As a consequence of improved management in acute coronary syndromes and improved longevity of the population, the number of patients with heart failure is growing. The prevalence and incidence in industrialised countries are estimated to be around 1% and 0.15% of the population, respectively.w1 Up to 10% of people with heart failure are at an advanced stage, amounting to 300 000 patients in the USA and 60 000 in the UK. In parallel, research w2–5 has led to the concept of cardiac replacement by transplantation. Following the first successful heart transplantation in 1967 in the Groote-Schuur-Hospital, Kapstadt, South Africa,w6 the first successful US heart transplant was performed in 1968 at Stanford University. In the same year, an ad hoc committee at Harvard University established the criteria of brain death.w7 More than 55 000 cardiac transplants have now been performed in more than 200 hospitals worldwide ( www.ishlt.org). The combination of good surgical success rates and the presence of a growing number of well equipped cardiac transplant programmes has created an enormous flux of heart failure patients towards these centres. Since the annual cardiac transplantation rate will likely remain below 4500 worldwide, with < 3000 in the USA and < 300 in the UK, it is evident that cardiac transplantation will continue to play only a very limited quantitative role in the treatment of the advanced heart failure syndrome.1 Yet, its importance will continue to reside with its role as the option of last resort for patients with advanced heart failure, offered within centres with a complete spectrum of medical and surgical treatment options. The aim of this review is to outline a contemporary perspective on cardiac transplantation with respect to recipient and donor management, as well as an appropriate organisational policy. For further background reading, excellent material is available.w8–15
EMERGING TREATMENTS IN ADVANCED HEART FAILURE
There has been progress in medical treatment including angiotensin converting enzyme (ACE) inhibitors w16 and β blockers.2 Surgical therapies including coronary artery bypass grafting with or without surgical anterior ventricular endocardial restoration,w17 mitral reconstruction in cardiomyopathy patients with severe mitral regurgitation,w18 combined with partial left ventriculectomy in idiopathic dilated cardiomyopathy,w19 and left ventricular assist device therapy3w20–24 are evolving. The current status of surgical therapies for advanced heart failure has recently been reviewed in this series.w25 Antiarrhythmic heart failure therapy with implantable defibrillators has also improved survival.w26
CURRENT SURVIVAL BENEFIT WITH CARDIAC TRANSPLANTATION
The appropriate identification of heart transplant candidates is based on the expected gain in survival and quality of life compared to all organ conserving medical and surgical treatment options in advanced heart failure. Selection criteria have been addressed in expert consensus guidelines.4 They are a matter of increasing controversy. The assumption of a survival benefit across the entire spectrum of advanced heart failure may not be valid any longer because of two opposing trends. One trend is the increasing survival with emerging organ saving treatments. The other trend is that outcomes after cardiac transplantation have not consistently improved, due to listing of more critically ill patients, use of so-called marginal donor hearts from an extended donor pool,1 and the initiation of new heart transplantation centres with an inevitable learning phase.w27
The death rates of advanced heart failure patients on the waiting list of the United Network for Organ Sharing (UNOS), the US organisation in charge of organ transplantation ( www.unos.org), have decreased dramatically over time, from 432.2 per 1000 patient years in 1990 to 172.4 per 1000 patient years in 1999. For patients with advanced medical urgency status (status 1A, defined as haemodynamic instability requiring ventricular assist device implantation or high dose intravenous inotropes) in 1999, it was 581.9 per 1000 patient years, as compared with 204.7 per 1000 patient years for medical urgency status (status 1B, defined as requirement of low dose intravenous inotropes) and 130.7 per 1000 patient years for regular urgency status (status 2, defined as stable outpatient condition) registrants. In comparison to waiting list outcomes, for the 1997-98 UNOS heart transplant cohort the one year post-transplantation survival rate was 86%. Recipients in medical urgency status 1 at the time of transplant had slightly lower one year post-transplantation survival rates (mean (SD) 84.8 (0.7)% v 87.5 (1.0)%) than those in status 2 at the time of transplant.
The International Society for Heart and Lung Transplantation (ISHLT) Registry ( www.ishlt.org) indicates an improvement of one year survival after cardiac transplantation from 74.4% between 1980-86 to 85.6% between 1996-99. It does not provide data on waiting list mortality. Thus, the survival benefit with cardiac transplantation cannot be estimated from the ISHLT registry data.
The COCPIT (comparative outcomes and clinical profiles in transplantation) study by the German Transplantation Society and Eurotransplant International Foundation ( www.eurotransplant.org)5 found in a complete national cohort of all 889 adult patients listed for a first heart transplant in Germany in the year 1997 that patients with a predicted high risk of dying from heart failure according to the Heart Failure Survival Score (HFSS), using heart rate, mean blood pressure, aetiology, QRS duration, serum sodium, left ventricular ejection fraction, and peak oxygen uptake,6 experienced not only the highest risk of dying on the waiting list (32%, 20%, 20% for high, medium, and low risk patients, respectively; p = 0.0003), but were the only group that had a survival benefit from transplantation. Limitations of this cohort study included a short observation period, and incomplete data in the HFSS which was in part a result of the fact that the COCPIT data collection was started before the HFSS was published.
