Abstract
Background: The “warm up” effect in angina may represent ischaemic preconditioning, which is mediated by adenosine A1 receptors in most models.
Objective: To investigate the effect of a selective A1 agonist, GR79236 (GlaxoSmithKline), on exercise induced angina and ischaemic left ventricular dysfunction in patients with coronary artery disease.
Design: A double blind crossover study.
Patients: 25 patients with multivessel coronary artery disease.
Interventions: On mornings one week apart, patients received intravenous GR79236 10 μg/kg or placebo, and then carried out two supine bicycle exercise tests separated by 30 minutes. Equilibrium radionuclide angiography was done before and during exercise.
Results: The onset of chest pain or 1 mm ST depression was delayed and occurred at a higher rate–pressure product during the second exercise test following either placebo or GR79236. Compared with placebo, GR79236 did not affect these indices during equivalent tests. GR79236 reduced resting global ejection fraction from (mean (SD)) 63 (7)% to 61 (5)% (p < 0.05) by a selective reduction in the regional ejection fraction of “ischaemic” left ventricular sectors (those where the ejection fraction fell during the first exercise test following placebo). Ischaemic sectors showed increased function during the second test following placebo (72 (21)% v 66 (20)%; p = 0.0001), or during the first test following GR79236 (69 (21)% v 66 (20)%; p = 0.0001). Sequential exercise further increased the function of ischaemic sectors even after drug administration.
Conclusions: GR79236 failed to mimic the warm up effect, and warm up occurred even in the presence of this agent. This suggests that ischaemic preconditioning is not an important component of this type of protection. The complex actions of the drug on regional left ventricular function at rest and during exercise suggest several competing A1 mediated actions.
Keywords: adenosine, angina, exercise, coronary artery disease
In most animal models, as well as in isolated superfused human right atrial trabeculae, ischaemic preconditioning is triggered by adenosine, acting through myocardial A1 receptors.1–3 This is followed by activation of intracellular signalling pathways and phosphorylation of effectors such as the ATP gated potassium (KATP) channel.4 On the basis of these laboratory observations, adenosine has been advocated for cardioprotection in various clinical settings. In acute myocardial infarction, it reduces infarct size when given before thrombolytic treatment.5 Administration of adenosine during cardiopulmonary bypass reduces postoperative inotropic requirements and improves regional wall motion.6 Intracoronary adenosine given before coronary angioplasty reduces chest pain and ST depression during balloon inflation to an even greater degree than sequential inflations.7
During sequential exercise testing, patients with coronary artery disease may develop less angina and ECG evidence of ischaemia—the “warm up” phenomenon.8 It is hypothesised that this represents a human form of ischaemic preconditioning. In this setting of demand ischaemia in stable coronary artery disease, adenosine or an orally available equivalent would not be therapeutically useful, as any A1 mediated myocardial protection would be offset by the coronary steal effect of A2 mediated coronary vasodilatation. Indeed adenosine is widely used to induce perfusion defects in radionuclide perfusion imaging. GR79236 (N-[(1S, trans)-2-hydroxycyclopentyl]adenosine) is a highly selective adenosine A1 receptor agonist developed for use in humans (GlaxoSmithKline). Its EC50 (concentration required for 50% of maximum response) for A2 receptors is approximately 100 times greater than that for A1 receptors in a range of preparations.9 This selectivity avoids A2 mediated coronary vasodilatation which would mask any protective, and potentially therapeutic, effect. In anaesthetised rabbits and conscious pigs, GR79236 mimics the effect of ischaemic preconditioning against infarction, maximum protection occurring at doses of 10 μg/kg and 3.5 μg/kg, respectively.10–12 Studies in normal human volunteers have shown that the drug is well tolerated when given in doses of up to 20 μg/kg as a slow intravenous bolus, with no adverse haemodynamic effects and a terminal elimination half life of one to two hours (GlaxoSmithKline, unpublished observations).
