Abstract
The transcription factor nuclear factor (NF)-κB is well recognised as a pivotal player in osteoclastogenesis and inflammation induced bone loss. Here, the authors discuss their recent results, obtained using a genetic approach in mice, that indicate the importance of IKKß, and not IKKα, as a transducer of signals from receptor activator of NF-κB (RANK) to NF-κB. Ablation of IKKß results in lack of osteoclastogenesis and unresponsiveness of IKKß deficient mice to inflammation induced bone loss. In the need of a more effective therapy for the treatment of inflammatory diseases causing bone resorption, specific inhibition of IKKß represents a logical alternative strategy to the current therapies.
Full Text
The Full Text of this article is available as a PDF (86.2 KB).
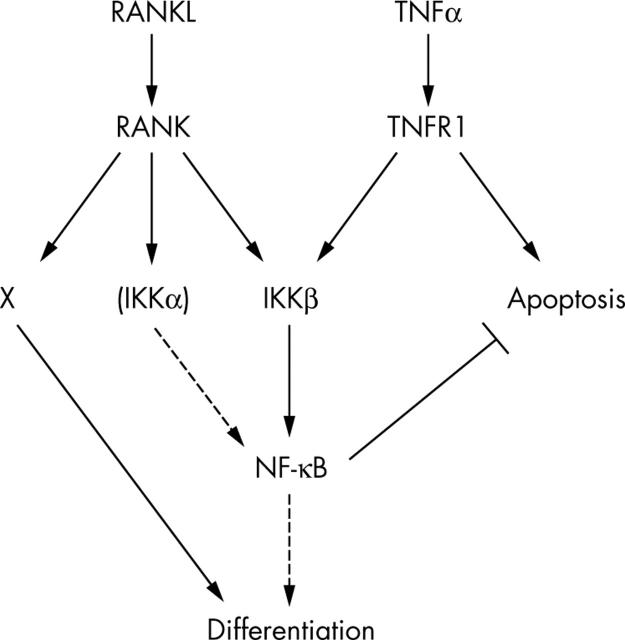
Figure 1.
Schematic model of receptor activator of nuclear factor (NF)-κB ligand (RANKL) and tumour necrosis factor α (TNFα) signalling during osteoclastogenesis and inflammation induced bone loss. X, a pathway other than IκB kinase (IKK)/NF-κB that is activated by RANKL binding to RANK and is essential for production of functional osteoclasts. IKKα function in RANK signalling is dispensable. TNFR1, TNF receptor 1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Amer Y., Erdmann J., Alexopoulou L., Kollias G., Ross F. P., Teitelbaum S. L. Tumor necrosis factor receptors types 1 and 2 differentially regulate osteoclastogenesis. J Biol Chem. 2000 Sep 1;275(35):27307–27310. doi: 10.1074/jbc.M003886200. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997 Apr 10;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bathon J. M., Martin R. W., Fleischmann R. M., Tesser J. R., Schiff M. H., Keystone E. C., Genovese M. C., Wasko M. C., Moreland L. W., Weaver A. L. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000 Nov 30;343(22):1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Bolon B., Campagnuolo G., Feige U. Duration of bone protection by a single osteoprotegerin injection in rats with adjuvant-induced arthritis. Cell Mol Life Sci. 2002 Sep;59(9):1569–1576. doi: 10.1007/s00018-002-8530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi Giuseppina, Karin Michael. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004 Jun;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Boyle William J., Simonet W. Scott, Lacey David L. Osteoclast differentiation and activation. Nature. 2003 May 15;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Emery P., Keystone E., Patel K., Furst D. E., Kalden J. R., St Clair E. W., Weisman M., Smolen J., Lipsky P. E. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004 Feb;63(2):149–155. doi: 10.1136/ard.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnuolo Giuseppe, Bolon Brad, Feige Ulrich. Kinetics of bone protection by recombinant osteoprotegerin therapy in Lewis rats with adjuvant arthritis. Arthritis Rheum. 2002 Jul;46(7):1926–1936. doi: 10.1002/art.10369. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Gerondakis S., O'Donnell K., Wicks I. P. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest. 2000 Jun;105(12):1799–1806. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. K., O'Donnell K., Lawlor K. E., Wicks I. P. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF. J Clin Invest. 2001 Jun;107(12):1519–1527. doi: 10.1172/JCI12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah M., Fitzgerald C., Alvarez U., Hruska K. c-Src is required for stimulation of gelsolin-associated phosphatidylinositol 3-kinase. J Biol Chem. 1998 May 8;273(19):11908–11916. doi: 10.1074/jbc.273.19.11908. [DOI] [PubMed] [Google Scholar]
- Danning C. L., Illei G. G., Hitchon C., Greer M. R., Boumpas D. T., McInnes I. B. Macrophage-derived cytokine and nuclear factor kappaB p65 expression in synovial membrane and skin of patients with psoriatic arthritis. Arthritis Rheum. 2000 Jun;43(6):1244–1256. doi: 10.1002/1529-0131(200006)43:6<1244::AID-ANR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dougall W. C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M. E., Maliszewski C. R. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999 Sep 15;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L. T., Lakkakorpi P. T., Nakamura I., Machwate M., Nagy R. M., Rodan G. A. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998 Sep 1;102(5):881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Epinat J. C., Gilmore T. D. Diverse agents act at multiple levels to inhibit the Rel/NF-kappaB signal transduction pathway. Oncogene. 1999 Nov 22;18(49):6896–6909. doi: 10.1038/sj.onc.1203218. [DOI] [PubMed] [Google Scholar]