All data currently available suggest that the survival benefit from cardiac transplantation is greatest in those patients who are at highest risk of dying from advanced heart failure without transplantation.
IMPROVEMENT OF MANAGEMENT COUNTERBALANCES SICKER PATIENT COHORT
Consistent with this trend, a shift toward more severely ill patients undergoing cardiac transplantation has been observed during the last 10 years.w28 With an increasing fraction of patients undergoing orthotopic heart transplantation after previous cardiac surgery, intraoperative management has become more challenging.w29 w30 The surgical challenges have specifically increased with an increasing fraction of patients undergoing ventricular assist implantation as bridge to transplantation.w31 w32 Furthermore, traditional contraindications for transplant listing are being questioned—for example, with respect to a history of Hodgkin or non-Hodgkin lymphoma,w33 elevated pulmonary vascular resistance requiring sophisticated medical bridging,w34 increased pretransplantation panel reactive antibody (PRA) concentrations,w35 and left ventricular assist device (LVAD) use w36 w37—implying that advances in transplantation management have offset the increasing severity of transplant recipients.
CURRENT INDICATIONS/CONTRAINDICATIONS AND EVALUATION PROCEDURE
Current indication criteria are a modification of the 1993 American College of Cardiology Bethesda guidelines,4 mainly based on the availability of the HFSS6 (table 1). Conditions considered contraindications for cardiac transplantation, based on evidence of reduced short term and long term survival benefit after transplantation, are listed in table 2.
Table 1.
Cardiac transplantation indication criteria
| 1. Accepted | Heart Failure Survival Score (HFSS, Aaronson 19976) high risk |
| Peak Vo2 <10 ml/kg/min after reaching anaerobic threshold | |
| NYHA class III/IV heart failure refractory to maximal medical treatment | |
| Severely limiting ischaemia not amenable to interventional or surgical revascularisation | |
| Recurrent symptomatic ventricular arrhythmias refractory to medical, ICD, and surgical treatment | |
| 2. Probable | HFSS medium risk |
| Peak Vo2 <14 ml/kg/min and severe functional limitations | |
| Instability of fluid status and renal function despite good compliance, daily weights, salt and fluid restriction and flexible diuretics | |
| Recurrent unstable ischaemia not amenable to revascularisation | |
| 3. Inadequate | HFSS low risk alone |
| Peak Vo2 >15–18 ml/kg/min without other indications | |
| Left ventricular ejection fraction <20 % alone | |
| History of NYHA class III/IV symptoms alone | |
| History of ventricular arrhythmias alone | |
ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association; Vo2, oxygen consumption.
Table 2.
Cardiac transplantation contraindication criteria
| Cardiac disease | Irreversible pulmonary hypertension (PVR >6 WU despite standardised reversibility testing protocol) |
| Other diseases | Active infection |
| Pulmonary infarction within the last 6–8 weeks | |
| Significant chronic renal impairment with persistent creatine >2.5 or clearance <25 ml/min | |
| Significant chronic hepatic impairment with persistent bilirubin >2.5 or ALT/AST >×2 | |
| Active or recent malignancy | |
| Systemic diseases such as amyloidosis | |
| Significant chronic lung disease | |
| Significant symptomatic carotid or peripheral vascular disease | |
| Significant coagulopathies | |
| Recent peptic ulcer disease | |
| Major chronic disabling disease | |
| Diabetes with end organ damage and/or brittle diabetes | |
| Excessive obesity (e.g. >30% over normal) | |
| Psychosocial | Active mental illness |
| Evidence of drug, tobacco, of alcohol abuse within the last six months refractory to expert intervention | |
| Psychosocial instability refractory to expert intervention | |
| Age | > 65 years |
ALT/AST, ratio of serum alanine aminotransferase to aspartate aminotransferase; PVR, pulmonary vascular resistance; WU woods units.
Patients are evaluated for transplantation after referral by a cooperating cardiologist. At the initial evaluation, a mutual long term working relationship between patient, relatives, and the team is established. The evaluation includes the tests summarised in table 3. The listing decision involves a recommendation by the team and decision by the patient. The complexity of the evaluation process mandates a team approach. For the patient with permanent contraindications the team offers continued care with the same intensity as for a transplant candidate, in conjunction with the primary care physician and cardiologist. At the time of listing, the patient and family are informed about the peculiarities of the waiting time, the perioperative period, the long term maintenance medication, and the rules of living with the new heart. A flexible schedule of outpatient appointments constitutes the cornerstone of waiting time surveillance. Deteriorating heart failure may precipitate organ failure. The bridging of organ function is part of the management of heart transplant candidates. If irreversible organ dysfunction ensues, the termination of life support must be considered, incorporating the patient's preferences. The patient must know that in case of a donor organ offer, acceptance of the organ depends on the judgment of donor organ quality by the donor surgical team.