The effect of selective adenosine A1 receptor activation on myocardial function at rest and during exercise in patients with stable coronary disease is unknown. We hypothesised that if the warm up effect is a manifestation of ischaemic preconditioning, GR79236 would protect against exercise induced ischaemia in patients with coronary artery disease in a similar way to previous exercise. Moreover, sequential exercise following drug administration should provide no additional benefit. We investigated these hypotheses in a human model of the warm up effect, using supine bicycle exercise testing and equilibrium radionuclide angiography (ERNA), which has been reported previously.13
METHODS
Patients
Twenty five patients with stable angina pectoris (mean (SD) age 63 (7) years; 22 men, 3 women) were studied a median of 12 weeks after day case coronary angiography. All had three vessel coronary artery disease but preserved left ventricular function (global ejection fraction by contrast ventriculography ≥ 50%). Patients were excluded if they had severe resting ECG abnormalities, were in atrial fibrillation, or were diabetic and being treated with a sulfonylurea drug. The study protocol was approved by the local research ethics committee, and all patients gave informed consent before participating. Some of the control data have been reported in a previous paper on the warm up effect.13
Study protocol
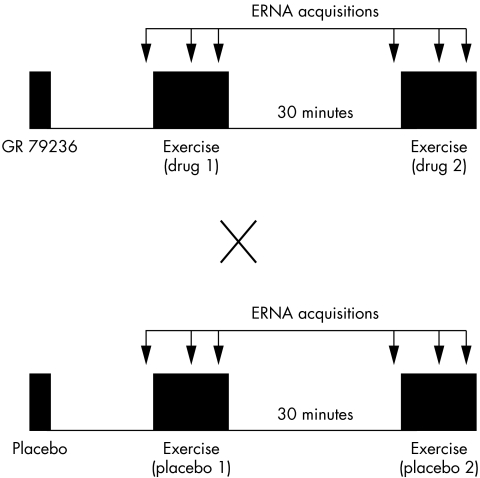
The study was conducted on two mornings, one week apart, using a double blind, placebo controlled crossover design (fig 1). The patients received intravenous injections over 10 minutes of either 10 μg/kg GR79236, or placebo. Thirteen patients received active drug on day 1, and 12 patients on day 2. Patients performed two maximal symptom limited supine bicycle exercise tests on each day, 30 minutes from end to start. Approximately 15 minutes elapsed from the end of the drug or placebo infusion to the beginning of the first exercise test. ERNA was performed just before and during each test. Exercise acquisitions were begun at the same exercise time for all four tests—at least one minute into a stage to allow stabilisation of heart rate—and the workload was allowed to increase during a scan as required by the exercise protocol. As many exercise acquisitions as possible were obtained, usually two or three, and the final acquisition provided the “exercise” indices described in Results. Camera positions were reproduced on each study day, and patients remained on the camera table throughout the protocol.
Figure 1.
Design of the study. ERNA, equilibrium radionuclide angiography.
Exercise stress testing
Patients underwent supine bicycle exercise testing, beginning at a workload of 25 W and increasing by 25 W every three minutes until limited by symptoms. The ECG lead showing the most profound ST depression during the first exercise test following placebo was analysed for all four tests. Patients performed two practice tests on separate days at least one week before the study, to familiarise them with the technique. All tests were performed at a room temperature of 22°C, and at the same time of day to minimise diurnal variation in exercise capacity. All antianginal drugs were discontinued 72 hours before each study day.
ERNA acquisition
Following in vivo blood pool labelling with 750–800 MBq of 99mTc-pertechnetate on each day, frame mode (24 frames per cycle) equilibrium radionuclide angiography was performed in the best septal left anterior oblique projection according to the usual protocol for our laboratory.14 All resting ERNA acquisitions were continued to a count rate of 200 000 per frame, and exercise acquisitions to 150 000 per frame. An acquisition was interrupted during the first test on the first study day if the RR interval increased beyond the preset gating window (mean ±10% at rest, ±15% during exercise), or a second peak started to appear in the RR histogram. During all subsequent tests, the equivalent acquisition was then continued only as far as the number of counts obtained during the first test. In one patient it proved impossible to obtain an adequate exercise ERNA acquisition during the first exercise test following placebo; this individual was excluded from the analysis of ERNA indices.
ERNA processing
ERNA processing was automated using commercially available software (XT), but each step was carefully checked and if necessary corrected by the observer. Global ejection fraction was calculated from the background corrected time–activity curve using a variable region of interest. Regional ejection fractions were calculated for nine radial sectors of equal angle (40°) using a fixed centroid method. Sectors that included counts from the aortic root and mitral valve were not used in the analysis. Sectors were classified as “ischaemic” or “non-ischaemic” depending on whether or not the regional ejection fraction fell by at least one point during the first exercise test following placebo.13
Statistical analysis
Data are expressed as mean (SD) unless otherwise specified. Continuous variables were compared using paired and unpaired Student's t tests, or Wilcoxon signed rank and Mann–Whitney U tests when there was deviation from the normal distribution. Two way parametric or non-parametric analyses of variance with the Friedman test were used to compare measurements between the four exercise tests. Multiple pairwise comparisons of indices were corrected using the Bonferroni method (p value multiplied by the number of comparisons). A probability value of p < 0.05 is taken as significant throughout the paper.
