before and after development of visual function. J Neurochem. 1960;5:253–276. doi: 10.1111/j.1471-4159.1960.tb13363.x. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. Glial strategy for metabolic shuttling and neuronal function. Bioessays. 2000;22:747–752. doi: 10.1002/1521-1878(200008)22:8<747::AID-BIES8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Demestre M, Boutelle M, Fillenz M. Stimulated release of lactate in freely moving rats is dependent on the uptake of glutamate. J Physiol. 1997;499:825–832. doi: 10.1113/jphysiol.1997.sp021971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J Neurosci Res. 1995;42:131–143. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Dagani F. Relationships between the neuronal sodium/potassium pump and energy metabolism: effects of K, Na, and adenosine triphosphate in isolated brain synaptosomes. J Gen Physiol. 1990;95:591–616. doi: 10.1085/jgp.95.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Ions and energy in mammalian brain. Progr Neurobiol. 1994;43:37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Dagani F, Nelson D, Deas J, Silver IA. Relations between intracellular ions and energy metabolism: a study with monensin in synaptosomes, neurons, and C6 glioma cells. J Neurosci. 1991;11:2410–2421. doi: 10.1523/JNEUROSCI.11-08-02410.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson G, Peterson A, Iverfeldt K, Walum E. Sodium-dependent glutamate uptake as an activator of oxidative metabolism in primary astrocyte cultures from newborn rat. Glia. 1995;15:152–156. doi: 10.1002/glia.440150207. [DOI] [PubMed] [Google Scholar]
- Graymore C, Tansley K, Kerly M. Metabolism of developing retina. II The effect of an inherited retina degeneration on the development of glycolysis in the rat retina. Biochem J. 1959;72:459–461. doi: 10.1042/bj0720459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Dienel GA. Energy metabolism in the brain. Int Rev Neurobiol. 2002;51:1–102. doi: 10.1016/s0074-7742(02)51003-5. [DOI] [PubMed] [Google Scholar]
- Hertz L, Swanson RA, Newman GC, Mariff H, Juurlink BHJ, Peng L. Can experimental conditions explain the discrepancy whether or not glutamate stimulates aerobic glycolysis? Dev Neurosci. 1998;20:339–347. doi: 10.1159/000017329. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: Glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci USA. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116. [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retinal Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- McGillem GS, Guidry C, Dacheux RF. Antigenic changes of rabbit retinal Müller cells in culture. Invest Ophthalmol Vis Sci. 1998;39:1453–1461. [PubMed] [Google Scholar]
- McKenna M, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- Newman E, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–311. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Darrow RA, Lininger LA. In: Environmental and age-related changes in retinal proteins, in Photostasis and Related phenomena. Williams TP, Thistle D, editors. Plenum; New York: 1998. pp. 79–92. [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Stolz M, Sorg O, Martin JL, Deschepper CF, Magistretti PJ. Regulation of energy metabolism by neurotransmitters in astrocytes in primary culture and in an immortalized cell line. Glia. 1997;21:74–83. doi: 10.1002/(sici)1098-1136(199709)21:1<74::aid-glia8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Peng L, Swanson RA, Hertz L. Effects of L-glutamate, D-aspartate, and monensin on glycolytic and oxidative metabolism in mouse astrocyte cultures: further evidence that glutamate uptake is metabolically driven by oxidative metabolism. Neurochem Int. 2001;38:437–443. doi: 10.1016/s0197-0186(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M. Mechanisms of glutamate metabolic signaling in retinal glial (Müller) cells. J Neurosci. 2000;20:1809–1821. doi: 10.1523/JNEUROSCI.20-05-01809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman BC. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Pressman BC, Fahim M. Pharmacology and toxicology of the monovalent carboxylic ionophores. Annu Rev Pharmacol Toxicol. 1982;22:465–490. doi: 10.1146/annurev.pa.22.040182.002341. [DOI] [PubMed] [Google Scholar]
- Qu H, Eloqayli H, Unsgard G, Sonnewald U. Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. J Neurosci Res. 2001;66:1127–1132. doi: 10.1002/jnr.10032. [DOI] [PubMed] [Google Scholar]
- Rauen T, Taylor WR, Kuhlbrodt K, Wiessner M. High-affinity glutamate transporters in the rat retina: a major role of the glial glutamate transporter GLAST-1 in transmitter clearance. Cell Tissue Res. 1998;291:19–31. doi: 10.1007/s004410050976. [DOI] [PubMed] [Google Scholar]
- Reichelt W, Stabel-Burow J, Pannicke T, Weichert H, Heinemann LL. The glutathione level of retinal Müller glial cells is dependent on the high-affinity sodium-dependent uptake of glutamate. Neuroscience. 1997;77:1213–1224. doi: 10.1016/s0306-4522(96)00509-x. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenberg MD. Müller cell localization of glutamine synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Meister A. Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine. Proc Natl Acad Sci USA. 1970;66:500–506. doi: 10.1073/pnas.66.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy VP, Ripps H. The Retinal Müller Cell. Kluwer Academic/Plenum; New York: 2001. [Google Scholar]
- Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Dehar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Takahashi S, Gotoh J, Driscoll BF, Law MJ. Contribution of astroglia to functionally activated energy metabolism. Dev Neurosci. 1996;18:343–352. [PubMed] [Google Scholar]
- Swanson RA, Bennington JH. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci. 1996;18:515–521. doi: 10.1159/000111448. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1:366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- Wang L, Kondo M, Bill A. Glucose metabolism in cat outer retina. Invest Ophthal Vis Sci. 1997;38:48–55. [PubMed] [Google Scholar]
- White RD, Neal MJ. The uptake of L-glutamate by the retina. Brain Res. 1976;111:79–93. doi: 10.1016/0006-8993(76)91050-7. [DOI] [PubMed] [Google Scholar]
- Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS. A quantitative assessment of glucose metabolism in the isolated rat retina. In: Christen Y, Doly CY, Droy-Lefaix M-T, editors. Les Seminaires Ophthalmologiques D’IP-SEN: Vision and Adaptation. Vol. 6. 1995. pp. 78–96. [Google Scholar]
- Winkler BS, Riley MV. Na+–K+ and HCO3− ATPase activity in retina: dependence on calcium and sodium. Invest Ophthal Vis Sci. 1977;16:1151–1154. [PubMed] [Google Scholar]
- Winkler BS, Kapousta-Bruneau N, Arnold MJ, Green DG. The glutamate–glutamine cycle in Müller cells is not critical for b-wave maintenance in the isolated rat retina. Vis Neurosci. 1999;16:345–353. doi: 10.1017/s095252389916214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Arnold MJ, Brassell MA, Puro DG. Energy metabolism in human Müller cells. Invest Ophthalmol Vis Sci. 2000;41:3183–3190. [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Sauer MW, Starnes CA. Modulation of the Pasteur effect in retinal cells: implications for understanding compensatory metabolic mechanisms. Exp Eye Res. 2003a;76:715–723. doi: 10.1016/s0014-4835(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Pourcho RG, Starnes C, Slocum J, Slocum N. Metabolic mapping in mammalian retina: a biochemical and 3H-2-deoxyglucose autoradiography study. Exp Eye Res. 2003b;77:327–337. doi: 10.1016/s0014-4835(03)00147-7. [DOI] [PubMed] [Google Scholar]
- Yarowsky P, Boyne AF, Wierwille R, Brookes N. Effect of monensin on deoxyglucose uptake in cultured astrocytes: energy metabolism is coupled to sodium entry. J Neurosci. 1986;6:859–866. doi: 10.1523/JNEUROSCI.06-03-00859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