Table 3.
Cardiac transplant evaluation tests
| Laboratory | Creatinine, blood urea nitrogen, electrolytes, liver panel, lipid panel, calcium, phosphorus, total protein, albumin, uric acid, complete blood count with differential and platelet count, thyroid panel, antinuclear antibodies, erythrocyte sedimentation rate, rapid plasma reagin, iron binding, partial thromboplastin time, prothrombin time |
| Blood type | |
| IgG and IgM antibodies against cytomegalovirus, herpes simplex virus, HIV, varicella-zoster virus, hepatitis B surface antigen, hepatitis C antigen, toxoplasmosis, other titres when indicated | |
| Prostate specific antigen (male >50 years), mammogram and pap smear (female >40 years) | |
| Screening against a panel of donor antigens (panel reactive antibodies) and human leucocyte antigen phenotype | |
| 24 hour urine for creatinine clearance and total protein, urinalysis, urine culture | |
| Baseline bacterial and fungal cultures if indicated | |
| Cardiac | 12 lead ECG, 24 hour Holter monitor |
| Echocardiogram | |
| Thallium scan if indicated | |
| Exercise stress test with oxygen uptake measurements | |
| Right and left heart catheterisation | |
| Myocardial biopsy on selected cases where aetiology of heart failure is in question | |
| Vascular | Transcranial Doppler |
| Peripheral vascular studies | |
| Carotid Doppler >55 years | |
| Renal | Intravenous pyelogram if indicated |
| Pulmonary | Chest x ray |
| Pulmonary function tests | |
| Chest computer tomogram >65 years (thoracic aorta) | |
| Gastrointestinal | Abdominal ultrasound >55 years |
| Upper gastrointestinal series if indicated | |
| Barium enema if indicated | |
| Liver biopsy if indicated | |
| Metabolic | Bone densitometry |
| Neurologic | Screening evaluation |
| Psychiatric | Screening evaluation |
| Dental | Complete dental evaluation |
| Cardiothoracic surgery | Evaluation |
| Physical therapy | Evaluation |
| Social work | Patient attitude and family support, medical insurance, and general financial resources |
| Transplant coordinator | Education |
EXPANSION OF DONOR CRITERIA
Donor heart acceptance criteria need to be continuously revised in order to responsibly increase the donor pool. These extended criteria include advanced donor–recipient size match, donor age, donor heart dysfunction, donor heart structural changes, donor malignancies, and donor infection. A normal sized adult male (> 70 kg) donor is suitable for most recipients, despite an increased risk associated with small donor size relative to the recipient. Safe expansion of donor age criteria to >60 years has been reported.w38 Donors older than 55 years may be used selectively in certain higher risk recipients. Other donor factors such as left ventricular hypertrophy and ischaemic time may synergistically increase the mortality risk.w39 Donor hearts with myocardial dysfunction can recover after transplantation.w40 Donors with mild coronary artery disease may be considered for selected higher risk recipients. A small series of donor hearts treated with bypass grafting for obstructive coronary lesions at the time of transplantation with a good long term survival has been reported.w41 The transplantation of 37 and 363 hepatitis B surface antigen+ donor hearts and anti-hepatitis B core antibody positive donor organs, respectively, based on 13 309 heart transplants in the time period between 1994 and 1999 in the UNOS registry—on the assumption that patients were at similar risk of dying from heart failure—was associated with a similar five year post-transplantation survival.w42 Current donor contraindications are listed in table 4.
Table 4.
Donor contraindications
| Finding | Rationale |
| Age >55–60 years | Reduced allograft function |
| Diffuse coronary artery disease | |
| Documented myocardial infarction | |
| Documented other heart disease | |
| Refractory ventricular arrhythmias | |
| Malignancies (except CNS) | Tumour spread in recipient |
| Refractory generalised infection | Infection spread in recipient |
CNS, central nervous system.
DONOR MANAGEMENT PRINCIPLES
Optimal management of the haemodynamic, metabolic, and respiratory status of the donor is essential in order to maximise the yield of suitable thoracic donor organs.7 Specifically, this has been advocated by the group at Papworth Hospital in Cambridge, UK.w43 Brain death is associated with an “autonomic and cytokine storm”. The release of noradrenaline (norepinephrine) leads to subendocardial ischaemia. Subsequent cytokine release results in further myocardial depression. This is accompanied by pronounced vasodilation and loss of temperature control. Vasodilation and myocardial depression are compounded by changes in volume status, specifically by relative hypovolaemia which is usually a consequence of aggressive diuretic treatment used to minimise donor cerebral oedema. Optimal donor haemodynamic management includes a pulmonary artery catheter to achieve the goals of euvolaemia and normal cardiac output, minimising the use of α agonists.8 Metabolic management aims at correcting acid-base imbalances and hormonal perturbations. Substitution of insulin, corticosteroids,w44 triiodothyronine,w45 and arginine vasopressin w46 has been shown to be of benefit. A committed transplantation coordination network such as developed in Australia contributes to optimal donor management and recruitment.w47
GENERAL PRINCIPLES OF POST-TRANSPLANTATION MANAGEMENT
The post-transplantation management serves a fourfold purpose: control of allograft rejection, minimisation of side effects of immunosuppressants, coping with the transplantation process, and reintegration of the patient into society. The main challenges in the early postoperative period are the management of rejection and infection. In the long term course after transplantation, the main challenges are management of vasculopathy and malignancies. The denervation physiology characteristic of orthotopic cardiac transplantation requires a distinct modification of pharmacotherapy (table 5).
