Full Text
The Full Text of this article is available as a PDF (278.3 KB).
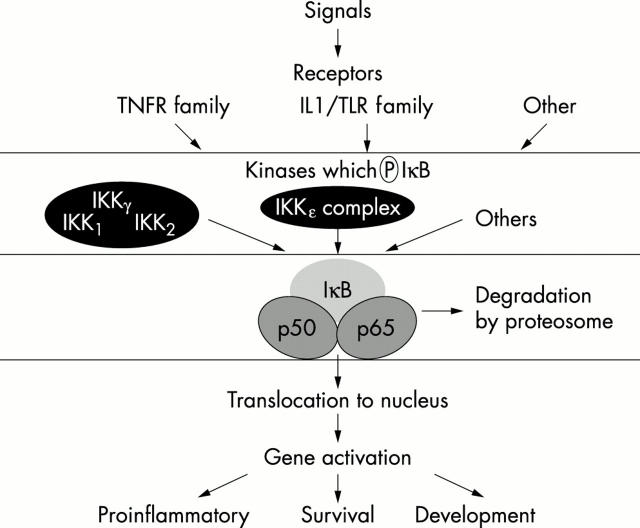
Figure 1 .
Schematic diagram of NF-κB activation.
Figure 2 .
Requirements for a good therapeutic target.
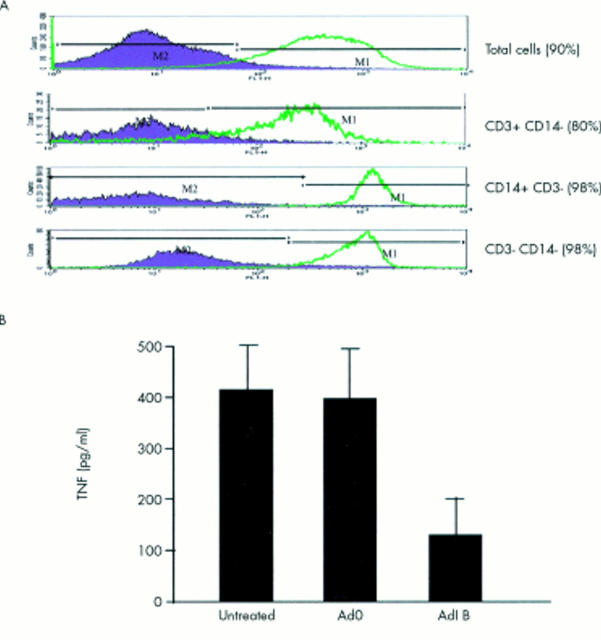
Figure 3.
TNFα production in rheumatoid synovial cell cultures is NF-κB dependent. (A) More than 90% of rheumatoid synovial cells can be infected with replication deficient adenoviruses at an m.o.i. of 40 as shown by measuring ß-galactosidase activity by FACS. Rheumatoid T cells, macrophage-like and fibroblast-like cells are all efficiently infected. (B) Rheumatoid synovial cells were infected with an adenovirus overexpressing IκBα (AdvIκBα) or a control adenovirus without insert (Adv0) at an m.o.i. of 40. Supernatants were collected after 48–72 hours and examined for TNFα production by ELISA.
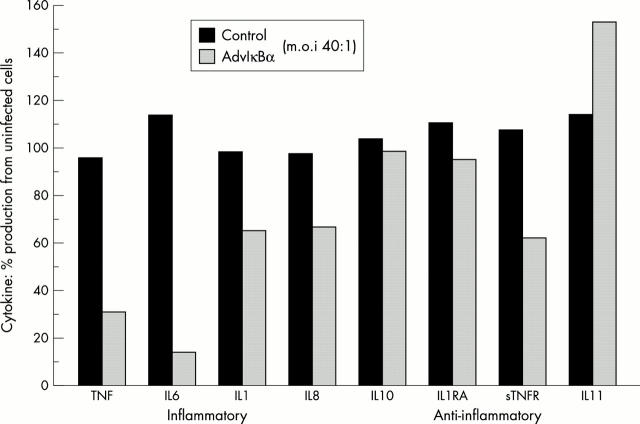
Figure 4 .
