Abstract
Neural and stem cell transplantation is emerging as a potential treatment for neurodegenerative diseases. Transplantation of specific committed neuroblasts (fetal neurons) to the adult brain provides such scientific exploration of these new potential therapies. Huntington's disease (HD) is a fatal, incurable autosomal dominant (CAG repeat expansion of huntingtin protein) neurodegenerative disorder with primary neuronal pathology within the caudate–putamen (striatum). In a clinical trial of human fetal striatal tissue transplantation, one patient died 18 months after transplantation from cardiovascular disease, and postmortem histological analysis demonstrated surviving transplanted cells with typical morphology of the developing striatum. Selective markers of both striatal projection and interneurons such as dopamine and c-AMP-related phosphoprotein, calretinin, acetylcholinesterase, choline acetyltransferase, tyrosine hydroxylase, calbindin, enkephalin, and substance P showed positive transplant regions clearly innervated by host tyrosine hydroxylase fibers. There was no histological evidence of immune rejection including microglia and macrophages. Notably, neuronal protein aggregates of mutated huntingtin, which is typical HD neuropathology, were not found within the transplanted fetal tissue. Thus, although there is a genetically predetermined process causing neuronal death within the HD striatum, implanted fetal neural cells lacking the mutant HD gene may be able to replace damaged host neurons and reconstitute damaged neuronal connections. This study demonstrates that grafts derived from human fetal striatal tissue can survive, develop, and are unaffected by the disease process, at least for 18 months, after transplantation into a patient with HD.
Recent findings in genetics, stem cell biology, and neural transplantation suggest that brain repair will be possible for the treatment of neurodegenerative diseases (1, 2). Before initiating large clinical trials that test the efficacy of novel donor cells, it is important to determine the clinical feasibility of such cell-based therapies. Transplantation of specific committed neuroblasts (fetal neurons) to the adult human brain provides such a scientific exploration of feasibility of cell-based therapies.
The underlying genetic mutation of Huntington's disease (HD) is a polyglutamine repeat in the N-terminal region of the huntingtin gene (3). This mutation results in brain pathology dominated by massive neuronal loss of the medium spiny projection neurons of the caudate and putamen (4). Recent studies of HD postmortem brain tissue show that the N-terminal region of the mutant huntingtin protein aggregates in nuclear inclusions in both cortical and striatal neurons (5–7). These aggregates may represent evidence of ongoing cellular pathology (5–7). Implanted fetal neural cells lacking the mutant HD gene may be able to replace dead or dysfunctional host neurons and reconstitute disrupted neuronal connections (8, 9).
Physiological and anatomical evidence in animal studies shows that all striatal cell types can survive transplantation, grow, and establish functional afferent and efferent connections with the host brain (10–16). In animal models, behavioral signs analogous to HD (abnormal locomotion, chorea, dystonia, and subcortical dementia) can be improved by transplantation of embryonic striatal tissue into the degenerated striatum (10, 17–21). In primates, striatal allografts and xenografts have been shown to survive and improve motor function (19, 21). Several studies have demonstrated the viability and growth potential of human striatal cell implants using rodent models. For example, Wictorin (12) demonstrated long-distance growth of human striatal cells in a rodent model of HD. Critically, normal development of cellular components have been shown in cell implants derived from cell preparations identical to those used for this clinical trial (22).
Based on these studies and the fact that current therapies do not prevent the unrelenting clinical course of HD, a clinical trial of human fetal striatal tissue transplantation for the treatment of HD was initiated at the University of South Florida (9). In this series, one patient died 18 months after transplantation from causes unrelated to surgery. The current report provides the first demonstration that human fetal cells can survive and develop appropriately in the HD brain. The results also show that the expression of the mutant huntingtin protein in the host brain is confined to the host tissue and is not expressed in the genetically unrelated fetal graft, which conceptually supports the use of striatal tissue implantation as a novel therapy for patients with HD.
Methods
Patient Evaluation.
A 54-year-old male with HD was evaluated for 12 months preoperatively and 18 months postoperatively according to a modified CAPIT-HD protocol (23, 24). The diagnosis was confirmed genetically for CAG repeat length (25).
Donor Tissue Preparation and Transplantation.
