Abstract
The most surprising feature of the inflammatory response in rheumatoid arthritis is not that it occurs but that it does not resolve. The persistence of the chronic inflammatory response in conjunction with ongoing joint destruction is an all too familiar finding in many patients with rheumatoid arthritis. Despite the use of effective anti-inflammatory agents and disease modifying drugs, a significant proportion of patients with rheumatoid arthritis continue to have resistant disease. Complete clinical remission is unusual for more than six months and a formal cure of the disease remains elusive. In this report we focus on how attempts to address the question of why rheumatoid arthritis persists have led to a different interpretation of the pathogenesis of rheumatoid disease; one in which alterations in stromal cells such as fibroblasts play an important role in the switch from resolving to persistent disease.
Full Text
The Full Text of this article is available as a PDF (154.2 KB).
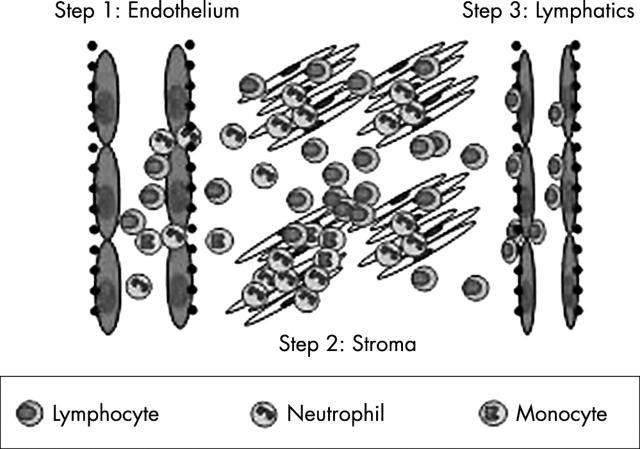
Figure 1.
Leucocyte–stromal interactions are involved at every stage in the trafficking of leucocytes into, within, and out of tissues. The molecular basis by which leucocytes leave the circulation and migrate across endothelium has been well studied (step 1, endothelium). How leucocytes interact with stromal cells (step 2) and how they exit tissue into the lymphatics (step 3) remains poorly understood.
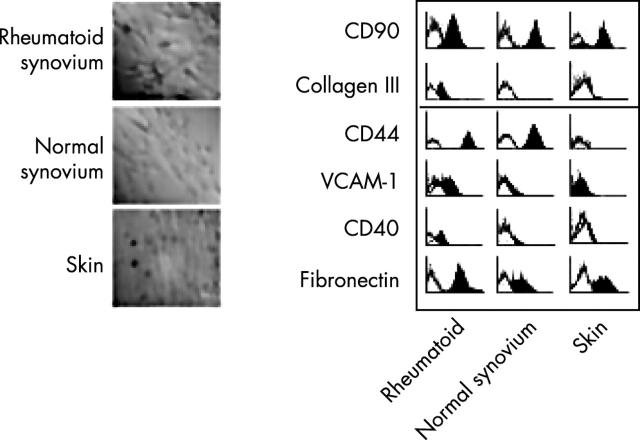
Figure 2.
Not all fibroblasts are the same. Fibroblasts from the rheumatoid, or normal synovium look different from fibroblasts from the skin and express different patterns of adhesion and matrix molecules compared with fibroblasts derived from the skin. Pictures (left) show fibroblasts labelled for fibronectin with nuclei counter stain. A range of markers (CD90, collagen III, CD44, VCAM-1 (CD106), CD40, and fibronectin) have been used to label either rheumatoid, normal synovium, or skin fibroblasts which have then been examined by flow cytometry.
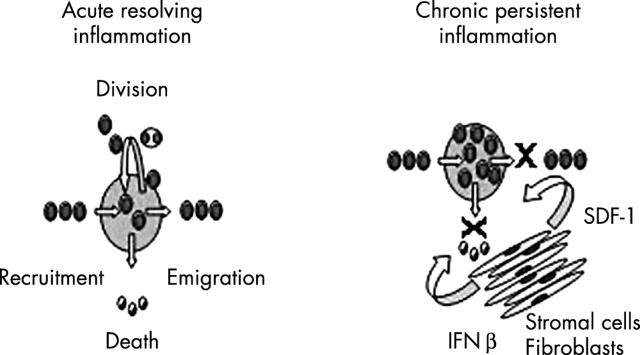
Figure 3.
