Full Text
The Full Text of this article is available as a PDF (112.6 KB).
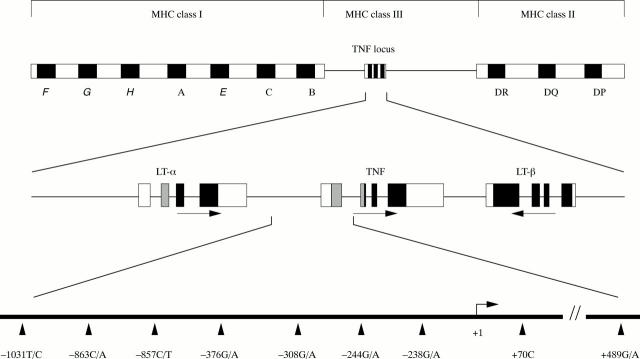
Figure 1 .
Schematic representation of the location of the TNF gene within the major histocompatibility complex. The middle row represents the intron/exon organisation of the TNF and LT genes. The arrows indicate the transcriptional orientation of the TNF and LT genes. At the bottom the 5' region of the gene including the transcription initiation site is depicted. The position of the SNPs within this part of the gene is indicated.
Figure 2 .
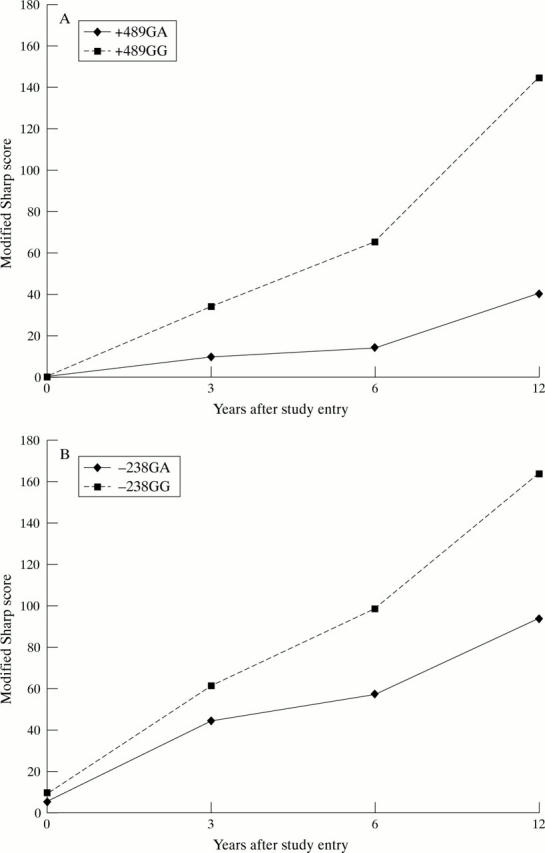
Joint damage in relation to the TNF +489 and −238 polymorphism in a prospective cohort of RA patients. The median radiographic damage score expressed as modified Sharp score for radiographs of hands and feet for the TNF +489 GG and GA patients (panel A) and the TNF −238 GG and GA patients (panel B) is presented. Modified Sharp scores at onset (t=0), at 3, 6 and 12 years is depicted.
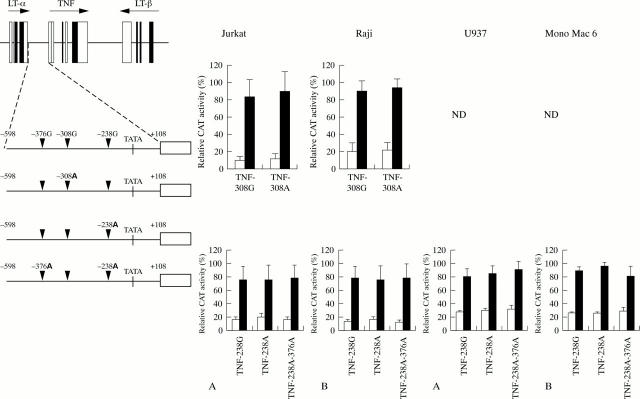
Figure 3 .
Schematic representation of the human TNF locus and the TNF promoter/reportergene constructs used to study the functional relevance of the −376, −308 and −238 gene variants. In the right panel the results of the transfections are shown. Presented are the results from at least three independent transfections with three different batches of DNA. Reportergene expression is expressed as the relative activity of the plasmid with the highest activity (100%) reached at after stimulation in each experiment. Relative reportergene expression levels were averaged (SD) and plotted in histograms. Jurkat cells were unstimulated (open bars) or stimulated for 24 hours with anti-CD3 (0.5 µg/ml) and anti-CD28 (5 µg/ml) (filled bars). Raji cells were unstimulated (open bars) or stimulated for 24 hours with PMA (50 ng/ml) filled bars. U937 and Mono Mac 6 were unstimulated (open bars) or stimulated (filled bars) for four hours with LPS (1 µg/ml)/PMA (10 ng/ml) and LPS, respectively. ND = not determined.