RESULTS
Effects of GR79236 injection
There were no significant adverse events following injection of either GR79236 or placebo. Ten patients (40%) described vague feelings of heaviness or warmth following administration of the active drug, but none did so following placebo. GR79236 did not affect heart rate (drug 66 (2) to 64 (3) beats/min; placebo 65 (2) to 66 (2) beats/min), but did cause prolongation of the PR interval on the ECG (drug 168 (4) to 190 (6) ms, p < 0.0001; placebo 168 (5) to 170 (5) ms, NS). No patient developed second or third degree heart block following drug administration.
Haemodynamic and ECG data during the four exercise tests
These data are shown in table 1. There were no significant differences in total exercise time, peak rate–pressure product (RPP) achieved, or maximum ST depression between the four study exercise tests. Following either placebo or drug, the onset of chest pain or 1 mm ST depression was delayed and occurred at a higher RPP during the second exercise test than during the first. However, these indices were comparable between the first test following GR79236 and that following placebo.
Table 1.
Exercise ECG data for the four exercise tests
| Placebo 1 | Placebo 2 | Drug 1 | Drug 2 | p Value | |
| Baseline | |||||
| Heart rate (beats/min) | 65 (2) | 66 (2) | 65 (3) | 68 (2) | NS |
| SBP (mm Hg) | 155 (4) | 151 (5) | 160 (4) | 154 (4) | 0.01 |
| RPP (×103 mm Hg/min) | 10.1 (2.0) | 9.9 (1.9) | 10.5 (2.5) | 10.5 (2.4) | NS |
| ST depression (mm) | −0.1 (0.1) | −0.1 (0.1) | −0.1 (0.1) | −0.1 (0.1) | NS |
| Chest pain onset | |||||
| Time (min) | 5.9 (0.7) | 6.8 (0.7)* | 6.5 (0.6) | 7.2 (0.6)† | 0.03 |
| Heart rate (beats/min) | 98 (4) | 103 (4)* | 96 (3) | 100 (3)† | 0.02 |
| SBP (mm Hg) | 168 (4) | 173 (6) | 177 (5) | 174 (4) | NS |
| RPP (×103 mm Hg/min) | 16.6 (2.9) | 17.8 (3.6) | 17.1 (3.5) | 17.5 (3.0) | NS |
| ST depression (mm) | 1.3 (0.3) | 1.1 (0.3) | 1.3 (0.3) | 1.2 (0.3) | NS |
| 1 mm ST depression | |||||
| Time (min) | 6.0 (0.8) | 6.9 (0.8)* | 6.5 (0.8) | 7.2 (0.8)† | 0.009 |
| Heart rate (beats/min) | 99 (4) | 105 (4)* | 98 (4) | 105 (5)† | <0.0001 |
| SBP (mm Hg) | 171 (5) | 177 (6) | 177 (5) | 178 (4) | NS |
| RPP (×103 mm Hg/min) | 17.0 (3.7) | 18.6 (4.3)* | 17.5 (4.0) | 18.8 (4.3)† | 0.003 |
| Peak exercise | |||||
| Time (min) | 8.9 (0.6) | 9.1 (0.6) | 9.2 (0.7) | 9.4 (0.7) | NS |
| Heart rate (beats/min) | 107 (3) | 110 (4)* | 107 (4) | 110 (4)† | 0.03 |
| SBP (mm Hg) | 187 (5) | 185 (5) | 190 (4) | 185 (4) | NS |
| RPP (×103 mm Hg/min) | 20.0 (4.0) | 20.3 (4.6) | 20.4 (4.4) | 20.5 (4.8) | NS |
| ST depression (mm) | 1.7 (0.3) | 1.5 (0.3) | 1.6 (0.2) | 1.5 (0.3) | NS |
Values are mean (SD).
Comparisons between all four exercise tests using two way parametric analysis of variance; pairwise comparisons using Student's t test for paired data with Bonferroni correction.