IκBα overexpression selectively inhibits cytokine expression in RA joint cultures. It blocks proinflammatory but not anti-inflammatory cytokines and mediators (IL10, IL1RA, sTNFR, IL11).
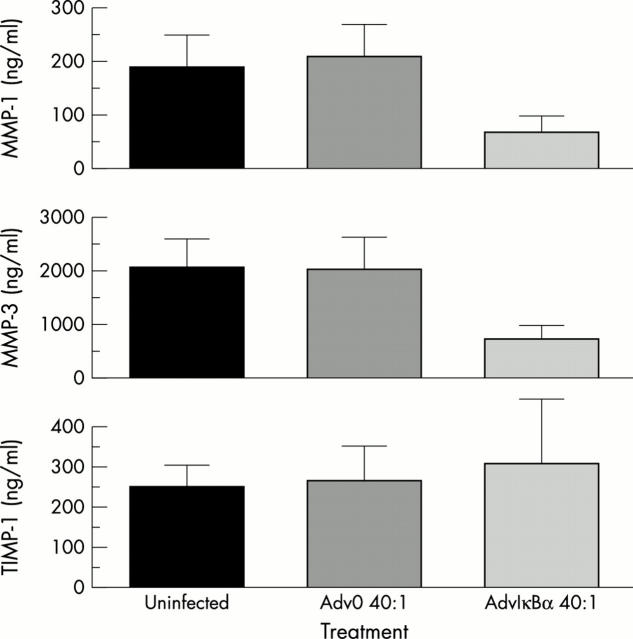
Figure 5 .
IκBα down regulates the production of MMP-1 and MMP-3 but not TIMP-1. Rheumatoid synovial cells were infected with an adenovirus overexpressing IκBα (AdvIκBα) or a control adenovirus without insert (Adv0) at an m.o.i. of 40. Supernatants were collected after 48–72 hours and examined by ELISA for the presence of MMPs and TIMP-1.
Figure 6 .
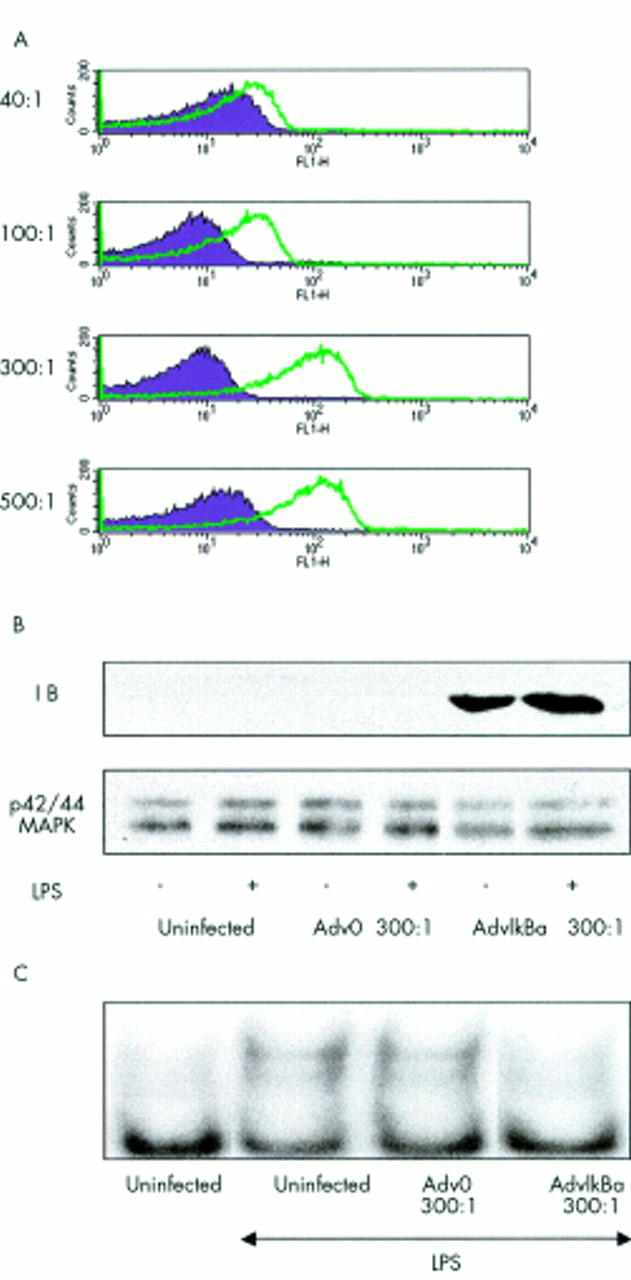
Dendritic cells take up adenovirus effectively. (A) Mature dendritic cells were generated by five days' culture in 50 ng/ml GM-CSF and 10 ng/ml IL4 and a further three days in monocyte conditioned medium. Then they were plated on a 96 well, flat bottom plate at a density of 2x105 cells/well and left either uninfected with an m.o.i. ranging from 40 to 500 of an adenovirus without insert (Adv0) or an adenovirus overexpressing ß-Gal expression. Fluorescein-di-(ß-D)-galactopyranoside (Sigma) was used as a substrate of ß-galactosidase and cell fluorescence was analysed by FACS analysis. (B) and (C) 10x106 cells for each condition were left either uninfected or infected with Adv0 or AdvIκBα at an m.o.i. of 300. Two days after infection, cells were left unstimulated or stimulated with LPS (50 ng/ml) for 60 minutes. Cytosolic and nuclear extracts were then prepared and examined for IκBα or p42/44MAPK (loading control) expression by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (B), or for NF-κB DNA binding activity by electrophoretic mobility shift assay (C).
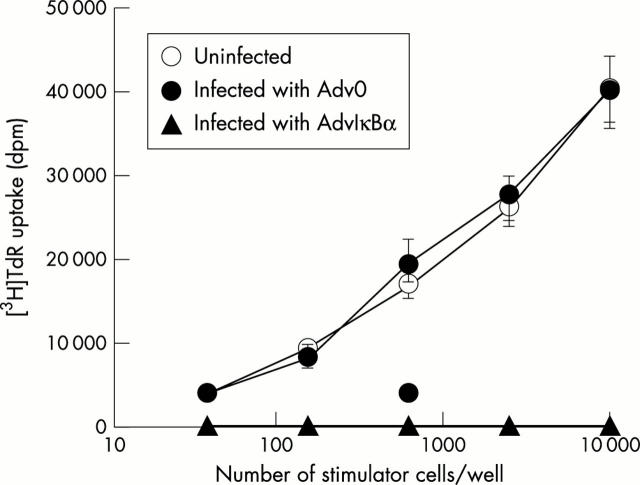
Figure 7 .
AdvIκBα inhibits the mixed lymphocyte reaction. Mature DC were left uninfected, infected with Adv0, or infected with AdvIκBα at an m.o.i. of 300. DC were then plated in graded doses for 105 purified, allogeneic T cells in triplicate in a 96 cell, round bottom microtitre plate on day 1 after adenovirus infection. Proliferation was determined on day six by a [3H]thymidine ([3H]TdR) uptake assay. Each point represents the mean (SEM) of three separate experiments.