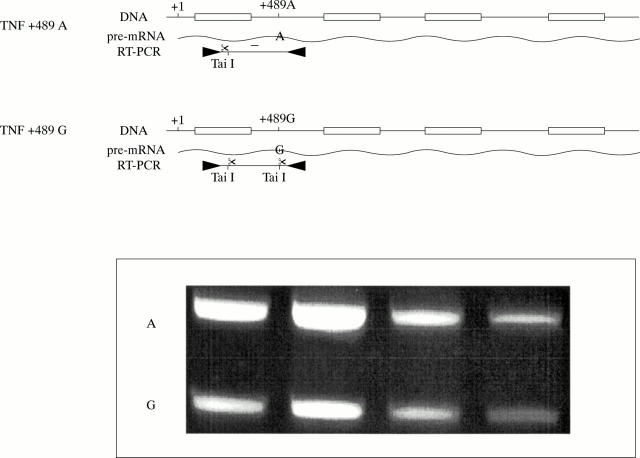
Figure 4 .
Analysis of the relative contribution of the two +489 alleles in the total TNF precursor mRNA synthesis.Upper panel gives a schematic representation of the genotype of a person heterozygous for the +489 polymorphism. The G at position +489 creates a Tai1 restriction site that is not cleavable in the A allele. PBMC of heterozygous subjects are stimulated with LPS (10 ng/ml) for four hours and RNA is isolated. RNA is reversed transcribed and PCR amplified by two primers spanning the TNF region of interest. Precursor TNF RT-PCR product is digested with Tai to discriminate between the relative contribution of the respective alleles in total TNF precursor production. On the gel results are shown from three people tested. Lane 1; Analysis on genomic DNA from one of the subjects as control (theoretically 1:1), lane 2-4: results from cells stimulated with LPS 10 ng/ml. The A indicates the fragment representing the noncleavable +489A fraction of the transcript. The G the cleavable +489G fraction.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho K., Koskenvuo M., Tuominen J., Kaprio J. Occurrence of rheumatoid arthritis in a nationwide series of twins. J Rheumatol. 1986 Oct;13(5):899–902. [PubMed] [Google Scholar]
- Alexander H. R., Sheppard B. C., Jensen J. C., Langstein H. N., Buresh C. M., Venzon D., Walker E. C., Fraker D. L., Stovroff M. C., Norton J. A. Treatment with recombinant human tumor necrosis factor-alpha protects rats against the lethality, hypotension, and hypothermia of gram-negative sepsis. J Clin Invest. 1991 Jul;88(1):34–39. doi: 10.1172/JCI115298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtzen K., Morling N., Fomsgaard A., Svenson M., Jakobsen B., Odum N., Svejgaard A. Association between HLA-DR2 and production of tumour necrosis factor alpha and interleukin 1 by mononuclear cells activated by lipopolysaccharide. Scand J Immunol. 1988 Nov;28(5):599–606. doi: 10.1111/j.1365-3083.1988.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Braun N., Michel U., Ernst B. P., Metzner R., Bitsch A., Weber F., Rieckmann P. Gene polymorphism at position -308 of the tumor-necrosis-factor-alpha (TNF-alpha) in multiple sclerosis and it's influence on the regulation of TNF-alpha production. Neurosci Lett. 1996 Sep 6;215(2):75–78. [PubMed] [Google Scholar]
- Brennan F. M., Maini R. N., Feldmann M. TNF alpha--a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992 May;31(5):293–298. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- Brinkman B. M., Giphart M. J., Verhoef A., Kaijzel E. L., Naipal A. M., Daha M. R., Breedveld F. C., Verweij C. L. Tumor necrosis factor alpha-308 gene variants in relation to major histocompatibility complex alleles and Felty's syndrome. Hum Immunol. 1994 Dec;41(4):259–266. doi: 10.1016/0198-8859(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Brinkman B. M., Huizinga T. W., Breedveld F. C., Verweij C. L. Allele-specific quantification of TNFA transcripts in rheumatoid arthritis. Hum Genet. 1996 Jun;97(6):813–818. doi: 10.1007/BF02346195. [DOI] [PubMed] [Google Scholar]
- Brinkman B. M., Huizinga T. W., Kurban S. S., van der Velde E. A., Schreuder G. M., Hazes J. M., Breedveld F. C., Verweij C. L. Tumour necrosis factor alpha gene polymorphisms in rheumatoid arthritis: association with susceptibility to, or severity of, disease? Br J Rheumatol. 1997 May;36(5):516–521. doi: 10.1093/rheumatology/36.5.516. [DOI] [PubMed] [Google Scholar]
- Brinkman B. M., Kaijzel E. L., Huizinga T. W., Giphart M. J., Breedveld F. C., Verweij C. L. Detection of a C-insertion polymorphism within the human tumor necrosis factor alpha (TNFA) gene. Hum Genet. 1995 Oct;96(4):493–493. doi: 10.1007/BF00191815. [DOI] [PubMed] [Google Scholar]
- Brinkman B. M., Zuijdeest D., Kaijzel E. L., Breedveld F. C., Verweij C. L. Relevance of the tumor necrosis factor alpha (TNF alpha) -308 promoter polymorphism in TNF alpha gene regulation. J Inflamm. 1995;46(1):32–41. [PubMed] [Google Scholar]