*p<0.05 v placebo 1; †p<0.05 v drug 1.
RPP, rate–pressure product; SBP, systolic blood pressure.
Regional left ventricular systolic function
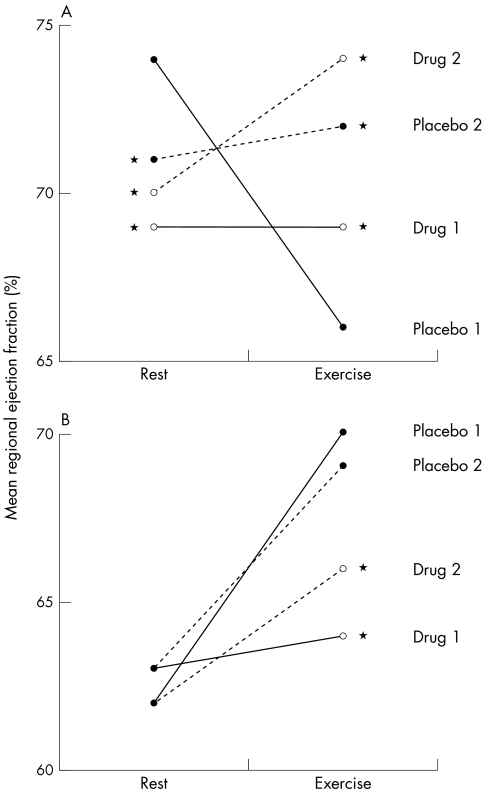
These results are shown in table 2 and figs 2 and 3. Following placebo, during the first exercise test an “ischaemic” response was seen in 55 left ventricular sectors (38%) and a “non-ischaemic” response in 89 (62%). Non-ischaemic sectors showed comparable responses during both exercise tests. Ischaemic sectors showed an increase in both exercise ejection fraction and change in ejection fraction between rest and exercise (ΔEF) during the second test compared with the first.
Table 2.
Regional ejection fraction (%) of ischaemic and non-ischaemic left ventricular sectors before and during the four exercise tests
| Exercise test | Rest | Exercise | Change |
| Ischaemic sectors (n=55) | |||
| Placebo 1 | 74 (20) | 66 (20) | −9 (7) |
| Placebo 2 | 71 (19)* | 72 (21)* | +1 (9)* |
| Drug 1 | 69 (20)* | 69 (21)* | −1 (10)* |
| Drug 2 | 70 (21)* | 74 (19)* | +1 (10)* |
| p Value | 0.0003 | 0.005 | <0.0001 |
| Non-ischaemic sectors (n=89) | |||
| Placebo 1 | 62 (21) | 70 (22) | +8 (7) |
| Placebo 2 | 63 (24) | 69 (22) | +8 (10) |
| Drug 1 | 63 (23) | 64 (23)* | +2 (10)* |
| Drug 2 | 62 (23) | 66 (22)* | +5 (8)* |
| p Value | NS | <0.0001 | <0.0001 |
Values are mean (SD).
Comparisons between all four exercise tests using Friedman test; pairwise comparisons using Wilcoxon signed rank test with Bonferroni correction.
*p<0.05 v placebo 1.
Figure 2.
Mean regional ejection fraction for (A) ischaemic (n = 55) and (B) non-ischaemic (n = 80) left ventricular sectors before and during the four exercise tests. Comparisons carried out using the Wilcoxon signed rank test with Bonferroni correction (*p < 0.05 v placebo 1).
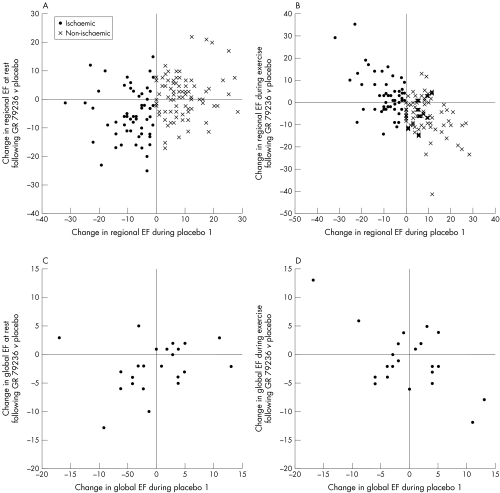
Figure 3.