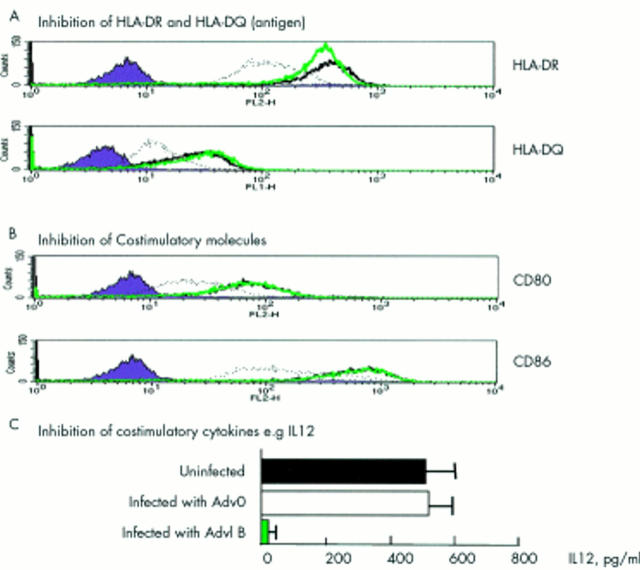
Figure 8 .
AdvIκBα inhibits three critical components of antigen presentation. The effect of AdvIκBα infection on dendritic cell antigen presenting and costimulatory molecules as well as the costimulatory cytokine IL12 was studied. IκBα was overexpressed in mature DC for two days and then cells were collected and stained by FACS for HLA (A) or CD80/86 (B), or stimulated by soluble CD40L for 24 hours, and then IL12 production was assayed by ELISA (C).
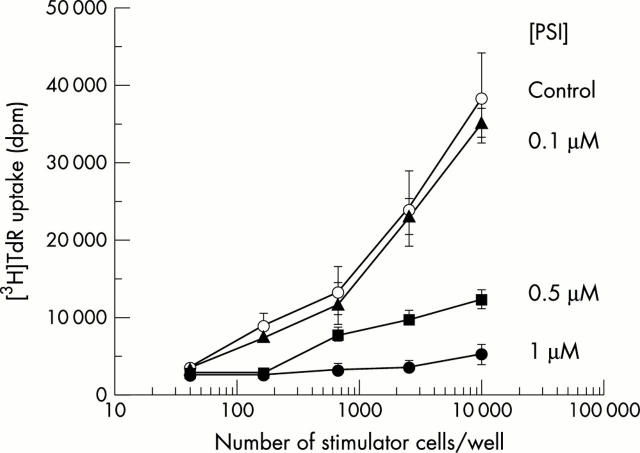
Figure 9 .
PSI pretreatment inhibits DC antigen presentation. DC were pretreated with vehicle or PSI (0.1 µM, 0.5 µM, 1 µM) for four hours. After washing, graded numbers from these four batches of DC were plated with 105 allo-lymphocytes on a 96 well plate. Proliferation was determined on day 6 using the [3H]TdR uptake assay. Each point represents the mean (SEM) of five separate experiments.
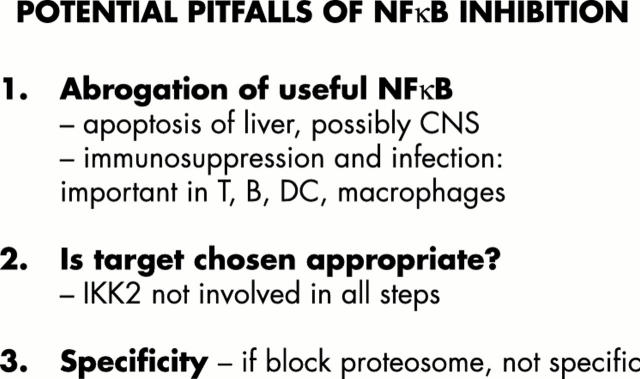
Figure 10 .