Table 5.
Effect of denervation on cardiac pharmacology
| Substance | Effect on recipient | Mechanism |
| Digitalis | Normal increase of contractility; minimal effect on AV node | Direct myocardial effect; denervation |
| Atropine | None | Denervation |
| Adrenaline | Increased contractility; increased chronotropy | Denervation hypersensitivity |
| Noradrenaline | Increased contractility; increased chronotropy | Denervation hypersensitivity |
| Isoproterenol | Normal increase in contractility; normal increase in chronotropy | No neuronal uptake |
| Quinidine | No vagolytic effect | Denervation |
| Verapamil | AV block | Direct effect |
| Nifedipine | No reflex tachycardia | Denervation |
| Hydralazine | No reflex tachycardia | Denervation |
| β Blocker | Increased antagonist effect | Denervation |
AV, atrioventricular.
Allograft rejection
The alloimmune response of the recipient leads to destruction of the allograft (fig 1). The differentiation of this alloimmune response is orchestrated by a subtle regulation of soluble immune mediators, called cytokines.w48 An understanding of acute allograft rejection requires an appreciation of complex, adaptive networks like the interdigitated inflammatory, immune, and physiologic processes that are at work in transplanted allografts.w49
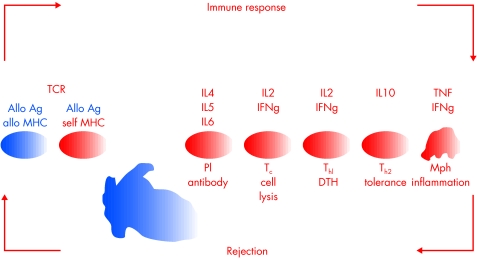
Figure 1.
The alloimmune response consists of antigen (Allo Ag) presentation in the context of major histocompatibility (MHC) molecules either by donor cells or by recipient cells to the recipient's T cells via T cell receptor (TCR). This leads to T lymphocyte differentiation to CD4 T cells of T helper1 (Th1) or T helper2 (Th2) phenotype, CD8 cytotoxic T cells (Tc), and B lymphocyte development into plasma cells producing specific clones of antibodies. These immunocompetent cells destroy the allogeneic graft cells. In addition, an inflammatory response by the innate immune system involving macrophages (Mph) participates in this networked alloimmune response. Cytokines (IL2-10, TNF, IFng) play an important role in this orchestrated response. The Th2 subset may favour graft acceptance.
Rejection management by immunosuppression
The three drug regimen of cyclosporine, azathioprine, and usually corticosteroids has been the mainstay of immunosuppression for patients undergoing cardiac transplantation since the early 1980s. However, this regimen has some inherent toxicities and does not prevent graft coronary artery disease. Thus there has been a widely perceived need for the introduction of improved immunosuppressive agents. The most commonly used drugs, their targets, selectivity, and main side effects are summarised in table 6.
Table 6.
Commonly used immunosuppressive drugs: targets, main side effects, and selectivity
| Method | Target | Major side effects | Selectivity |
| Steroids | Lymphocytes/RES | Osteoporosis, diabetes, psychosis, infection, obesity | + |
| Azathioprine | Lymphocytes | Marrow suppression, hepatopathy | ++ |
| Polyclonal antithymocyte globulin | T lymphocytes | Infection, malignancies | ++ |
| Monoclonal CD3 antibodies | CD3+ T lymphocytes | Infection, malignancies | +++ |
| Mycophenolate | De novo purine synthesis in lymphocytes | Gastrointestinal | ++++ |
| Cyclosporine | IL2 inhibition in T lymphocytes | Nephropathy | ++++ |
| Tacrolimus | IL2 inhibition in T lymphocytes | Nephropathy | ++++ |
| Daclizumab | IL2 receptor antibodies | None | ++++ |
RES, reticuloendothelial system.