The patient received cyclosporin (6 mg/kg/d p.o.; Sandoz Pharmaceutical) for 2 weeks before the first operation to 2 weeks after the second (contralateral) surgery, followed by 2 mg/kg/d p.o. for 5 more months (26). Surgical implant procedures were staged 1 month apart. Embryonic donors were obtained and stored using methods described (26). The developing striatum was dissected from the lateral half of the lateral ventricular eminence of donors 8–9 weeks postconception (9, 22, 27). Each striatal primordia was dissected into 0.5–1 mm3 pieces and deposited along a needle tract 9–15 mm in height. Three striata were transplanted stereotactically into the right putamen, and one was transplanted into the right caudate. The left side was implanted using similar technique with six striata from three donors transplanted into four sites in the putamen and two sites in the caudate nucleus. Deposits were separated by up to 5 mm in a three-dimensional array. An MRI scan performed after the left-sided procedure revealed a minor needle tract hemorrhage in one of the putaminal tracts. Surgical targeting of the left caudate nucleus proved to be difficult, due to its atrophy-associated intraoperative shift. Postoperative MRI scan revealed that both left caudate nucleus needle tracts were approximately 7 mm caudal to their planned target sites, which correlated with approximately 1 cm of hemispheric shrinkage (data not shown).
Postmortem Tissue Preparation and Histological Evaluation.
The patient died suddenly 18 months after surgery. At autopsy, there was evidence of acute and chronic aspiration, early bronchogenic pneumonia, severe (greater than 90%) three-vessel coronary artery disease without myocardial infarction, and severe peripheral vascular disease. The postmortem delay was 36 h, and the brain was stored at 4°C for the last 24 h. The brain was cut into 1.5-cm coronal sections, immersion-fixed in Zamboni's solution, and sliced to 40-μm-thick coronal sections as reported (28).
Series of adjacent sections were processed by immunohistochemical methodology described (29) for the visualization of dopamine and cAMP-associated receptor phosphoprotein (Research Biochemicals, Natick, MA) (dilution 1:20,000), calretinin (Swant, Bellinzona, Switzerland) (1:2,500), glial fibrillary acidic protein (Roche Molecular Biochemicals) (1:20), tyrosine hydroxylase (Pel-Freez Biologicals) (1:500), calbindin (Sigma) (1:2,500), 200-kDa human neurofilament (Biodesign International, Kennebunkport, ME) (1:50), enkephalin (Medicorp, Montréal, Canada) (1:50), substance P (Medicorp) (1:50), choline acetyltransferase (Chemicon) (1:250), huntingtin-associated protein (30) (1:500), EM-48 (6, 30) (1:3000), ubiquitin (Chemicon) (1:250) antibodies, acetylcholinesterase, and nicotinamide adenine dinucleotide phosphate diaphorase staining. Control sections were treated as above except that the primary antibody was omitted from the incubation medium.
Sections were digitally captured at 20× on a Zeiss Axioplan microscope and evaluated for the presence of transplanted embryonic human cells in Adobe photoshop 5.0 software. Percentage of areas containing striatal neuronal markers (see Fig. 1 b–k) were calculated using the National Institutes of Health Image program (version 1.61, using density slide function) for each stain in eight adjacent sections. Volumetric assessment was performed using Nissl-stained sections of the HD caudate–putamen, schematically illustrated in the three-dimensional reconstruction (Fig. 1a). Cell implant areas were multiplied by section thickness (40 μm) and the total number of sections comprising cell implants to obtain total volumes. The volumes of the five transplant sites within the caudate–putamen were also evaluated relative to previous volumetric measurements of the normal human caudate–putamen (31).
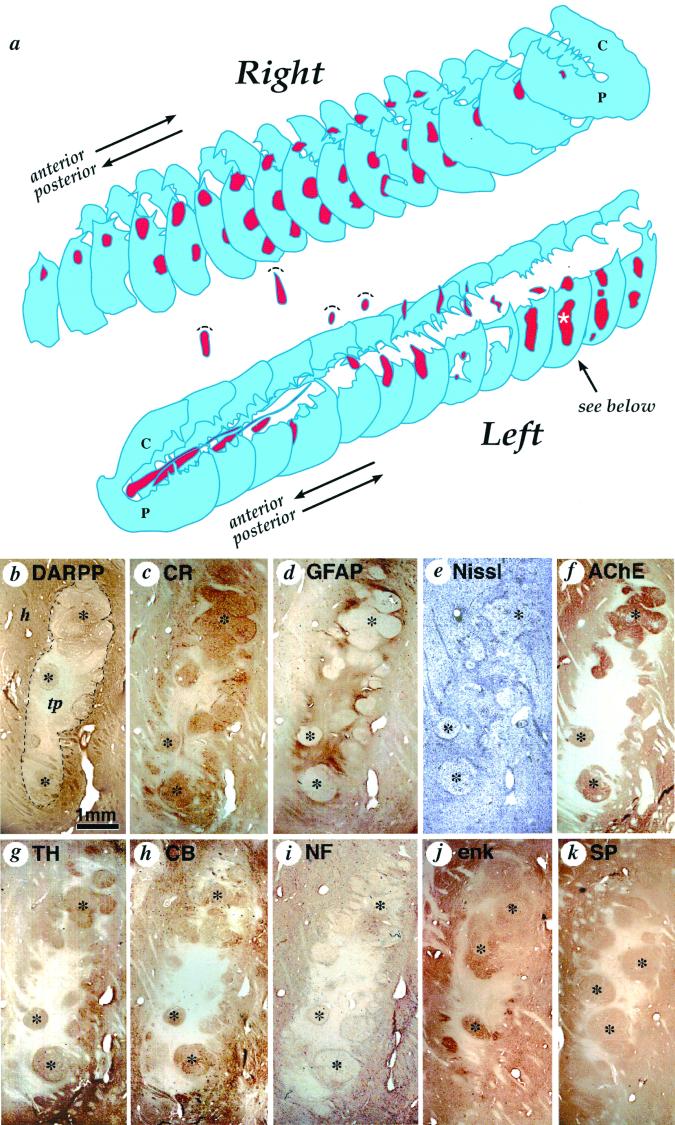
Figure 1.