Scatter plots showing the effect of GR79236 on left ventricular function at rest and during exercise as a function of the response during the first exercise test following placebo. (A) Change in regional ejection fraction (EF) at rest. GR79236 decreased resting function of ischaemic sectors, but had no consistent effect on non-ischaemic sectors. (B) Change in regional EF during exercise. GR79236 increased exercise function of ischaemic sectors, but decreased exercise function of non-ischaemic sectors. (C) Change in global EF at rest. GR79236 tended to decrease resting global EF in patients whose EF decreased during exercise following placebo, but had no consistent effect in those whose EF increased. (D) Change in global EF during exercise. GR79236 tended to increase exercise global EF in patients whose EF decreased during exercise following placebo, but decreased exercise EF in those whose EF increased.
Before the first exercise test, GR79236 did not affect resting ejection fraction of non-ischaemic sectors compared with placebo, but reduced that of ischaemic sectors. During the first exercise test, GR79236 reduced ejection fraction and ΔEF for non-ischaemic sectors, but increased those indices for ischaemic sectors. During the second test compared with the first following GR79236, both non-ischaemic and ischaemic sectors showed non-significant increases in ejection fraction and ΔEF.
Global left ventricular systolic function
These results are shown in table 3 and fig 3. Global ejection fraction was comparable between the two exercise tests following either placebo or GR79236. GR79236 depressed baseline global ejection fraction compared with placebo, mainly affecting patients whose ejection fraction fell during subsequent exercise. The drug did not affect exercise ejection fraction or ΔEF when all patients were considered together. However, the scatter plot of individual patient data revealed a similar pattern to that observed with left ventricular sectors: GR79236 reduced exercise ejection fraction in patients with a positive (“non-ischaemic”) ΔEF following placebo, but increased exercise ejection fraction in patients with a negative (“ischaemic”) ΔEF following placebo.
Table 3.
Global ejection fraction (%) before and during the four exercise tests
| Resting EF | Exercise EF | ΔEF | |
| Placebo 1 | 63 (7) | 63 (7) | 0 (6) |
| Placebo 2 | 63 (9) | 64 (7) | +2 (5)* |
| Drug 1 | 61 (5)* | 62 (9) | +1 (6) |
| Drug 2 | 61 (7) | 64 (10) | +2 (6) |
| p Value | NS | NS | NS |
Values are mean (SD).
Comparisons between all four exercise tests using Friedman test; pairwise comparisons using Wilcoxon signed rank test with Bonferroni correction.
*p<0.05 v placebo 1. EF, ejection fraction.
DISCUSSION
We have shown that GR79236, a selective adenosine A1 receptor agonist, is unable to mimic the warm up effect. Moreover, warm up protection occurs even in the presence of the drug. The effects of GR79236 on regional left ventricular function at rest and during exercise were complex, and depended on the inducibility of ischaemic dysfunction.
Effect of GR79236 on baseline haemodynamic and ECG indices
GR79236 was well tolerated in our study, and had no effect on resting haemodynamic variables. The observed prolongation of the PR interval confirms that activation of adenosine A1 receptors in the atrioventricular node occurred at the dose used in the study. This negative dromotropic effect reflects activation of the inwardly rectifying potassium current, IK-Ado.15 Thus adenosine is used therapeutically to induce transient atrioventricular block in the termination of supraventricular tachyarrhythmias.
Effect of GR79236 on left ventricular contractile function
The classification of left ventricular sectors into “ischaemic” or “non-ischaemic” is robust, with a concordance of 78% for two tests performed one hour apart, with patients remaining on the gamma camera table throughout.13 In middle aged subjects with a low probability of coronary disease, only 9% of sectors showed a false positive “ischaemic” response. We have previously demonstrated that the ejection fraction of ischaemic sectors remains persistently reduced 15 and 30 minutes after exercise, consistent with myocardial stunning.13 The same sectors show increased function during the second of two exercise tests 30 minutes apart in association with the warm up effect. The reduction at 15 minutes and the increase during the second test are both proportional to the reduction during the first test. Non-ischaemic sectors show identical responses during the two exercise tests. Effects on global left ventricular function are apparent when the initial response to exercise is considered.