- Cabrera M., Shaw M. A., Sharples C., Williams H., Castes M., Convit J., Blackwell J. M. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995 Nov 1;182(5):1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Trowsdale J. Map of the human MHC. Immunol Today. 1993 Jul;14(7):349–352. doi: 10.1016/0167-5699(93)90234-C. [DOI] [PubMed] [Google Scholar]
- D'Alfonso S., Richiardi P. M. A polymorphic variation in a putative regulation box of the TNFA promoter region. Immunogenetics. 1994;39(2):150–154. doi: 10.1007/BF00188619. [DOI] [PubMed] [Google Scholar]
- D'Alfonso S., Richiardi P. M. An intragenic polymorphism in the human tumor necrosis factor alpha (TNFA) chain-encoding gene. Immunogenetics. 1996;44(4):321–322. doi: 10.1007/BF02602566. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Ferencik S., Lindemann M., Horsthemke B., Grosse-Wilde H. A new restriction fragment length polymorphism of the human TNF-B gene detected by AspHI digest. Eur J Immunogenet. 1992 Dec;19(6):425–430. doi: 10.1111/j.1744-313x.1992.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Fernandes D. M., Baldwin C. L. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun. 1995 Mar;63(3):1130–1133. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kühn R., Müller W., Trinchieri G., Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996 Jul 15;157(2):798–805. [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hajeer A. H., Worthington J., Silman A. J., Ollier W. E. Association of tumor necrosis factor microsatellite polymorphisms with HLA-DRB1*04-bearing haplotypes in rheumatoid arthritis patients. Arthritis Rheum. 1996 Jul;39(7):1109–1114. doi: 10.1002/art.1780390706. [DOI] [PubMed] [Google Scholar]
- Hamann A., Mantzoros C., Vidal-Puig A., Flier J. S. Genetic variability in the TNF-alpha promoter is not associated with type II diabetes mellitus (NIDDM). Biochem Biophys Res Commun. 1995 Jun 26;211(3):833–839. doi: 10.1006/bbrc.1995.1887. [DOI] [PubMed] [Google Scholar]
- Higuchi T., Seki N., Kamizono S., Yamada A., Kimura A., Kato H., Itoh K. Polymorphism of the 5'-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue Antigens. 1998 Jun;51(6):605–612. doi: 10.1111/j.1399-0039.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- Höhler T., Kruger A., Schneider P. M., Schopf R. E., Knop J., Rittner C., Meyer zum Büschenfelde K. H., Märker-Hermann E. A TNF-alpha promoter polymorphism is associated with juvenile onset psoriasis and psoriatic arthritis. J Invest Dermatol. 1997 Oct;109(4):562–565. doi: 10.1111/1523-1747.ep12337469. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., Fronek Z., Lewis G. D., Koo M., Hansen J. A., McDevitt H. O. Heritable major histocompatibility complex class II-associated differences in production of tumor necrosis factor alpha: relevance to genetic predisposition to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1233–1237. doi: 10.1073/pnas.87.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Briant L., Udalova I. A., Sevin A., Nedospasov S. A., Cambon-Thomsen A. Extensive genetic polymorphism in the human tumor necrosis factor region and relation to extended HLA haplotypes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9717–9721. doi: 10.1073/pnas.88.21.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijzel E. L., van Krugten M. V., Brinkman B. M., Huizinga T. W., van der Straaten T., Hazes J. M., Ziegler-Heitbrock H. W., Nedospasov S. A., Breedveld F. C., Verweij C. L. Functional analysis of a human tumor necrosis factor alpha (TNF-alpha) promoter polymorphism related to joint damage in rheumatoid arthritis. Mol Med. 1998 Nov;4(11):724–733. [PMC free article] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger K. M., Carville K. S., Abraham L. J. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997 Apr;34(5):391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lawrence J. S. Heberden Oration, 1969. Rheumatoid arthritis--nature or nurture? Ann Rheum Dis. 1970 Jul;29(4):357–379. doi: 10.1136/ard.29.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire W., Hill A. V., Allsopp C. E., Greenwood B. M., Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994 Oct 6;371(6497):508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- Messer G., Spengler U., Jung M. C., Honold G., Blömer K., Pape G. R., Riethmüller G., Weiss E. H. Polymorphic structure of the tumor necrosis factor (TNF) locus: an NcoI polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J Exp Med. 1991 Jan 1;173(1):209–219. doi: 10.1084/jem.173.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L., Morel Y. The molecular genetics of 21-hydroxylase deficiency. Annu Rev Genet. 1989;23:371–393. doi: 10.1146/annurev.ge.23.120189.002103. [DOI] [PubMed] [Google Scholar]