(a) Three-dimensional schematic reconstruction of the patient's caudate nucleus and putamen illustrating the sites and relative sizes of bilateral human fetal cell implants as they appeared 18 months after implantation. Caudate nucleus and putamen cross-sections are shown in blue, and transplant tissue is shown in red. Two identified cell implants in the left putamen and three identified transplants in the right putamen were columnar in shape and oriented horizontally following bilateral frontal tracks. The rostral-most left hemisphere tract, intended for the caudate nucleus, was confined to the internal capsule. Volume of this implant was comparatively reduced and more cell dense than any other graft. (b–k) Serial sections through the most posterior graft in the left putamen (indicated by a white asterisk in a), stained with different neurohistological markers to reveal transplant architecture and immunohistochemically stained with antibodies characteristic of striatal neural cell phenotypes: (b) dopamine and cAMP-associated receptor phosphoprotein, (c) calretinin, (d) glial fibrillary acidic protein for astrocytes, (e) cresyl violet (Nissl) stain for general cell perikarya, (f) acetylcholinesterase staining for cholinergic cells and innervation (also see fig. 2a for choline acetyltransferase), (g) tyrosine hydroxylase staining for donor tissue axons, (h) calbindin, (i) 200-kDa human neurofilament (NF-200) for mature axons, (j) enkephalin, and (k) substance P. Cell implant cytoarchitecture (delineated by a dotted line in a) is characterized by two distinct tissue types: roughly spherical zones containing clusters of larger lower density neurons that are acetylcholinesterase-positive (f), glial fibrillary acidic protein-negative (d), and lightly neurofilament-positive (i) (marked by black stars); surrounded by regions of relatively high cell density that are acetylcholinesterase-negative, glial fibrillary acidic protein-negative -positive, and neurofilament-negative. The remaining photomicrographs identify overlapping classes of striatal phenotypes, whereas tyrosine hydroxylase stains host axons that specifically innervate striatal but not nonstriatal tissue. h, host; tp, transplant. (Scale bar = 1 mm.)
Results
Clinical Data.
Molecular genetic testing confirmed the diagnosis of HD. CAG trinucleotide allele sizes were 16 and 42 (±1) (25). During the year before surgery, the patient's clinical unified Huntington disease rating scale scores were 30, 33, 26, 36, and 38 (12, 9, 6, and 3 months before surgery and at the baseline examination before surgery). Apparent changes in chorea and balance (reduced scores) occurred by 3 days after the first of the bilateral procedures and persisted for the duration of the patient's life. Scores were 29, 30, 27, 28, 32, and 30 at 1, 3, 6, 9, 12, and 15 months, respectively, after transplantation. A detailed analysis of the clinical data will be reported separately for the entire group of transplanted HD patients (32).
Histological Evaluation.
Gross examination of the brain after autopsy showed significant ventricular enlargement (grade II) (33) due to the atrophy associated with neuronal loss in the caudate–putamen. A total of six transplant sites were identified, three in the right putamen, two in the left putamen, and one in the left anterior limb of the internal capsule (Fig. 1a). Based on histological examination and volumetric analysis, we estimated that the total volume of cell implants occupied 9.8% of the total volume of the remaining left caudate–putamen and 7.6% of the remaining right caudate–putamen. The largest of individual graft sites (Fig. 1a) was observed in the left putamen and measured about 45 mm3. None of the implants distorted the host striatal cytoarchitecture.