- Foxwell B., Browne K., Bondeson J., Clarke C., de Martin R., Brennan F., Feldmann M. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci U S A. 1998 Jul 7;95(14):8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G., Carlson L., Xing L., Poljak L., Shores E. W., Brown K. D., Leonardi A., Tran T., Boyce B. F., Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997 Dec 15;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese Mark C., Bathon Joan M., Martin Richard W., Fleischmann Roy M., Tesser John R., Schiff Michael H., Keystone Edward C., Wasko Mary Chester, Moreland Larry W., Weaver Arthur L. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002 Jun;46(6):1443–1450. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- Ghosh S., May M. J., Kopp E. B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gravallese E. M. Bone destruction in arthritis. Ann Rheum Dis. 2002 Nov;61 (Suppl 2):ii84–ii86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H., Shu H. B., Pan M. G., Goeddel D. V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996 Jan 26;84(2):299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Hu Y., Baud V., Delhase M., Zhang P., Deerinck T., Ellisman M., Johnson R., Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999 Apr 9;284(5412):316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Hu Y., Baud V., Oga T., Kim K. I., Yoshida K., Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001 Apr 5;410(6829):710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Iotsova V., Caamaño J., Loy J., Yang Y., Lewin A., Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997 Nov;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Ji Hong, Pettit Allison, Ohmura Koichiro, Ortiz-Lopez Adriana, Duchatelle Veronique, Degott Claude, Gravallese Ellen, Mathis Diane, Benoist Christophe. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002 Jul 1;196(1):77–85. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi Eijiro, Aoki Kazuhiro, Saito Hiroaki, D'Acquisto Fulvio, May Michael J., Nakamura Ichiro, Sudo Testuo, Kojima Takefumi, Okamoto Fujio, Fukushima Hidefumi. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004 May 23;10(6):617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin Michael, Lin Anning. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002 Mar;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Karsenty Gerard, Wagner Erwin F. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002 Apr;2(4):389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Takahashi N., Jimi E., Udagawa N., Takami M., Kotake S., Nakagawa N., Kinosaki M., Yamaguchi K., Shima N. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000 Jan 17;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. Y., Feige U., Sarosi I., Bolon B., Tafuri A., Morony S., Capparelli C., Li J., Elliott R., McCabe S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 Nov 18;402(6759):304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999 Jan 28;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Kremer Joel M., Weinblatt Michael E., Bankhurst Arthur D., Bulpitt Ken J., Fleischmann Roy M., Jackson Christopher G., Atkins Kelly M., Feng Anyang, Burge Daniel J. Etanercept added to background methotrexate therapy in patients with rheumatoid arthritis: continued observations. Arthritis Rheum. 2003 Jun;48(6):1493–1499. doi: 10.1002/art.11142. [DOI] [PubMed] [Google Scholar]
- Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998 Apr 17;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lam J., Takeshita S., Barker J. E., Kanagawa O., Ross F. P., Teitelbaum S. L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000 Dec;106(12):1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence Toby, Willoughby Derek A., Gilroy Derek W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002 Oct;2(10):787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Lee S. E., Woo K. M., Kim S. Y., Kim H. M., Kwack K., Lee Z. H., Kim H. H. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002 Jan;30(1):71–77. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- Leisen J. C., Duncan H., Riddle J. M., Pitchford W. C. The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J Rheumatol. 1988 Jan;15(1):17–22. [PubMed] [Google Scholar]