Potential pitfalls of NF-κB inhibition.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondeson J., Foxwell B., Brennan F., Feldmann M. Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci U S A. 1999 May 11;96(10):5668–5673. doi: 10.1073/pnas.96.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski Cathleen J., Andreakos Evangelos, Foxwell Brian M. J., Feldmann Marc. TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion. Eur J Immunol. 2002 Jul;32(7):2037–2045. doi: 10.1002/1521-4141(200207)32:7<2037::AID-IMMU2037>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Conron Matthew, Andreakos Evangelos, Pantelidis Panagiotis, Smith Clive, Beynon Huw L. C., Dubois Roland M., Foxwell Brian M. J. Nuclear factor-kappaB activation in alveolar macrophages requires IkappaB kinase-beta, but not nuclear factor-kappaB inducing kinase. Am J Respir Crit Care Med. 2002 Apr 1;165(7):996–1004. doi: 10.1164/ajrccm.165.7.2107058. [DOI] [PubMed] [Google Scholar]
- DiDonato J. A., Hayakawa M., Rothwarf D. M., Zandi E., Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997 Aug 7;388(6642):548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Foxwell B. M., Yoshimura S., Bondeson J., Brennan F. M., Feldmann M. High efficiency gene transfer is an efficient way of defining therapeutic targets: a functional genomics approach. Ann Rheum Dis. 2001 Nov;60 (Suppl 3):iii13–iii17. doi: 10.1136/ard.60.90003.iii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxwell B., Browne K., Bondeson J., Clarke C., de Martin R., Brennan F., Feldmann M. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci U S A. 1998 Jul 7;95(14):8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., May M. J., Kopp E. B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995 Jun;3(3):207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- Handel M. L., McMorrow L. B., Gravallese E. M. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995 Dec;38(12):1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Marok R., Winyard P. G., Coumbe A., Kus M. L., Gaffney K., Blades S., Mapp P. I., Morris C. J., Blake D. R., Kaltschmidt C. Activation of the transcription factor nuclear factor-kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996 Apr;39(4):583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Zhu H., Murray B. W., Shevchenko A., Bennett B. L., Li J., Young D. B., Barbosa M., Mann M., Manning A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997 Oct 31;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Miagkov A. V., Kovalenko D. V., Brown C. E., Didsbury J. R., Cogswell J. P., Stimpson S. A., Baldwin A. S., Makarov S. S. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzinger W., Hofer-Warbinek R., Schmid J. A., Koshelnick Y., Binder B. R., de Martin R. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001 Mar 15;97(6):1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Smith C., Andreakos E., Crawley J. B., Brennan F. M., Feldmann M., Foxwell B. M. NF-kappaB-inducing kinase is dispensable for activation of NF-kappaB in inflammatory settings but essential for lymphotoxin beta receptor activation of NF-kappaB in primary human fibroblasts. J Immunol. 2001 Nov 15;167(10):5895–5903. doi: 10.4049/jimmunol.167.10.5895. [DOI] [PubMed] [Google Scholar]
- Thomas R. Antigen-presenting cells in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20(1-2):53–72. doi: 10.1007/BF00831999. [DOI] [PubMed] [Google Scholar]
- Thomas R., Lipsky P. E. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol Today. 1996 Dec;17(12):559–564. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- Tomita T., Takeuchi E., Tomita N., Morishita R., Kaneko M., Yamamoto K., Nakase T., Seki H., Kato K., Kaneda Y. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor kappaB decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 1999 Dec;42(12):2532–2542. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Woronicz J. D., Gao X., Cao Z., Rothe M., Goeddel D. V. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997 Oct 31;278(5339):866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Bondeson J., Brennan F. M., Foxwell B. M., Feldmann M. Role of NFkappaB in antigen presentation and development of regulatory T cells elucidated by treatment of dendritic cells with the proteasome inhibitor PSI. Eur J Immunol. 2001 Jun;31(6):1883–1893. doi: 10.1002/1521-4141(200106)31:6<1883::aid-immu1883>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Bondeson J., Foxwell B. M., Brennan F. M., Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001 May;13(5):675–683. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- Zandi E., Rothwarf D. M., Delhase M., Hayakawa M., Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997 Oct 17;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]