Clearly demarcated grafts were well integrated with the host tissue and lacked gliotic borders. All grafts showed similar tissue organization and were essentially composed of two distinct zones. The first zone was immunoreactive for markers selective of typical large- and medium-sized striatal neurons (29, 34), including dopamine and cAMP-associated receptor phosphoprotein (Fig. 1b), calretinin (Fig. 1c), acetylcholinesterase staining (Fig. 1f), calbindin (Figs. 1h and 2c), enkephalin (Fig. 1j), substance P (Fig. 1k), and glutamic acid decarboxylase and preproenkephalin (data not shown) and also contained cells displaying markers for striatal interneurons such as choline acetyltransferase (Fig. 2a), nicotinamide adenine dinucleotide phosphate diaphorase (Fig. 2b), and parvalbumin (data not shown). These zones of striatal neuronal markers occupied between 36 and 56% (mean 50%, SD 7.5%) of the surviving cell implant (Fig. 1 b–k). The other transplant zone lacked many of these neuronal markers. These areas contained sparse neurons interspersed with astrocytes as demonstrated by glial fibrillary acidic protein immunostaining (Fig. 1d). Host-derived dopaminergic fibers grew into the cell implant, as demonstrated by tyrosine hydroxylase immunoreactivity (Fig. 1g). These axons overlapped with graft-derived striatal-like tissue zones of the transplants as seen on adjacent sections. Because tract tracing studies were not possible in this material, definite evidence of neuritic outgrowth from the intrastriatal grafts to the adjacent globus pallidus could not be obtained.
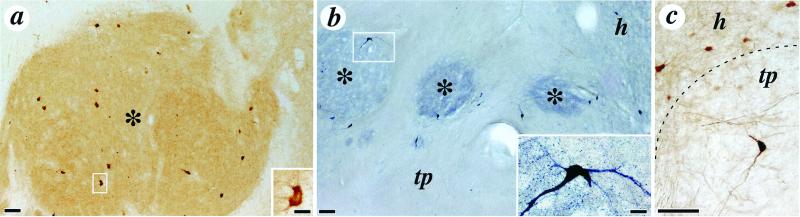
Figure 2.
Photomicrographs depicting distinctive striatal neuronal phenotypes within the cell implants. (a) Low power photomicrographs illustrating numerous choline acetyltransferase immunoreactive neurons within one striatal graft zone; the morphology of a choline acetyltransferase positive neuron is shown at higher magnification in the Inset. (b) Low-power photomicrograph illustrating nicotinamide adenine dinucleotide phosphate diaphorase immunoreactivity in the same transplant site. The typical morphology of these neurons is illustrated at higher magnification in the Inset. (c) Higher magnification of calbindin-immunoreactive neurons in the host (h) and within the transplant (tp). Striatal zones of the implants are indicated by asterisks in a and b, and the interface of the host and the transplant is delineated by the dotted line in c. (Scale bars: panels, 100 μm; Insets, 20 μm.)
Huntingtin-associated protein immunostaining was evident in both the graft and host tissue (data not shown). In contrast, EM-48, an antibody that recognizes the N-terminal region of the abnormal human huntingtin protein and specifically labels nuclear inclusions (7), was abundant in host tissue, but expression was minimal within the human fetal allograft (Fig. 3 a and b). These data were consistent with huntingtin nuclear inclusions in the host tissue using the anti-ubiquitin staining (Fig. 3c).
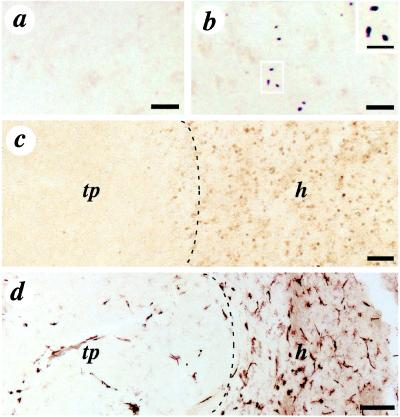
Figure 3.
Immunohistochemical staining for the mutant huntingtin protein (EM-48) (a and b), ubiquitin (nuclear inclusion marker) (c), and HLA-DR (microglia and macrophages marker) (d) in the transplant and the host tissue. EM-48 staining of mutant human huntingtin protein, specifically labeling nuclear inclusions, is abundant in the host tissue (b) but not in the transplant (a). Inclusions in b are illustrated at higher magnification in the Inset. (c) Low-power photomicrograph of ubiquitin staining in the host (h) and absence of staining in the transplant (tp) (interface of the transplant and the host indicated by the dotted line). The transplant (left) is clearly devoid of ubiquitin expression. (d) Low-power photomicrographs of HLA-DR staining (interface of the transplant and the host indicated by a dotted line). The transplant contains few HLA-DR positive cells (tp) compared with the host tissue (h), which contains numerous HLA-DR immunoreactive cells. (Scale bars: a and b, 50 μm; Inset, 25 μm; c, 500 μm; d, 100 μm.)