The observed effect of GR79236 on global and regional left ventricular function during exercise is similar to the effect of β blockade. In patients whose global ejection fraction increased “normally” with exercise following placebo, the drug decreased the exercise ejection fraction. A similar effect was seen on non-ischaemic sectors. Conversely, in patients whose global ejection fraction decreased “abnormally” with exercise following placebo, GR79236 increased the exercise ejection fraction. A similar effect was seen on ischaemic sectors. Likewise, patients undergoing exercise ERNA while taking β blockers show an impaired increase in global ejection fraction in the absence of coronary disease, or an attenuated fall in the presence of coronary disease.16
At rest, GR79236—unlike β blockers—did not affect the global ejection fraction in patients with a normal exercise response, or the regional ejection fraction of non-ischaemic sectors. These observations are consistent with data from basic research showing that adenosine has a negative inotropic effect on the left ventricle, but only in the setting of β1 adrenergic stimulation (that is, in the context of our study, during exercise but not at rest).17,18 Dobson and colleagues studied isolated, perfused, normoxic rat hearts and showed that the inotropic effect of isoprenaline (isoproterenol) was attenuated by an exogenous adenosine agonist, but was potentiated by breakdown of endogenous adenosine by adenosine deaminase. Sato and colleagues obtained similar results in open chest dogs during coronary hypoperfusion18. The inotropic effect of isoprenaline was attenuated by exogenous adenosine but was potentiated by an adenosine receptor antagonist or an inhibitor of endogenous adenosine release. Thus adenosine modulating agents did not appear to affect left ventricular function in the absence of β1 stimulation.
GR79236 depressed the function of ischaemic sectors at rest without affecting that of non-ischaemic sectors. This resulted in a reduction in global left ventricular function overall, but specifically in patients whose global ejection fraction fell with exercise. Given the selectivity of GR79236 for adenosine A1 receptors, this is unlikely to have been the result of a gross A2 mediated steal effect, as occurs when adenosine stress is used in myocardial perfusion imaging. Our observation may instead reflect a transmural steal effect, the opposite of which has been invoked to explain the protection against exercise induced myocardial ischaemia that is seen with the A1 receptor antagonists theophylline and the more selective bamiphylline.19,20 Activation of prejunctional A1 receptors on sympathetic perivascular nerves is known to inhibit catecholamine release. This would lead to a selective dilatation of subepicardial vessels, with a transmural steal of coronary blood flow away from the subendocardium in regions supplied by stenosed coronary arteries. The subendocardium would become ischaemic, resulting in resting hypocontractility.
Relation to other studies of the pharmacology of the warm up effect
Importantly, GR79236 did not significantly affect the time to, or the rate–pressure product at, the onset of angina or ST depression, and did not therefore mimic the warm up effect. Moreover, warm up protection occurred during sequential exercise testing despite previous administration of the drug. These observations suggest that activation of adenosine A1 receptors is not an essential event in triggering the warm up effect. Consistent with these findings, other workers have failed to prevent the phenomenon using the adenosine A1 antagonists theophylline and bamiphylline.21,22
It has been suggested that the KATP channel is a possible end effector in ischaemic preconditioning. The KATP channel blocker glibenclamide prevents ischaemic preconditioning in many animal models, but studies of the warm up effect have drawn conflicting conclusions. Ovunc showed complete abolition of the warm up effect in diabetic patients following a dose of glibenclamide.23 Tomai and colleagues were only able to show loss of the increase in rate–pressure product at, but not the time to, 1.5 mm ST depression.24 They interpreted these findings as evidence that “KATP channels play a role in the component of the warm up phenomenon related to ischaemic preconditioning”. Other workers have reported frankly negative results, with the warm up effect persisting after a single dose of glibenclamide in a study by Correa and Schaefer, or in diabetic patients taking long term glibenclamide in a study by Bogaty and colleagues.25,26
During coronary angioplasty in humans, there is a reduction in chest pain severity and ST depression during the second of two consecutive balloon inflations.27 The use of total coronary occlusion rather than demand ischaemia equates directly to laboratory studies of ischaemic preconditioning. The pharmacology of this “model” is more consistent with ischaemic preconditioning than that of the warm up effect. Adenosine and bradykinin have been shown to reproduce protection in this setting, while bamiphylline, glibenclamide, and naloxone (a non-selective opioid receptor antagonist) prevent it.7,28–31
Limitations of the study
Ischaemic preconditioning is a direct myocardial protective effect, and is not caused by improved blood supply through recruitment of collaterals as it occurs even in species which lack such channels.32 However, the warm up effect could be explained by acute recruitment of coronary collaterals leading to improved myocardial perfusion. The inability to measure transmural myocardial perfusion accurately in an exercising patient is an important confounding factor in most studies of the warm up effect. Bogaty and colleagues addressed this issue using quantitative thallium-201 SPECT in patients with coronary disease performing treadmill exercise.33 They were unable to detect a difference in the severity or extent of perfusion defects between a single exercise test performed on one day, and the second of two exercise tests performed 10 minutes apart on another day. However, the ability of SPECT to resolve subtle differences in transmural myocardial perfusion is uncertain.