The dynamics of the synovial inflammatory infiltrate. The dynamic balance of cells in any tissue compartment depends on the balance of cells that enter, exit, proliferate, or die. Homoeostasis is maintained during normal inflammatory responses leading to resolution of the inflammatory infiltrate. In chronic persistent inflammation inappropriate accumulation of leucocytes is caused by stromal production of prosurvival (interferon ß) and proretentive (stromal cell derived factor 1 (SDF-1); CXCL12) factors by fibroblasts and other resident tissue stromal cells.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Donnelly S. C., Peng T., Bucala R., Metz C. N. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001 Jun 15;166(12):7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997 Feb;18(2):72–76. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- Amft N., Curnow S. J., Scheel-Toellner D., Devadas A., Oates J., Crocker J., Hamburger J., Ainsworth J., Mathews J., Salmon M. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjögren's syndrome. Arthritis Rheum. 2001 Nov;44(11):2633–2641. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bianco P., Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000 Jun;105(12):1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady H. R., Lamas S., Papayianni A., Takata S., Matsubara M., Marsden P. A. Lipoxygenase product formation and cell adhesion during neutrophil-glomerular endothelial cell interaction. Am J Physiol. 1995 Jan;268(1 Pt 2):F1–12. doi: 10.1152/ajprenal.1995.268.1.F1. [DOI] [PubMed] [Google Scholar]
- Brouty-Boyé D., Pottin-Clémenceau C., Doucet C., Jasmin C., Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000 Mar;30(3):914–919. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Buckley C. D., Amft N., Bradfield P. F., Pilling D., Ross E., Arenzana-Seisdedos F., Amara A., Curnow S. J., Lord J. M., Scheel-Toellner D. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000 Sep 15;165(6):3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Buckley C. D. Michael Mason prize essay 2003. Why do leucocytes accumulate within chronically inflamed joints? Rheumatology (Oxford) 2003 Jun 27;42(12):1433–1444. doi: 10.1093/rheumatology/keg413. [DOI] [PubMed] [Google Scholar]
- Buckley C. D., Pilling D., Lord J. M., Akbar A. N., Scheel-Toellner D., Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001 Apr;22(4):199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- Buckley C. D. Science, medicine, and the future. Treatment of rheumatoid arthritis. BMJ. 1997 Jul 26;315(7102):236–238. doi: 10.1136/bmj.315.7102.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender D., Haskard D., Yu C. L., Iguchi T., Miossec P., Oppenheimer-Marks N., Ziff M. Pathways to chronic inflammation in rheumatoid synovitis. Fed Proc. 1987 Jan;46(1):113–117. [PubMed] [Google Scholar]
- Dooley M. A., Hogan S. L. Environmental epidemiology and risk factors for autoimmune disease. Curr Opin Rheumatol. 2003 Mar;15(2):99–103. doi: 10.1097/00002281-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Firestein G. S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996 Nov;39(11):1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Firestein Gary S. Evolving concepts of rheumatoid arthritis. Nature. 2003 May 15;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Gilroy Derek W., Lawrence Toby, Perretti Mauro, Rossi Adriano G. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004 May;3(5):401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Hogaboam C. M., Steinhauser M. L., Chensue S. W., Kunkel S. L. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int. 1998 Dec;54(6):2152–2159. doi: 10.1046/j.1523-1755.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- Jones Elena A., Kinsey Sally E., English Anne, Jones Richard A., Straszynski Liz, Meredith David M., Markham Alex F., Jack Andrew, Emery Paul, McGonagle Dennis. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002 Dec;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998 Oct;153(4):1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova-Mutafchieva L., Taylor P., Funa K., Maini R. N., Zvaifler N. J. Mesenchymal cells expressing bone morphogenetic protein receptors are present in the rheumatoid arthritis joint. Arthritis Rheum. 2000 Sep;43(9):2046–2055. doi: 10.1002/1529-0131(200009)43:9<2046::AID-ANR16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L., Blaser A., Kappeler A., Schärli P., Laissue J. A., Baggiolini M., Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999 Nov;104(10):R49–R54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner U., Gay R. E., Gay S. Cellular pathways of joint destruction. Curr Opin Rheumatol. 1997 May;9(3):213–220. doi: 10.1097/00002281-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Pap T., Müller-Ladner U., Gay R. E., Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000 Jun 8;2(5):361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage Greg, Falciani Francesco, Burman Angela, Filer Andrew, Ross Ewan, Bofill Margarita, Martin Stuart, Salmon Mike, Buckley Christopher D. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Haemost. 2003 Oct;90(4):688–697. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]
- Pilling D., Akbar A. N., Girdlestone J., Orteu C. H., Borthwick N. J., Amft N., Scheel-Toellner D., Buckley C. D., Salmon M. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999 Mar;29(3):1041–1050. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Salmon M., Scheel-Toellner D., Huissoon A. P., Pilling D., Shamsadeen N., Hyde H., D'Angeac A. D., Bacon P. A., Emery P., Akbar A. N. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997 Feb 1;99(3):439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon Mike, Akbar Arne N. Telomere erosion: a new link between HLA DR4 and rheumatoid arthritis? Trends Immunol. 2004 Jul;25(7):339–341. doi: 10.1016/j.it.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Silman Alan J. Commentary: Do genes or environment influence development of rheumatoid arthritis? BMJ. 2002 Feb 2;324(7332):264–264. [PubMed] [Google Scholar]
- Smith R. S., Smith T. J., Blieden T. M., Phipps R. P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997 Aug;151(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- Smolen Josef S., Steiner Günter. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003 Jun;2(6):473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- Vaday G. G., Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000 Feb;67(2):149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- Vallejo Abbe N., Weyand Cornelia M., Goronzy Jörg J. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004 Mar;10(3):119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Vallejo Abbe N., Yang Hongyu, Klimiuk Piotr A., Weyand Cornelia M., Goronzy Jörg J. Synoviocyte-mediated expansion of inflammatory T cells in rheumatoid synovitis is dependent on CD47-thrombospondin 1 interaction. J Immunol. 2003 Aug 15;171(4):1732–1740. doi: 10.4049/jimmunol.171.4.1732. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Graham G. J. Homing and mobilization in the stem cell niche. Trends Cell Biol. 1999 Jun;9(6):233–238. doi: 10.1016/s0962-8924(99)01559-7. [DOI] [PubMed] [Google Scholar]
- Zanelli E., Breedveld F. C., de Vries R. R. HLA association with autoimmune disease: a failure to protect? Rheumatology (Oxford) 2000 Oct;39(10):1060–1066. doi: 10.1093/rheumatology/39.10.1060. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J., Firestein G. S. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994 Jun;37(6):783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J., Marinova-Mutafchieva L., Adams G., Edwards C. J., Moss J., Burger J. A., Maini R. N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000 Aug 31;2(6):477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]