Recently several new immune pharmacological agents have become available. For induction therapy beyond the polyclonal antithymocyte globulin or monoclonal OKT3, antibody preparations which specifically bind to the interleukin 2 (IL2) receptor, basiliximab and daclizumab, are being evaluated. The new maintenance immunosuppressive drugs are either inhibitors of de novo synthesis of purine or pyrimidine nucleotides, or are immunophilin binding drugs that inhibit signal transduction in lymphocytes. The newer inhibitors of de novo nucleotide synthesis include mycophenolate mofetil, mizoribine, brequinar, and leflunomide. The immunophilin binding drugs are cyclosporine, tacrolimus, and rapamycin. Out of these, four agents have been introduced recently into clinical cardiac transplantation. Mycophenolate mofetil is used as a substitute for azathioprine and has been shown to result in lower mortality and rejection rates in heart transplant recipients.9 Tacrolimus can be used as a substitute for cyclosporine.w50 Specific blockade of the high affinity IL2 receptor (CD25) with the human IgG1 monoclonal antibody daclizumab reduces the frequency and severity of cardiac allograft rejection during the induction period.10 Rapamycin/sirolimus has been shown to reverse refractory cardiac allograft rejection.w51
Rejection monitoring
Several methods of rejection monitoring have evolved over the last decades (table 7). The endomyocardial biopsy has long been the preferred technique for monitoring the rejection status of the cardiac allograft,w52 based on the ISHLT cardiac allograft rejection grading system.11w53 Different non-invasive algorithms for cardiac allograft rejection monitoring have been proposed. Early after cardiac transplantation, raised concentrations of inflammatory cells and soluble inflammatory molecules and lower concentrations of immunocompetence markers are associated with impaired allograft function in the absence of cellular rejection. Based on this, an algorithm incorporating clinical status, graft function, mononuclear cell subset analysis, and endomyocardial biopsy has been proposed.12 Use of an algorithm combining endomyocardial biopsy, lymphocyte growth assays, and anti-HLA antibody measurements enables prospective stratification of cardiac transplant recipients into risk categories for progression to high grade rejection. Low risk individuals require fewer biopsies, moderate risk individuals require an ongoing schedule of surveillance biopsies, and high risk individuals require rational organisation of interventional strategies aimed at preventing rejection.13
Table 7.
Methods of cardiac allograft rejection monitoring
| Method | Delay | Serial assessment | Costs | Indication of rejection | Sensitivity | Specificity |
| History | None | Yes | + | Dyspnoea, weight gain, discomfort | + | + |
| Physical exam | None | Yes | + | Arrhythmia, S3, crackles, JVD, oedema | + | + |
| ECG | None | Yes | + | Atrial arrhythmias, low voltage | + | + |
| Pacemaker | Telemetry | Every night | +++ | Voltage ↓ 7% | +++ | +++ |
| Echo | 30 mins | Yes | +++ | Isovolumic relaxation time ↓, fractional shortening ↓ | +++ | +++ |
| Biopsy | 24 hours | Max 1/week | +++ | Cell infiltrates, haemorrhage | +++ | +++ |
| Myosin scan | 24 hours | Max 1/3 month | +++ | Heart/lung ratio >1.6 | +++ | +++ |
| MRI | 1 hour | Max 1/week | +++ | Signal ↑ due to oedema | +++ | +++ |
| Immune monitoring | 4 hours | Yes | +++ | CD4/CD8↑, HLA-DR+/CD14 ↑, IL2↑ | +++ | +++ |
JVD, jugular venous distension.
Prophylaxis and treatment of infection
Opportunistic infections after transplantation continue to constitute a challenge for management (table 8). Cytomegalovirus (CMV) remains the most important infection affecting heart transplant recipients. In the prevention of CMV disease, those at risk of primary disease (donor seropositive, recipient seronegative) should receive prophylaxis.w54 In many units, oral ganciclovir is now the preferred route of prophylactic treatment. To monitor activity of CMV infection, assessment of viral load has become a valuable tool. Legionella pneumophila may cause pneumonia of variable severity after cardiac transplantation. Chlorination and heating of water is an important preventive measure. Specific cultures in outbreak situations should be considered to identify less frequent L pneumophila serotypes and the non-pneumophila Legionella species.w55 Pneumocystis carinii pneumonia,w56 tuberculosis,w57 toxoplasmosis,w58 pulmonary aspergillosis,w59 and other fungal infections w60 w61 continue to constitute challenges for the immunocompromised heart transplant recipient. Sulfamethoxazole/trimethoprine is used in most units for P carinii pneumonia prophylaxis.
Table 8.
Management of opportunistic infections after cardiac transplantation
| Organism | Test | Treatment |
| Cytomegalovirus | IE-Gene, PCR, IgM | Gancyclovir, if severe additional CMV antibodies |
| Herpes simplex virus | IgM | Aciclovir |
| Varicella-zoster virus | IgM | Aciclovir |
| Hepatitis B virus | IgM | Lamivudine |
| Legionella species | Urine antigen, x ray | Erythromycin |
| Mycobacterium tuberculosis | Ziehl-Neelson | Rifampicin, isoniazid, myambutol |
| Nocardia asteroides | Brain CT | Sulfamethoxazole/trimethoprim |
| Pneumocystis carinii | x ray | Sulfamethoxazole/trimethoprim |
| Toxoplasma gondii | X ray, IFT, CFT, IgA, IgM | Pyrimethamine + sulfadiazine, folic acid |
| Candida albicans | Direct | Fluconazole, itraconazole |
| Aspergillus fumigatus | X ray | Itraconazole, amphotericin B, flucytosine |
| Cryptococcus neoformans | Brain CT | Itraconazole, amphotericin B, flucytosine, or fluconazole |
| Listeria monocytogenes | CNS: ampicillin + gentamycin |
CMV, cytomegalovirus; CT computed tomography; IFT immunofluoresence test; CFT, complement fixation test.