- Li Q., Van Antwerp D., Mercurio F., Lee K. F., Verma I. M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999 Apr 9;284(5412):321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Li Z. W., Chu W., Hu Y., Delhase M., Deerinck T., Ellisman M., Johnson R., Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999 Jun 7;189(11):1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., van der Heijde D. M., St Clair E. W., Furst D. E., Breedveld F. C., Kalden J. R., Smolen J. S., Weisman M., Emery P., Feldmann M. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000 Nov 30;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Liu Z. G., Hsu H., Goeddel D. V., Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996 Nov 1;87(3):565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Hutchinson N. I., Parsons J. N., Bayne E. K., Tocci M. J. Analysis of IL-1 and TNF-alpha gene expression in human rheumatoid synoviocytes and normal monocytes by in situ hybridization. J Immunol. 1990 Dec 15;145(12):4154–4166. [PubMed] [Google Scholar]
- Maeda Shin, Chang Lufen, Li Zhi-Wei, Luo Jun-Li, Leffert Hyam, Karin Michael. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003 Nov;19(5):725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- Maini R. N., Breedveld F. C., Kalden J. R., Smolen J. S., Davis D., Macfarlane J. D., Antoni C., Leeb B., Elliott M. J., Woody J. N. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Maini R., St Clair E. W., Breedveld F., Furst D., Kalden J., Weisman M., Smolen J., Emery P., Harriman G., Feldmann M. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999 Dec 4;354(9194):1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Zhu H., Murray B. W., Shevchenko A., Bennett B. L., Li J., Young D. B., Barbosa M., Mann M., Manning A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997 Oct 31;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Miagkov A. V., Kovalenko D. V., Brown C. E., Didsbury J. R., Cogswell J. P., Stimpson S. A., Baldwin A. S., Makarov S. S. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland L. W., Baumgartner S. W., Schiff M. H., Tindall E. A., Fleischmann R. M., Weaver A. L., Ettlinger R. E., Cohen S., Koopman W. J., Mohler K. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997 Jul 17;337(3):141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Schiff M. H., Baumgartner S. W., Tindall E. A., Fleischmann R. M., Bulpitt K. J., Weaver A. L., Keystone E. C., Furst D. E., Mease P. J. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999 Mar 16;130(6):478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- Mostov K., Werb Z. Journey across the osteoclast. Science. 1997 Apr 11;276(5310):219–220. doi: 10.1126/science.276.5310.219. [DOI] [PubMed] [Google Scholar]
- Nakashima Tomoki, Wada Teiji, Penninger Josef M. RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol. 2003 May;15(3):280–287. doi: 10.1097/00002281-200305000-00016. [DOI] [PubMed] [Google Scholar]
- Novack Deborah Veis, Yin Li, Hagen-Stapleton Amanda, Schreiber Robert D., Goeddel David V., Ross F. Patrick, Teitelbaum Steven L. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003 Aug 25;198(5):771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit A. R., Ji H., von Stechow D., Müller R., Goldring S. R., Choi Y., Benoist C., Gravallese E. M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001 Nov;159(5):1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H. Bone morphogenesis and modeling: soluble signals sculpt osteosomes in the solid state. Cell. 1997 Apr 18;89(2):159–161. doi: 10.1016/s0092-8674(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Redlich Kurt, Hayer Silvia, Ricci Romeo, David Jean-Pierre, Tohidast-Akrad Makiyeh, Kollias George, Steiner Günter, Smolen Josef S., Wagner Erwin F., Schett Georg. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002 Nov;110(10):1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas Evan, Sims Natalie A., Hards Daphne K., Lindsay Mandy, Quinn Julian W. M., Ryan Peter F. J., Dunstan Colin R., Martin T. John, Gillespie Matthew T. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002 Oct;161(4):1419–1427. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman G. D. Cell biology of the osteoclast. Exp Hematol. 1999 Aug;27(8):1229–1241. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- Ruocco Maria Grazia, Maeda Shin, Park Jin Mo, Lawrence Toby, Hsu Li-Chung, Cao Yixue, Schett Georg, Wagner Erwin F., Karin Michael. I{kappa}B kinase (IKK){beta}, but not IKK{alpha}, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005 May 16;201(10):1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Selleri C., Anderson S., Young N. S., Maciejewski J. P. Expression and modulation of cellular receptors for interferon-gamma, tumour necrosis factor, and Fas on human bone marrow CD34+ cells. Br J Haematol. 1997 May;97(2):356–365. doi: 10.1046/j.1365-2141.1997.562704.x. [DOI] [PubMed] [Google Scholar]
- Schett Georg, Redlich Kurt, Hayer Silvia, Zwerina Jochen, Bolon Brad, Dunstan Colin, Görtz Birgit, Schulz Andreas, Bergmeister Helga, Kollias Giorgos. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003 Jul;48(7):2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- Seetharaman R., Mora A. L., Nabozny G., Boothby M., Chen J. Essential role of T cell NF-kappa B activation in collagen-induced arthritis. J Immunol. 1999 Aug 1;163(3):1577–1583. [PubMed] [Google Scholar]
- Senftleben U., Cao Y., Xiao G., Greten F. R., Krähn G., Bonizzi G., Chen Y., Hu Y., Fong A., Sun S. C. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001 Aug 24;293(5534):1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Senftleben U., Li Z. W., Baud V., Karin M. IKKbeta is essential for protecting T cells from TNFalpha-induced apoptosis. Immunity. 2001 Mar;14(3):217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet. 1999 May;22(1):74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997 Apr 18;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nishikaku F., Nakatuka M., Koga Y. Osteoclast-like cells in murine collagen induced arthritis. J Rheumatol. 1998 Jun;25(6):1154–1160. [PubMed] [Google Scholar]
- Tak P. P., Firestein G. S. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001 Jan;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Amling M., Neff L., Peyman A., Uhlmann E., Levy J. B., Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. 1996 Oct 10;383(6600):528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Kremer J. M., Bankhurst A. D., Bulpitt K. J., Fleischmann R. M., Fox R. I., Jackson C. G., Lange M., Burge D. J. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999 Jan 28;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Weinblatt Michael E., Keystone Edward C., Furst Daniel E., Moreland Larry W., Weisman Michael H., Birbara Charles A., Teoh Leah A., Fischkoff Steven A., Chartash Elliot K. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003 Jan;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- Wong B. R., Besser D., Kim N., Arron J. R., Vologodskaia M., Hanafusa H., Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999 Dec;4(6):1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- Zandi E., Rothwarf D. M., Delhase M., Hayakawa M., Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997 Oct 17;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Heulsmann A., Tondravi M. M., Mukherjee A., Abu-Amer Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001 Jan 5;276(1):563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- Zou W., Hakim I., Tschoep K., Endres S., Bar-Shavit Z. Tumor necrosis factor-alpha mediates RANK ligand stimulation of osteoclast differentiation by an autocrine mechanism. 2001 Jun 26-Jul 25J Cell Biochem. 83(1):70–83. doi: 10.1002/jcb.1202. [DOI] [PubMed] [Google Scholar]
- Zwerina Jochen, Hayer Silvia, Tohidast-Akrad Makiyeh, Bergmeister Helga, Redlich Kurt, Feige Ulrich, Dunstan Colin, Kollias Giorgos, Steiner Günter, Smolen Josef. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004 Jan;50(1):277–290. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]
- den Broeder A. A., Joosten L. A. B., Saxne T., Heinegård D., Fenner H., Miltenburg A. M. M., Frasa W. L. H., van Tits L. J., Buurman W. A., van Riel P. L. C. M. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002 Apr;61(4):311–318. doi: 10.1136/ard.61.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Broeder Alfons, van de Putte Leo, Rau Rolf, Schattenkirchner Manfred, Van Riel Piet, Sander Oliver, Binder Christina, Fenner Helmut, Bankmann Yvonne, Velagapudi Raja. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002 Nov;29(11):2288–2298. [PubMed] [Google Scholar]