Macrophage and T-cell infiltration was evaluated with HLA-DR, CD-4, and CD-8 immunohistochemistry, respectively. There was a marked difference in HLA-DR (microglia or macrophages marker) expression between the host and the transplant. Whereas the degenerated HD host caudate–putamen showed considerable HLA-DR staining throughout (no increase at the interface of the cell implant), the cell implant contained much fewer HLA-DR positive cells than the host (Fig. 3d). There appeared to be little difference in T-helper cell density (CD-4), cytotoxic T-cell density (CD-8) (data not shown), and infiltration between the graft and host tissues, and there was a lack of perivascular cuffing.
Discussion
This study demonstrates that human fetal tissue derived from striatal primordia can survive transplantation into the putamen in a patient with HD. Notably, the disease process does not appear to induce HD-like neurodegeneration within the cell implants, and there is no evidence of immune rejection of the cell implants by the host.
These histological findings are consistent with previous results in both rodent and primate models of HD (19–21) as well as autopsy studies of human dopaminergic ventral mesencephalon allografts in patients with Parkinson's disease (28, 35, 36). In the latter studies, fetal neural allografts also survived in patients following withdrawal of immunosuppression for 12 months, and the disease process (Parkinson's disease) did not affect the grafts. A recent positron-emission tomography imaging study demonstrated survival and function of human fetal allograft in a Parkinson's patient for at least 10 years (37).
There is a correlation between the amount of neuronal loss in HD and the degree of caudate–putamen atrophy (33). In attempts to replace the striatal neurons lost to the disease process, the cell implants need to survive in sufficient numbers. In this study, we estimate that 10 striata from five fetal donors grew to a total transplant size similar to 5–10% of the total (left and right) normal human caudate–putamen tissue volume (31). Given that there was limited prior tissue loss of the caudate–putamen in this particular patient, the total transplant volume of 5–10% could reflect a reconstitution of this prior atrophy. Alternatively, it may represent poor cell survival due to cell preparation and surgical procedures.
Selective dissection of the fetal striatum always includes adjacent nonstriatal tissue (15, 20, 22, 27, 38). To determine the survival, internal organization, and morphology of the implanted fetal striatal cells, specific cellular and molecular markers can be used to distinguish between transplanted striatal and nonstriatal cells. Striatal zones within each transplant are believed to correspond to selective aggregation of striatal cells, interspersed with tissue derived from other brain regions such as cortex, pallidum, and amygdala (16, 22, 27, 38, 39). These nonstriatal regions are also present in the proliferative layers of the embryonic ventricular eminence. In the present study, 50%, on average, of surviving cell implants were composed of phenotypically normal striatal tissue. This is comparable to the percentage of striatal tissue zones demonstrated in rat-to-rat, pig-to-rat, and primate-to-primate cell transplant studies using the fetal striatal primordia as a donor source (12–14, 20, 21, 40, 42) and human-to-rat transplant studies using selective dissection of the developing striatum (22) similar to those used in the present study. In summary, transplants contained cells and an organization typical of the developing caudate–putamen (27, 39).
Implants may be beneficial through several cellular mechanisms. In animal models, the proposed mechanisms of transplant-induced improvement observed include graft-derived transmitter release as well as the production of trophic factors (11, 13, 16, 43–46). Previous animal studies have demonstrated reconnection of the cortical, thalamic and dopaminergic system with implanted fetal striatum (12, 13, 40, 47). We demonstrate here that host neuronal systems that normally project to the striatum (for example dopaminergic neurons) can grow into the human striatal tissue grafts. The dopaminergic fiber growth from the host was observed predominantly within the striatal zones of the transplants and therefore likely represent appropriate dopaminergic growth into caudate–putamen regions. Similar observations of host dopaminergic axonal reconnections have been reported in rodent and human striatal allograft and xenograft models (12–14, 20, 22, 40–42). Although it has been previously demonstrated that experimental human striatal fetal xenografts produce neuritic extensions to the rodent host brain (12, 22), we cannot demonstrate efferent connectivity at autopsy because such specific efferent tract labeling could not be reliably performed in the postmortem human tissue. Other important therapeutic actions may be the protection of striatal host neurons from further degeneration by neuronal or glial production of various growth factors provided by the implanted cells (44, 46–50). Further, animal studies have demonstrated that only fetal striatal tissue (as compared with control grafts) is able to restore functional globus pallidus γ-aminobutyric acid (GABA) release and neuronal firing and reduce behavioral deficits typical of striatal lesions (13, 16, 43, 51). Finally, recent studies demonstrate that animals with brain lesions can learn to use the implanted neural cells (52), which may provide a behavioral mechanism for sustained functional recovery.