Transplant vasculopathy
Cardiac allograft vasculopathy (CAV), an unusually accelerated and diffuse form of obliterative coronary arteriosclerosis, determines long term function of the transplanted heart and is the major cause of death in the long term after cardiac transplantation. CAV is a complicated interplay between immunologic and non-immunologic factors resulting in repetitive vascular injury and a localised sustained inflammatory response.w62 Dyslipidemia, oxidant stress, immunosuppressive drugs, and viral infection w63 appear to be important contributors to disease development. Endothelial dysfunction is an early feature of CAV and progresses over time after transplantation. Early identification of CAV is essential if long term prognosis is to be improved. Annual coronary angiography is performed for diagnostic and surveillance purposes. Intravascular ultrasound is a more sensitive diagnostic tool for early disease stages and has revealed that progressive luminal narrowing in CAV is in part caused by negative vascular remodelling. Because of the diffuse nature of CAV, percutaneous and surgical revascularisation procedures have a limited role. If annual coronarograms demonstrate rapid progression, retransplantation may be considered. Prevention of CAV progression is a primary therapeutic goal.w64 Unfortunately, conventional preventative measures have limited effects. Several pharmacological agents, including the calcium channel blocker diltiazem w65 and statins such as pravastatin14 or simvastatin,w66 have been shown to be effective.
Malignancies
Malignancies play a major role as cause of death after cardiac transplantation. In the long term course after cardiac transplantation, the risk of malignancies occurring is 1–2% per year. This risk is 10–100 fold higher than the risk in an age matched control population. Malignant tumours of the skin and lymphomas are the most frequent types, but any solid organ tumour may occur. The incidence of post-transplant lymphoproliferative disorder with a cyclosporine based immunosuppressive regimen is estimated to be around 2–4%w67 ( www.ctstransplant.org) and is a frequent and often fatal complication of organ transplantation. It most often results from an Epstein-Barr virus transformed B cell clone, which expresses B cell surface markers such as CD20. While these lymphomas may respond to reduction of immunosuppression, they may be successfully treated with the CD20 antibody rituximab.w68
TRANSPLANT CENTRE PRACTICE AND INFRASTRUCTURE
Improvements in perioperative and post-transplantation care have permitted a safe expansion of both the donor pool and recipient criteria for transplantation in experienced individual centres,w69 and in multicentre registries such as the Cardiac Transplant Research Database.w70 At large centres including Columbia University where more than 1200 cardiac transplants have been performed since 1977, with a one year survival rate of approximately 90% and a five year survival rate of approximately 75%, an extensive experience with recipients bridged to transplantation by mechanical assist devices has evolved.w71 The increasing challenge of providing advanced heart failure care founded on evidence based practice patterns requires reliable outcome data including identification of between centre variability and its causes.w72 There are only a few reports on this subject. Early data suggested an effect of centre volume,15 potentially as a surrogate for centre experience.w27 Recently, a total of 662 patients listed between 1992 and 1995 as UNOS status 1 for heart transplantation by four adult US cardiac transplant centres in an organ procurement organisation were analysed. These cardiac transplant centres demonstrated significant variability in the likelihood of transplantation and survival for patients listed as UNOS status 1.w73 In Europe, prompted by results from the German COCPIT study,5 a Eurotransplant wide analysis of centre specific heart transplant outcomes is currently being undertaken, applying empirical Bayes methods.w74
ALLOCATION BASED ON MEDICAL URGENCY VERSUS WAITING TIME
The discussion on the respective roles of medical urgency and waiting time in the listing and allocation cascade was started a decade ago following the finding that the survival benefit of transplantation decreases as the waiting time lengthens.16 Improvements in medical treatment and identification of risk factors for early mortality may make it possible to defer or avoid transplantation in many patients with advanced heart failure w75 while selecting those patients for transplantation who are at high risk of dying from heart failure without it. To test the hypothesis that cardiac transplantation confers a higher survival benefit in patients with a high risk of dying from heart failure, randomised clinical trial designs have been discussed.17 w76–79 If evidence in support of this hypothesis can be established, an allocation policy may either restrict the waiting list to high risk patients from the beginning or accept all potential candidates on the waiting list and subsequently prioritise according to medical urgency, thereby decreasing the impact of waiting time in the allocation algorithm for cardiac transplantation. The latter change has been suggested by the German Transplantation Societyw80 and the US Department of Health and has been reinforced by the Institute of Medicine of the US National Institutes of Health.18 As an example for a national allocation system incorporating medical urgency, the UK is divided into donor zones with the size of the zone allocated to each transplant centre based on their activity. Each centre then has local autonomy for allocation of donors within their zone to patients on their waiting list. In addition, approximately 15% of the donor hearts in the UK are allocated through a national high urgency system into which each centre can place a fixed number of patients depending on their transplant activity. The quota mechanism helps prevent abuse of the urgency waiting system ( http://www.exeter.nhsia.nhs.uk/products/core/donor/donor.asp ).