- Mulcahy B., Waldron-Lynch F., McDermott M. F., Adams C., Amos C. I., Zhu D. K., Ward R. H., Clegg D. O., Shanahan F., Molloy M. G. Genetic variability in the tumor necrosis factor-lymphotoxin region influences susceptibility to rheumatoid arthritis. Am J Hum Genet. 1996 Sep;59(3):676–683. [PMC free article] [PubMed] [Google Scholar]
- Mullighan C. G., Fanning G. C., Chapel H. M., Welsh K. I. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997 Dec 15;159(12):6236–6241. [PubMed] [Google Scholar]
- Partanen J., Koskimies S. Low degree of DNA polymorphism in the HLA-linked lymphotoxin (tumour necrosis factor beta) gene. Scand J Immunol. 1988 Sep;28(3):313–316. doi: 10.1111/j.1365-3083.1988.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Pociot F., Briant L., Jongeneel C. V., Mölvig J., Worsaae H., Abbal M., Thomsen M., Nerup J., Cambon-Thomsen A. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-alpha and TNF-beta by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol. 1993 Jan;23(1):224–231. doi: 10.1002/eji.1830230135. [DOI] [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Silman A. J., MacGregor A. J., Thomson W., Holligan S., Carthy D., Farhan A., Ollier W. E. Twin concordance rates for rheumatoid arthritis: results from a nationwide study. Br J Rheumatol. 1993 Oct;32(10):903–907. doi: 10.1093/rheumatology/32.10.903. [DOI] [PubMed] [Google Scholar]
- Stuber F., Udalova I. A., Book M., Drutskaya L. N., Kuprash D. V., Turetskaya R. L., Schade F. U., Nedospasov S. A. -308 tumor necrosis factor (TNF) polymorphism is not associated with survival in severe sepsis and is unrelated to lipopolysaccharide inducibility of the human TNF promoter. J Inflamm. 1995;46(1):42–50. [PubMed] [Google Scholar]
- Udalova I. A., Nedospasov S. A., Webb G. C., Chaplin D. D., Turetskaya R. L. Highly informative typing of the human TNF locus using six adjacent polymorphic markers. Genomics. 1993 Apr;16(1):180–186. doi: 10.1006/geno.1993.1156. [DOI] [PubMed] [Google Scholar]
- Uglialoro A. M., Turbay D., Pesavento P. A., Delgado J. C., McKenzie F. E., Gribben J. G., Hartl D., Yunis E. J., Goldfeld A. E. Identification of three new single nucleotide polymorphisms in the human tumor necrosis factor-alpha gene promoter. Tissue Antigens. 1998 Oct;52(4):359–367. doi: 10.1111/j.1399-0039.1998.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp R. G., Langermans J. A., Huizinga T. W., Elouali A. H., Verweij C. L., Boomsma D. I., Vandenbroucke J. P., Vandenbrouke J. P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997 Jan 18;349(9046):170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. HLA-DRB1 alleles as severity markers in RA. Bull Rheum Dis. 1994 Jun;43(4):5–8. [PubMed] [Google Scholar]
- Wilson A. G., Symons J. A., McDowell T. L., McDevitt H. O., Duff G. W. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. G., de Vries N., Pociot F., di Giovine F. S., van der Putte L. B., Duff G. W. An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med. 1993 Feb 1;177(2):557–560. doi: 10.1084/jem.177.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth P., Bell J. Polygenic susceptibility in rheumatoid arthritis. Ann Rheum Dis. 1991 Jun;50(6):343–346. doi: 10.1136/ard.50.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeben D., Hazes J. M., Zwinderman A. H., Cats A., Schreuder G. M., D'Amaro J., Breedveld F. C. Association of HLA-DR4 with a more progressive disease course in patients with rheumatoid arthritis. Results of a followup study. Arthritis Rheum. 1991 Jul;34(7):822–830. doi: 10.1002/art.1780340707. [DOI] [PubMed] [Google Scholar]