The quantification of perfusion within the layers of myocardium from subendocardium to subepicardium is impossible in an exercising patient. Therefore our interpretation of the effects of GR79236 on left ventricular function remains speculative. Nevertheless, the A1 selectivity of the drug appears to exclude a gross A2 mediated coronary steal effect.
Conclusions
GR79236 failed to reproduce “warm up” protection against angina and ECG changes. This suggests that adenosine A1 receptor triggered ischaemic preconditioning is not an important component of this type of protection in man. GR79236 had complex effects on left ventricular function at rest and during exercise, suggesting multiple competing A1 mediated actions.
Acknowledgments
ADK is supported by a British Heart Foundation Junior Research Fellowship.
Abbreviations
ERNA, equilibrium radionuclide angiography; KATP, ATP gated potassium channel; RPP, rate
pressure product; SPECT, single photon emission computed tomography; ΔEF; change in ejection fraction between rest and exercise
REFERENCES
- 1.Liu GS, Thornton J, VanWinkle DM, et al. Protection against infarction afforded by preconditioning is mediated by A1-adenosine receptors in the rabbit heart. Circulation 1991;84:350–6. [DOI] [PubMed] [Google Scholar]
- 2.Auchampach JA, Gross GJ. Adenosine A1 receptors, KATP channels, and ischemic preconditioning in dogs. Am J Physiol 1993;264:H1327–36. [DOI] [PubMed] [Google Scholar]
- 3.Walker DM, Walker JM, Pugsley WB, et al. Preconditioning in isolated superfused human muscle. J Mol Cell Cardiol 1995;27:1349–57. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol 2000;62:79–109. [DOI] [PubMed] [Google Scholar]
- 5.Mahaffey KW, Puma JA, Barbagelata A, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction. J Am Coll Cardiol 1999;34:1711–20. [DOI] [PubMed] [Google Scholar]
- 6.Mentzer RM, Rahko PS, Molina-Viamonte V, et al. Safety, tolerance, and efficacy of adenosine as an additive to blood cardioplegia in humans during coronary artery bypass surgery. Am J Cardiol 1997;79:38–43. [DOI] [PubMed] [Google Scholar]
- 7.Leesar MA, Stoddard M, Ahmed M, et al. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation 1997;95:2500–7. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe MD, Quinn NK. Warm-up phenomenon in angina pectoris. Lancet 1980;ii:934–6. [DOI] [PubMed] [Google Scholar]
- 9.Gurden MF, Coates J, Ellis F, et al. Functional characterization of three adenosine receptor types. Br J Pharmacol 1993;109:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheldrick A, Gray KM, Drew GM, et al. The effect of body temperature on myocardial protection conferred by ischaemic preconditioning or the selective adenosine A1 receptor agonist GR79236, in an anaesthetized rabbit model of myocardial ischaemia and reperfusion. Br J Pharmacol 1999;128:385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louttit JB, Hunt AA, Maxwell MP, et al. The time course of cardioprotection induced by GR79236, a selective adenosine A1-receptor agonist, in myocardial ischaemia-reperfusion injury in the pig. J Cardiovasc Pharmacol 1999;33:285–91. [DOI] [PubMed] [Google Scholar]
- 12.Huang C-H, Kim S-J, Ghaleh B, et al. An adenosine agonist and preconditioning shift the distribution of myocardial blood flow in conscious pigs. Am J Physiol 1999;276:H368–75. [DOI] [PubMed] [Google Scholar]
- 13.Kelion AD, Webb TP, Gardner MA, et al. The warm-up effect protects against ischaemic left ventricular dysfunction in patients with angina. J Am Coll Cardiol 2001;37:705–10. [DOI] [PubMed] [Google Scholar]
- 14.Kelion AD, Banning AP, Ormerod OJM. Does exercise radionuclide angiography still have a role in clinical cardiac assessment? J Nucl Cardiol 1999;6:540–6. [DOI] [PubMed] [Google Scholar]