FUTURE DIRECTIONS
Cell transplantation and regrowth of heart muscle
The concept of regenerating the failing heart is in the experimental stage. Several approaches including transplantation of embryonic cardiomyocytes,w81 cryopreserved w82 or bioengineered fetal cardiomyocytes,w83 neonatal cardiac myocytes, skeletal myoblasts,w84 autologous smooth muscle cells,w85 and dermal fibroblasts w86 have been proposed. Current problems include chronic rejection in allogeneic cells, lack of intercellular gap junction communication, and differential patterns in excitation–contraction coupling in skeletal and cardiac myocytes. Alternatively, lineage negative bone marrow cells19 or bone marrow derived endothelial precursor cells with phenotypic and functional characteristics of embryonic haemangioblasts have been proposed. The latter can be used to directly induce new blood vessel formation after experimental myocardial infarction, associated with decreased apoptosis of hypertrophied myocytes in the peri-infarct region, long term salvage and survival of viable myocardium, reduction in collagen deposition, and sustained improvement in cardiac function.20
Xenotransplantation
Xenotransplantation theoretically provides an unlimited supply of cells, tissues, and organs. The immunological challenge is that the favourite source animal of choice, the pig, and the human recipient were separated in their evolution 90 million years ago, during which time biological characteristics such as anatomy, physiology, and immunology have drifted far apart. The potential individual benefit of a xenograft has to be counterbalanced against the collective risk of xenozoonoses. Ethically, all three monotheistic religions and Hinduism support the idea of saving and improving human life with the help of an animal organ.w87 According to a committee of the ISHLT, the current experimental results do not presently justify initiating a clinical trial, but because of the immense potential, research in xenotransplantation should be encouraged.21
Mechanical circulatory support
Mechanical circulatory support systems are used nowadays frequently to support patients with severe heart failure to transplantation, to recovery, or as destination therapy. While the early totally artificial hearts and ventricular assist devices were mainly driven from an external pneumatic drive unit, the current generation of assist devices are electrically powered, ultracompact, totally implantable, and have small wearable drive/control consoles, allowing patients to return to their daily activities.w88 Successful bridging to recovery with ventricular support systems has been reported in postcardiotomy cardiogenic shock, acute myocarditis, and in the peri-infarction period. Benefit is related to reduction of left ventricular myocardial wall stress.w89 Since the REMATCH (randomized evaluation of mechanical assistance for the treatment of congestive heart failure) trial demonstrated a survival benefit from mechanical circulatory support therapy compared to all other options in non-transplant candidates,3 this will undoubtedly lead to a redefinition of its role in potential cardiac transplantation candidates in the near future.
Smaller rotationalw24 and completely implantable systemsw90 are under evaluation. In order to facilitate evidence based decision making in advanced heart failure therapy with mechanical circulatory support devices, the ISHLT recently inaugurated an International Mechanical Circulatory Support Device Database. This database provides the opportunity for online data entry via the internet and, as a service and motivation for every centre wordwide to participate, continuous centre specific outcome analyses enabling every participating centre to access its own data and view them in relation to the aggregate database ( http://www.ishlt.org/regist_mcsd_main.htm).22
Cardiac transplantation: key points .
Advanced heart failure is an increasing epidemiological problem wordwide
Cardiac transplantation has become the gold standard treatment in selected patients during the last 20 years
The numerical disparity between donors and recipients requires equitable solutions
Cardiac transplantation is increasingly restricted to patients at greatest risk of dying
Alternative treatments include neurohormonal blockers and mechanical support devices
CONCLUSION
A little more than three decades after the successful implementation of cardiac transplantation, this revolutionary concept of advanced heart failure treatment has gained tremendous momentum and is considered the gold standard treatment in selected patients. More specific modalities of immunosuppression continue to decrease the impact of acute and chronic rejection and immunosuppression related side effects. The success of cardiac transplantation has led to a widespread initiation of transplant programmes and an enlargement of cardiac transplantation waiting lists. The increasing numerical disparity between waiting list size and number of donor organ supply has stimulated research to identify those patients who benefit most from cardiac transplantation, as well to develop alternative treatments for advanced heart failure. The success of these new options, specifically the comprehensive blockers of the renin–angiotensin system and adrenergic system, defibrillators, and mechanical circulatory support devices creates the new challenge for cardiac transplantation to define its contemporary role. Against this background of established advanced heart failure management, organ saving surgical approaches (revascularisation, valve repair, ventricular restoration) and new paradigms such as cell transplantation and xenotransplantation must be tested using appropriately designed studies.