In HD, striatal neurons have a heightened sensitivity to the mutant form of the huntingtin protein (6). Using an antibody recognizing the human huntingtin-associated protein fragment (30), the normal protein was evident within the graft as well as surrounding host tissue. However, the specific EM-48 antibody (6, 7) and ubiquitin that recognize the abnormal neuronal nuclear inclusions were only visualized within the host brain (Fig. 3b) and not within the transplants (Fig. 3 a and c). It appears that transplanted cells (lacking the disease gene) are not affected by the HD disease process at least 18 months after surgery. The present findings are consistent with the view that implanted fetal cells are not vulnerable to the disease processes seen in neurodegenerative diseases (28, 36, 37).
Cell transplantation into the brain typically induces less immunological response than in peripheral sites (53). This may also be true in this study, where minimal macrophage and T-cell immunoreactivity was observed by HLA-DR, CD-4, and CD-8 immunohistochemistry within the graft. There was no evidence of perivascular cuffing, T-cell infiltration (data not shown), or the appearance of degenerating implanted neurons. Cyclosporine administered for only 6 months postoperatively was sufficient for graft survival, supporting previous findings of brain neural allograft survival in the presence of short-term immunosuppression (35) or even no immunosuppression (54).
This study supports the use of implanted fetal striatal tissue as a possible treatment for HD. Preliminary data from the cohort of seven patients involved in this open-label trial demonstrates that human fetal striatal grafts may at least provide short-term clinical benefit (32). This is also consistent with recent clinical work (Dr. Peschanski, personal communication) demonstrating similar short-term improvement, as well as recovery of cerebral metabolic function in patients with HD following fetal striatal transplantation. Further development of this transplant methodology with appropriate and abundant donor cells, such as neuronal stem and progenitor cells, may provide hope for functional brain repair in the treatment of HD.
Acknowledgments
We thank Dr. Thomas Mueller for his assistance with genetic testing. These studies were supported in part by National Institute of Neurological Disorders and Stroke Grant NS-30064, Tampa General Healthcare, the Hereditary Disease Foundation, the International Organization of Glutaric Acidemia, and Fonds de la Recherche en Santé du Québec.
Abbreviation
- HD
Huntington's disease
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dunnett S B. Neuropathol Appl Neurobiol. 1999;25:351–362. doi: 10.1046/j.1365-2990.1999.00207.x. [DOI] [PubMed] [Google Scholar]
- 2.Shihabuddin L S, Palmer T D, Gage F H. Mol Med Today. 1999;5:474–480. doi: 10.1016/s1357-4310(99)01596-8. [DOI] [PubMed] [Google Scholar]
- 3.The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 4.Ferrante R J, Kowall N W, Richardson E J., Jr J Neurosci. 1991;11:3877–3887. doi: 10.1523/JNEUROSCI.11-12-03877.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiFiglia M, Sapp E, Chase K O, Davies S W, Bates G P, Vonsattel J P, Aronin N. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 6.Li S H, Li X J. Hum Mol Genet. 1998;7:777–782. doi: 10.1093/hmg/7.5.777. [DOI] [PubMed] [Google Scholar]
- 7.Gutekunst C A, Li S H, Yi H, Mulroy J S, Kuemmerle S, Jones R, Rye D, Ferrante R J, Hersh S M, Li X J. J Neurosci. 1999;19:2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque N S, Borghesani P, Isacson O. Mol Med Today. 1997;3:175–183. doi: 10.1016/S1357-4310(97)01012-5. [DOI] [PubMed] [Google Scholar]