- 15.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol 1997;79:2–10. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay J, Nolan NG, Goldstein SA, et al. Effects of beta-adrenergic blocking drugs on sensitivity and specificity of radionuclide ventriculography during exercise in patients with coronary heart disease. Am Heart J 1983;106:271–8. [DOI] [PubMed] [Google Scholar]
- 17.Dobson JG, Ordway RW, Fenton RA. Endogenous adenosine inhibits catecholamine contractile responses in normoxic hearts. Am J Physiol 1986;251:H455–62. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Hori M, Kitakaze M, et al. Endogenous adenosine blunts β-adrenoceptor-mediated inotropic response in hypoperfused canine myocardium. Circulation 1992;85:1594–603. [DOI] [PubMed] [Google Scholar]
- 19.Crea F, Pupita G, Galassi AR, et al. Effect of theophylline on exercise-induced myocardial ischaemia. Lancet 1989;i:683–6. [DOI] [PubMed] [Google Scholar]
- 20.Gaspardone A, Crea F, Iamele M, et al. Bamiphylline improves exercise-induced myocardial ischemia through a novel mechanism of action. Circulation 1993;88:502–8. [DOI] [PubMed] [Google Scholar]
- 21.Kerensky RA, Franco E, Schlaifer JD, et al. Effect of theophylline on the warm-up phenomenon. Am J Cardiol 1999;84:1077–80. [DOI] [PubMed] [Google Scholar]
- 22.Tomai F, Crea F, Danesi A, et al. Effects of A1 adenosine receptor blockade on the warm-up phenomenon. Cardiologia 1997;42:385–92. [PubMed] [Google Scholar]
- 23.Ovunc K. Effects of glibenclamide, a K(ATP) channel blocker, on warm-up phenomenon in type II diabetic patients with chronic stable angina pectoris. Clin Cardiol 2000;23:535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomai F, Danesi A, Ghini AS, et al. Effects of KATP channel blockade by glibenclamide on the warm-up phenomenon. Eur Heart J 1999;20:196–202. [DOI] [PubMed] [Google Scholar]
- 25.Correa SD, Schaefer S. Blockade of KATP channels with glibenclamide does not abolish preconditioning during demand ischemia. Am J Cardiol 1997;79:75–8. [DOI] [PubMed] [Google Scholar]
- 26.Bogaty P, Kingma JG, Robitaille N-M, et al. Attenuation of myocardial ischemia with repeated exercise in subjects with chronic stable angina. J Am Coll Cardiol 1998;32:1665–71. [DOI] [PubMed] [Google Scholar]
- 27.Deutsch E, Berger M, Kussmaul WG, et al. Adaptation to ischemia during percutaneous transluminal coronary angioplasty. Clinical, hemodynamic, and metabolic features. Circulation 1990;82:2044–51. [DOI] [PubMed] [Google Scholar]
- 28.Leesar MA, Stoddard MF, Manchikalapudi S, et al. Bradykinin-induced preconditioning in patients undergoing coronary angioplasty. J Am Coll Cardiol 1999;34:639–50. [DOI] [PubMed] [Google Scholar]
- 29.Tomai F, Crea F, Gaspardone A, et al. Effects of A1 adenosine receptor blockade by bamiphylline on ischaemic preconditioning during coronary angioplasty. Eur Heart J 1996;17:846–53. [DOI] [PubMed] [Google Scholar]
- 30.Tomai F, Crea F, Gaspardone A, et al. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation 1994;90:700–5. [DOI] [PubMed] [Google Scholar]
- 31.Tomai F, Crea F, Gaspardone A, et al. Effects of naloxone on myocardial ischemic preconditioning in humans. J Am Coll Cardiol 1999;33:1863–9. [DOI] [PubMed] [Google Scholar]
- 32.Liu GS, Thornton J, VanWinkle DM, et al. Protection against infarction afforded by preconditioning is mediated by A1-adenosine receptors in the rabbit heart. Circulation 1991;84:350–6. [DOI] [PubMed] [Google Scholar]
- 33.Bogaty P, Kingma JG, Guimond J, et al. Myocardial perfusion imaging findings and the role of adenosine in the warm-up angina phenomenon. J Am Coll Cardiol 2001;37:463–9. [DOI] [PubMed] [Google Scholar]