Supplementary Material
Abbreviations
COCPIT, Comparative Outcomes and Clinical Profiles In Transplantation
CMV, cytomegalovirus
HFSS, Heart Failure Survival Score
ISHLT, International Society for Heart and Lung Transplantation
LVAD, left ventricular assist device
PRA, panel reactive antibody
REMATCH, Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart failure
UNOS, United Network for Organ Sharing
REFERENCES
- 1.Hosenpud JD, Bennett LE, Keck BM, et al. The registry of the International Society for Heart and Lung Transplantation: Eighteenth official report – 2000. J Heart Lung Transplant 2001;20:805–15. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Coats AJ, Fowler MB, et al for the Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. ▸ First demonstration of survival benefit by β blockers in an advanced heart failure population considered elective cardiac transplantation candidates. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. for the REMATCH Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43. ▸ First study to test in a randomised design the survival benefit of mechanical circulatory support in advanced heart failure patients. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA. 24th Bethesda conference: cardiac transplantation. J Am Coll Cardiol 1993;22 (suppl 1):1–64. [PubMed] [Google Scholar]
- 5.Deng MC, De Meester JMJ, Smits JMA, et al, on behalf of COCPIT Study Group. The effect of receiving a heart transplant: analysis of a national cohort entered onto a waiting list, stratified by heart failure severity. BMJ 2000;321:540–5. ▸ First national cohort study to suggest that survival benefit of cardiac transplantation is restricted to patients at highest risk of dying from heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaronson KD, Schwartz JS, Chen TMC, et al. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997;95:2660–7. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA, Baldwin J, Baumgartner W, et al. Cardiovascular management of a potential heart donor: a statement from the Transplantation Committee of the American College of Cardiology. Crit Care Med 1996;24:1599–601. [DOI] [PubMed] [Google Scholar]
- 8.Wheeldon DR, Potter CD, Oduro A, et al. Transforming the “unacceptable” donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant 1995;14:734–42. [PubMed] [Google Scholar]
- 9.Kobashigawa J, Miller L, Renlund D, et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate mofetil investigators. Transplantation 1998;66:507–15. [DOI] [PubMed] [Google Scholar]
- 10.Beniaminovitz A, Itescu S, Lietz K, et al. Prevention of rejection in cardiac transplantation by blockade of the interleukin-2 receptor with a monoclonal antibody. N Engl J Med 2000;342:613–9. [DOI] [PubMed] [Google Scholar]
- 11.Billingham ME, Cary NRB, Hammond ME, et al. A working formulation for the standardisation of nomenclature in the diagnosis of heart and lung rejection: heart rejection study group. J Heart Lung Transplant 1990;9:587–93. [PubMed] [Google Scholar]
- 12.Deng MC, Erren M, Roeder N, et al. T-Cell and monocyte subsets, inflammatory molecules, rejection and hemodynamics early after cardiac transplantation. Transplantation 1998;65:1255–6. [DOI] [PubMed] [Google Scholar]
- 13.Itescu S, Tung TC, Burke EM, et al. An immunological algorithm to predict risk of high-grade rejection in cardiac transplant recipients. Lancet 1998;352:263–70. [DOI] [PubMed] [Google Scholar]
- 14.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995;333:621–7. [DOI] [PubMed] [Google Scholar]
- 15.Hosenpud JD, Breen TJ, Edwards EB, et al. The effect of transplant center volume on cardiac transplant outcome. A report of the United Network for Organ Sharing Scientific Registry. JAMA 1994;271:1844–9. [PubMed] [Google Scholar]
- 16.Stevenson LW, Hamilton MA, Tillisch IH, et al. Decreasing survival benefit from cardiac transplantation for outpatients as the waiting list lengthens. J Am Coll Cardiol 1991;18:919–25. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein MO, Levin B, Robbins H. Clinical and prophylactic trials with assured new treatment for those at greater risk: I. A design proposal. Am J Public Health 1996;86:691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbons RD, Meltzer D, Duan N, and other members of the Institute of Medicine Committee on Organ Procurement and Transplantation. Waiting for organ transplantation. Science 2000;287:237–8. [DOI] [PubMed] [Google Scholar]
- 19.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–5. ▸ First demonstration of regeneration of infarcted myocardium by intracardiac injection of bone marrow derived stem cells. [DOI] [PubMed] [Google Scholar]
- 20.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–6. ▸ First demonstration of neovascularisation and sustained functional improvement of ischaemic myocardium by peripheral venous injection of autologous bone-marrow derived angioblasts. [DOI] [PubMed] [Google Scholar]
- 21.Cooper DK, Keogh AM. The potential role of xenotransplantation in treating endstage cardiac disease: a summary of the report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation. Curr Opin Cardiol 2001;16:105–9. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson LW, Kormos RL, Bourge RC, et al. Mechanical cardiac support 2000: current applications and future trial design. June 15-16, 2000 Bethesda, Maryland. J Am Coll Cardiol 2001;37:340–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.