- 9.Freeman T B, Hauser R A, Willing A E, Zigova T, Sanberg P R, Saporta S. Neural Transplantation in Neurodegenerative Disease. 2000. Novartis Foundation Symposium 231 (Wiley, Chichester, U.K.), in press. [Google Scholar]
- 10.Isacson O, Brundin P, Kelly P A, Gage F H, Björklund A. Nature (London) 1984;311:458–460. doi: 10.1038/311458a0. [DOI] [PubMed] [Google Scholar]
- 11.Isacson O, Brundin P, Gage F H, Björklund A. Neuroscience. 1985;16:799–817. doi: 10.1016/0306-4522(85)90095-8. [DOI] [PubMed] [Google Scholar]
- 12.Wictorin K. Prog Neurobiol. 1992;38:611–639. doi: 10.1016/0301-0082(92)90044-f. [DOI] [PubMed] [Google Scholar]
- 13.Campbell K, Kalen P, Wictorin K, Lundberg C, Mandel R J, Björklund A. Neuroscience. 1993;53:403–415. doi: 10.1016/0306-4522(93)90204-s. [DOI] [PubMed] [Google Scholar]
- 14.Sirinathsinghji D J, Heavens R P, Torres E M, Dunnett S B. Neuroscience. 1993;53:651–663. doi: 10.1016/0306-4522(93)90613-k. [DOI] [PubMed] [Google Scholar]
- 15.Isacson O, Deacon T W, Pakzaban P, Galpern W R, Dinsmore J, Burns L H. Nat Med. 1995;1:1189–1194. doi: 10.1038/nm1195-1189. [DOI] [PubMed] [Google Scholar]
- 16.Nakao N, Ogura M, Nakai K, Itakura T. Neuroscience. 1999;88:469–477. doi: 10.1016/s0306-4522(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 17.Deckel A W, Rominson R G, Coyle J T, Sanberg P R. Eur J Pharmacol. 1983;93:287–288. doi: 10.1016/0014-2999(83)90150-4. [DOI] [PubMed] [Google Scholar]
- 18.Sanberg P R, Henault M A, Deckel A W. Pharmacol Biochem Behav. 1986;25:297–300. doi: 10.1016/0091-3057(86)90269-8. [DOI] [PubMed] [Google Scholar]
- 19.Hantraye P, Riche D, Maziere M, Isacson O. Proc Natl Acad Sci USA. 1992;89:4187–4191. doi: 10.1073/pnas.89.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunnett S B, Svendsen C N. Curr Opin Neurobiol. 1993;3:790–796. doi: 10.1016/0959-4388(93)90155-r. [DOI] [PubMed] [Google Scholar]
- 21.Kendall A L, Rayment F D, Torres E M, Baker H F, Ridley R M, Dunnett S B. Nat Med. 1998;4:727–729. doi: 10.1038/nm0698-727. [DOI] [PubMed] [Google Scholar]
- 22.Freeman T B, Randall T S, Saporta S, Othberg A I, Nauert G M, Wiling A E, Scott D L, Sanberg P R. Exp Neurol. 1998;158:390. [Google Scholar]
- 23.Kieburtz K, MacDonald M, Shih C, Feigin A, Steinberg K, Bordwell K, Zimmerman C, Srinidhi J, Sotack J, Gusella J, et al. J Med Genet. 1994;31:872–874. doi: 10.1136/jmg.31.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn N, Brown R, Craufurd D, Goldman S, Hodges J, Kieburtz K, Lindvall O, MacMillan J, Roos R. Movement Disorders. 1996;11:143–150. doi: 10.1002/mds.870110205. [DOI] [PubMed] [Google Scholar]
- 25.Warner J P, Barron L H, Brock D J. Mol Cell Probes. 1993;7:235–239. doi: 10.1006/mcpr.1993.1034. [DOI] [PubMed] [Google Scholar]
- 26.Freeman T B, Olanow C W, Hauser R A, Nauert G M, Smith D A, Borlongan C V, Sanberg P R, Holt D A, Kordower J H, Vingerhoets F J G, et al. Ann Neurol. 1995;38:379–388. doi: 10.1002/ana.410380307. [DOI] [PubMed] [Google Scholar]
- 27.Freeman T B, Sanberg P R, Isacson O. Cell Transplant. 1995;4:539–545. doi: 10.1177/096368979500400604. [DOI] [PubMed] [Google Scholar]
- 28.Kordower J H, Freeman T B, Snow B J, Vingerhoets F J G, Mufson E J, Sanberg P R, Hauser R A, Smith D A, Nauert G M, Perl D M, et al. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 29.Cicchetti F, Beach T G, Parent A. Synapse. 1998;30:284–297. doi: 10.1002/(SICI)1098-2396(199811)30:3<284::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Li S H, Hossenin S H, Gutekunst C A, Ferrante R J, Hersch S M, Li X J. J Biol Chem. 1998;273:19220–19227. doi: 10.1074/jbc.273.30.19220. [DOI] [PubMed] [Google Scholar]
- 31.von Bonin G, Sharrif G A. J Comp Neurol. 1951;94:427–438. doi: 10.1002/cne.900940306. [DOI] [PubMed] [Google Scholar]
- 32.Hauser R A, Stoessl J A, Eichler S R, Schwartz S W, Sanberg P R, Saporta S, Nauert G M, Randall T S, Hahn M A, Scott D L, et al. Neurology. 2000;64:A153. [Google Scholar]
- 33.Vonsattel J P, Myers R H, Stevens T J, Ferrante R J, Bird E D, Richardson E P., Jr J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Kawagushi Y, Wilson C J, Augood S J, Emson P C. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 35.Kordower J H, Styren S, Clarke M, DeKosky S T, Olanow C W, Freeman T B. Cell Transplant. 1997;6:213–219. doi: 10.1177/096368979700600304. [DOI] [PubMed] [Google Scholar]
- 36.Kordower J H, Freeman T B, Chen E Y, Mufson E J, Sanberg P R, Hauser R A, Snow B, Olanow C W. Movement Disorders. 1998;13:383–393. doi: 10.1002/mds.870130303. [DOI] [PubMed] [Google Scholar]
- 37.Piccini P, Brooks D J, Björklund A, Gunn R N, Grasby P M, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, et al. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 38.Pakzaban P, Deacon T W, Burns L H, Isacson O. Exp Brain Res. 1993;97:13–22. doi: 10.1007/BF00228813. [DOI] [PubMed] [Google Scholar]
- 39.Deacon T W, Pakzaban P, Isacson O. Brain Res. 1994;668:211–219. doi: 10.1016/0006-8993(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 40.Pritzel M, Isacson O, Brundin P, Wiklund L, Björklund A. Exp Brain Res. 1986;65:112–126. doi: 10.1007/BF00243834. [DOI] [PubMed] [Google Scholar]
- 41.Labandeira-Garcia J L, Wictorin K, Cunningham E T, Jr, Björklund A. Neuroscience. 1991;42:407–426. doi: 10.1016/0306-4522(91)90385-2. [DOI] [PubMed] [Google Scholar]
- 42.Liu F C, Dunnett S B, Graybiel A M. J Neurosci. 1992;12:4281–4297. doi: 10.1523/JNEUROSCI.12-11-04281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirinathsinghji D J, Dunnett S B, Isacson O, Clarke D J, Kendrick K, Björklund A. Neuroscience. 1988;24:803–811. doi: 10.1016/0306-4522(88)90068-1. [DOI] [PubMed] [Google Scholar]
- 44.Levivier M, Gash D M, Przedborski S. Neuroscience. 1995;69:43–50. doi: 10.1016/0306-4522(95)00230-g. [DOI] [PubMed] [Google Scholar]
- 45.Sanberg P R, Borlongan C V, Koutouzis T K, Norgren R B, Jr, Cahill D W, Freeman T B. Ann NY Acad Sci. 1997;831:452–460. doi: 10.1111/j.1749-6632.1997.tb52217.x. [DOI] [PubMed] [Google Scholar]
- 46.Emerich D F, Bruhn S, Chu Y, Kordower J H. Cell Transplant. 1998;7:213–225. doi: 10.1177/096368979800700215. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Martin E, Caruncho H J, Rodrigez-Pallares J, Guerra M J, Labandeira-Garcia J L. J Comp Neurol. 1999;406:199–206. doi: 10.1002/(sici)1096-9861(19990405)406:2<199::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 48.Schumacher J M, Short M D, Hyman B T, Breakfield X O, Isacson O. Neuroscience. 1991;45:561–570. doi: 10.1016/0306-4522(91)90271-o. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Serrano A, Björklund A. J Neurosci. 1996;16:4604–4616. doi: 10.1523/JNEUROSCI.16-15-04604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kordower J H, Isacson O, Emerich D F. Exp Neurol. 1999;159:4–20. doi: 10.1006/exnr.1999.7156. [DOI] [PubMed] [Google Scholar]
- 51.Caruncho H J, Rodriguez-Pallares J, Guerra M J, Labandeira-Garcia J L. Brain Res Mol Brain Res. 1998;57:301–309. doi: 10.1016/s0169-328x(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 52.Brasted P J, Watts C, Robbins T W, Dunnett S B. Proc Natl Acad Sci USA. 1999;96:10524–10529. doi: 10.1073/pnas.96.18.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isacson O, Deacon T W, Schumacher J M. In: CNS Regeneration: Basic Science and Clinical Advances. Tuszynski M, Kordower J, editors. San Diego: Academic; 1999. pp. 365–387. [Google Scholar]
- 54.Freed C R, Breeze R E, Schneck S A. N Engl J Med. 1995;333:730–731. doi: 10.1056/NEJM199509143331112. [DOI] [PubMed] [Google Scholar]